Sex-Specific Aspects of Cardiac Electrophysiology and Arrhythmias
Abstract

Introduction
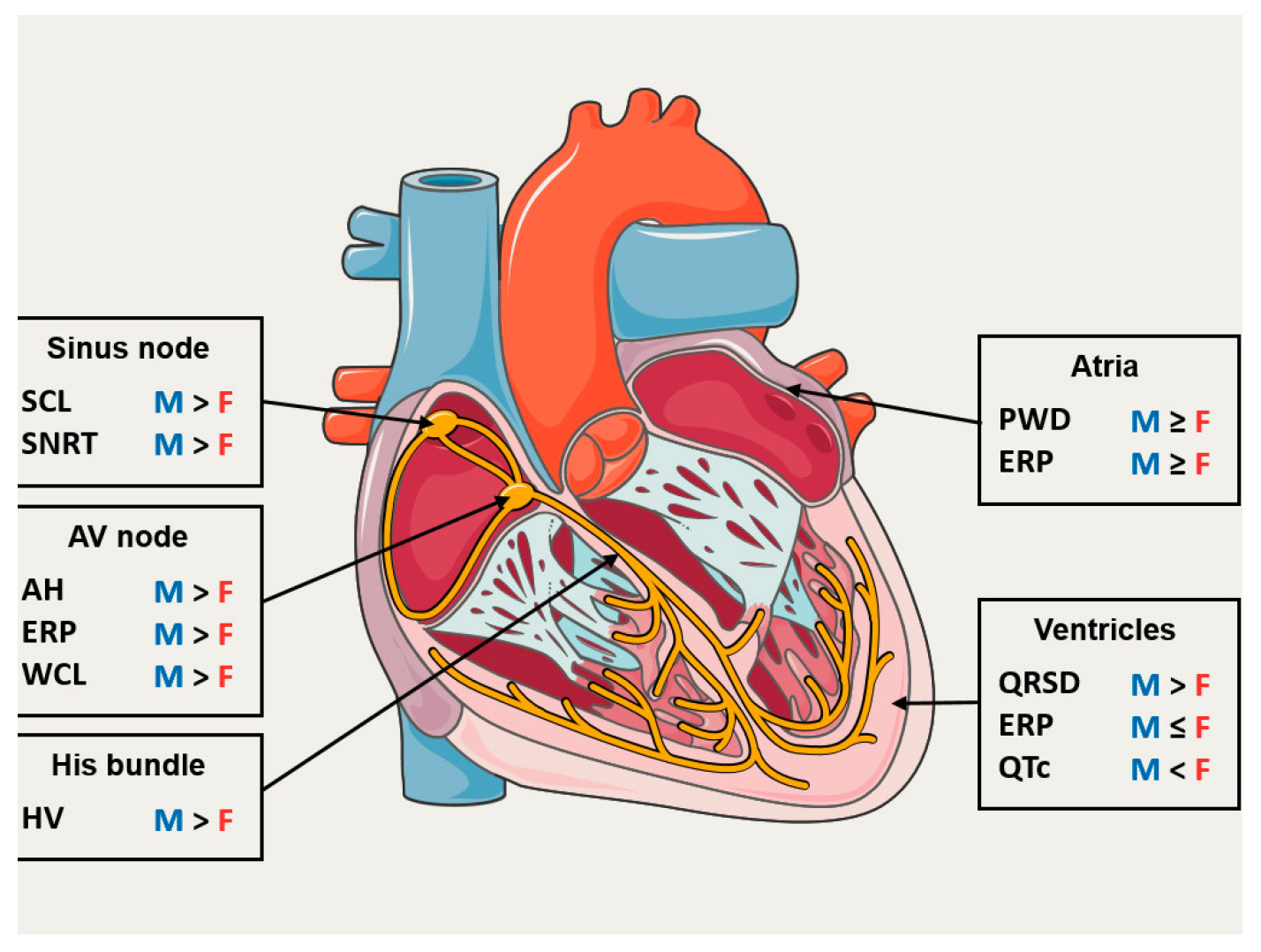
Sex Differences in Electrophysiological Properties of the Heart
Effect of the Menstrual Cycle
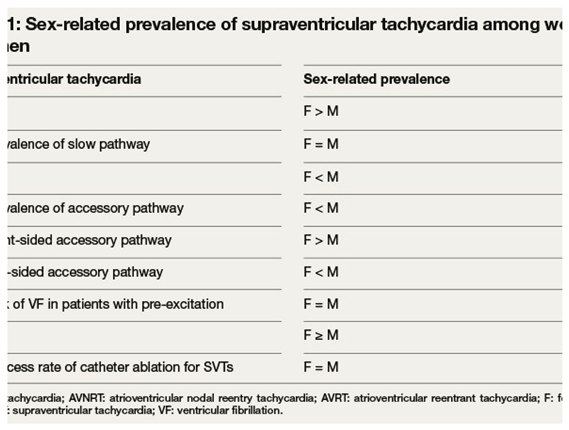
Supraventricular Tachycardias
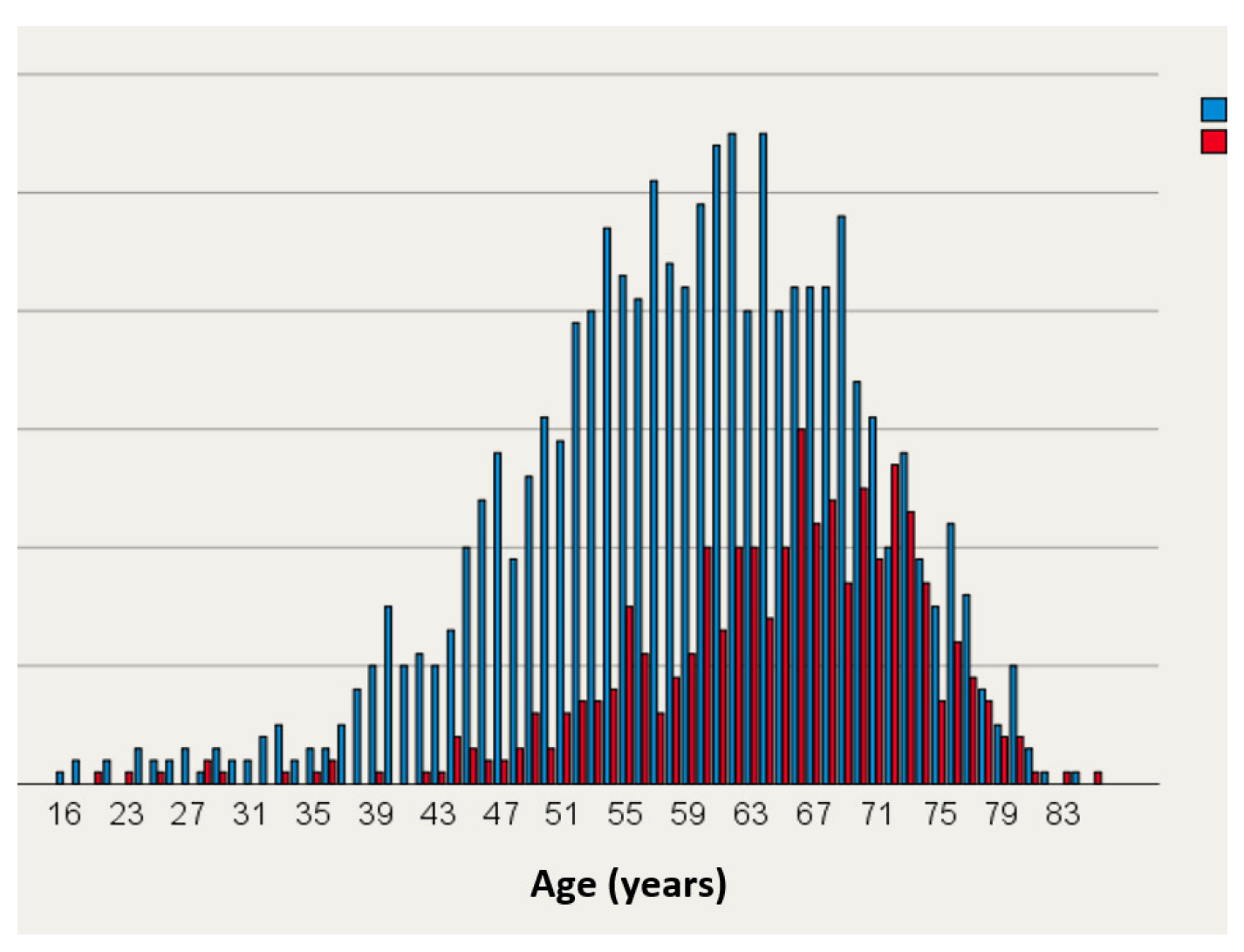
Atrial Fibrillation
Prevalence and Symptoms
Therapy of Atrial Fibrillation
Ventricular Tachycardia (VT)
Idiopathic Ventricular Arrhythmias
Ventricular Arrhythmias Associated with Structural Heart Disease
Inherited Cardia Arrhythmias
Long QT Syndrome
Brugada Syndrome
Conflicts of Interest
References
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; et al. Sex differences in cardiac arrhythmia: A consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. 2018, 20, 1565. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.V.; Robinson, R.B.; Danilo, P.; Jr Rosen, M.R. Effects of gonadal steroids on gender-related differences in transmural dispersion of L-type calcium current. Cardiovasc Res. 2002, 53, 752–762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Yuan, S.; Hertervig, E.; Kongstad, O.; Olsson, S.B. Gender and atrioventricular conduction properties of patients with symptomatic atrioventricular nodal reentrant tachycardia and Wolff-Parkinson-White syndrome. J Electrocardiol. 2001, 34, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hnatkova, K.; Smetana, P.; Toman, O.; Schmidt, G.; Malik, M. Sex and race differences in QRS duration. Europace. 2016, 18, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Zhou, S.H.; Wong, S.; Calhoun, H.P.; Berenson, G.S.; Prineas, R.; et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992, 8, 690–695. [Google Scholar] [PubMed]
- Nakagawa, M.; Ooie, T.; Takahashi, N.; Taniguchi, Y.; Anan, F.; Yonemochi, H.; et al. Influence of menstrual cycle on QT interval dynamics. Pacing Clin Electrophysiol. 