Stroke after Valve Intervention–How to Look For an Embolic Source
Abstract
Introduction
Epidemiology