2006, 29, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.S.; Demolis, J.L.; Riviere, R.; Strabach, S.; ChristinMaitre, S.; Funck-Brentano, C. Influence of endogenous oestrogens on QT interval duration. Eur Heart J. 2003, 24, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.H.; Ehlert, F.A.; Kruse, J.T.; Parker, M.A.; Goldberger, J.J.; Kadish, A.H. Gender-specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol. 1997, 79, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.; Leonardo, F.; Sarrel, P.M.; Beale, C.M.; De Luca, F.; Collins, P. Cyclical variation in paroxysmal supraventricular tachycardia in women. Lancet. 1996, 347, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Myerburg, R.J.; Cox, M.M.; Interian, A.; Jr Mitrani, R.; Girgis, I.; Dylewski, J.; et al. Cycling of inducibility of paroxysmal supraventricular tachycardia in women and its implications for timing of electrophysiologic procedures. Am J Cardiol. 1999, 83, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Tadros, R.; Ton, A.T.; Fiset, C.; Nattel, S. Sex differences in cardiac electrophysiology and clinical arrhythmias: Epidemiology, therapeutics, and mechanisms. Can J Cardiol. 2014, 30, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.J.; Morton, J.B.; Denman, R.; Lin, A.C.; Tierney, S.; Santucci, P.A.; et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004, 1, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; di Biase, L.; Pelargonio, G.; Natale, A. Outcome of invasive electrophysiological procedures and gender: Are males and females the same? J Cardiovasc Electrophysiol. 2011, 22, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet. 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roten, L.; Goulouti, E.; Lam, A.; Elchinova, E.; Nozica, N.; Spirito, A.; et al. Age and Sex Specific Prevalence of Clinical and Screen-Detected Atrial Fibrillation in Hospitalized Patients. J Clin Med. 2021, 10, 4871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lip, G.Y.; Laroche, C.; Boriani, G.; Cimaglia, P.; Dan, G.A.; Santini, M.; et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: A report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace. 2015, 17, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Pecen, L.; Ojeda, F.M.; Lucerna, M.; Rzayeva, N.; Blankenberg, S.; et al. Gender differences in clinical presentation and 1-year outcomes in atrial fibrillation. Heart. 2017, 103, 1024–1030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, D.; Mohanty, P.; Di Biase, L.; Sanchez, J.E.; Shaheen, M.H.; Burkhardt, J.D.; et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Roten, L.; Rimoldi, S.F.; Schwick, N.; Sakata, T.; Heimgartner, C.; Fuhrer, J.; et al. Gender differences in patients referred for atrial fibrillation management to a tertiary center. Pacing Clin Electrophysiol. 2009, 32, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, T.; Kaelin-Friedrich, M.; Makowski, K.; Noti, F.; Schaer, B.; Haeberlin, A.; et al. Sex-Related Differences in Patient Selection for and Outcomes after Pace and Ablate for Refractory Atrial Fibrillation: Insights from a Large Multicenter Cohort. J Clin Med. 2022, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michowitz, Y.; Rahkovich, M.; Oral, H.; Zado, E.S.; Tilz, R.; John, S.; et al. Effects of sex on the incidence of cardiac tamponade after catheter ablation of atrial fibrillation: Results from a worldwide survey in 34 943 atrial fibrillation ablation procedures. Circ Arrhythm Electrophysiol. 2014, 7, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019, 21, 1143–1144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakagawa, M.; Takahashi, N.; Nobe, S.; Ichinose, M.; Ooie, T.; Yufu, F.; et al. Gender differences in various types of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 2002, 13, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Baldinger, S.H.; Kumar, S.; Romero, J.; Fujii, A.; Epstein, L.M.; Michaud, G.F.; et al. A Comparison of Women and Men Undergoing Catheter Ablation for Sustained Monomorphic Ventricular Tachycardia. J Cardiovasc Electrophysiol. 2017, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Frankel, D.S.; Tung, R.; Santangeli, P.; Tzou, W.S.; Vaseghi, M.; Di Biase, L.; et al. Sex and Catheter Ablation for Ventricular Tachycardia: An International Ventricular Tachycardia Ablation Center Collaborative Group Study. JAMA Cardiol. 2016, 1, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Crotti, L.; Insolia, R. Long-QT syndrome: From genetics to management. Circ Arrhythm Electrophysiol. 2012, 5, 868–877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vink, A.S.; Neumann, B.; Lieve, K.V.V.; Sinner, M.F.; Hofman, N.; El Kadi, S.; et al. Determination and Interpretation of the QT Interval. Circulation. 2018, 138, 2345–2358. [Google Scholar] [CrossRef] [PubMed]
- Locati, E.H.; Zareba, W.; Moss, A.J.; Schwartz, P.J.; Vincent, G.M.; Lehmann, M.H.; et al. Ageand sex-related differences in clinical manifestations in patients with congenital longQT syndrome: Findings from the International LQTS Registry. Circulation. 1998, 97, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.H.; Timothy, K.W.; Frankovich, D.; Fromm, B.S.; Keating, M.; Locati, E.H.; et al. Age-gender influence on the rate-corrected QT interval and the QT-heart rate relation in families with genotypically characterized long QT syndrome. Journal of the American College of Cardiology. 1997, 29, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, B.; Yee, L.; Ben-Haim, Y.; Dobner, S.; Servatius, H.; Roten, L.; et al. Sex-Related Differences in Cardiac Channelopathies: Implications for Clinical Practice. Circulation. 2021, 143, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Mason, J.W.; Akiyama, T. New ageand sex-specific criteria for QT prolongation based on rate correction formulas that minimize bias at the upper normal limits. Int J Cardiol. 2014, 174, 535–540. [Google Scholar] [CrossRef] [PubMed]


© 2023 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Mühl, A.; Asatryan, B.; Tanner, H. Sex-Specific Aspects of Cardiac Electrophysiology and Arrhythmias. Cardiovasc. Med. 2023, 26, 698. https://doi.org/10.4414/cvm.2023.1243790698
Mühl A, Asatryan B, Tanner H. Sex-Specific Aspects of Cardiac Electrophysiology and Arrhythmias. Cardiovascular Medicine. 2023; 26(5):698. https://doi.org/10.4414/cvm.2023.1243790698
Chicago/Turabian StyleMühl, Aline, Babken Asatryan, and Hildegard Tanner. 2023. "Sex-Specific Aspects of Cardiac Electrophysiology and Arrhythmias" Cardiovascular Medicine 26, no. 5: 698. https://doi.org/10.4414/cvm.2023.1243790698
APA StyleMühl, A., Asatryan, B., & Tanner, H. (2023). Sex-Specific Aspects of Cardiac Electrophysiology and Arrhythmias. Cardiovascular Medicine, 26(5), 698. https://doi.org/10.4414/cvm.2023.1243790698



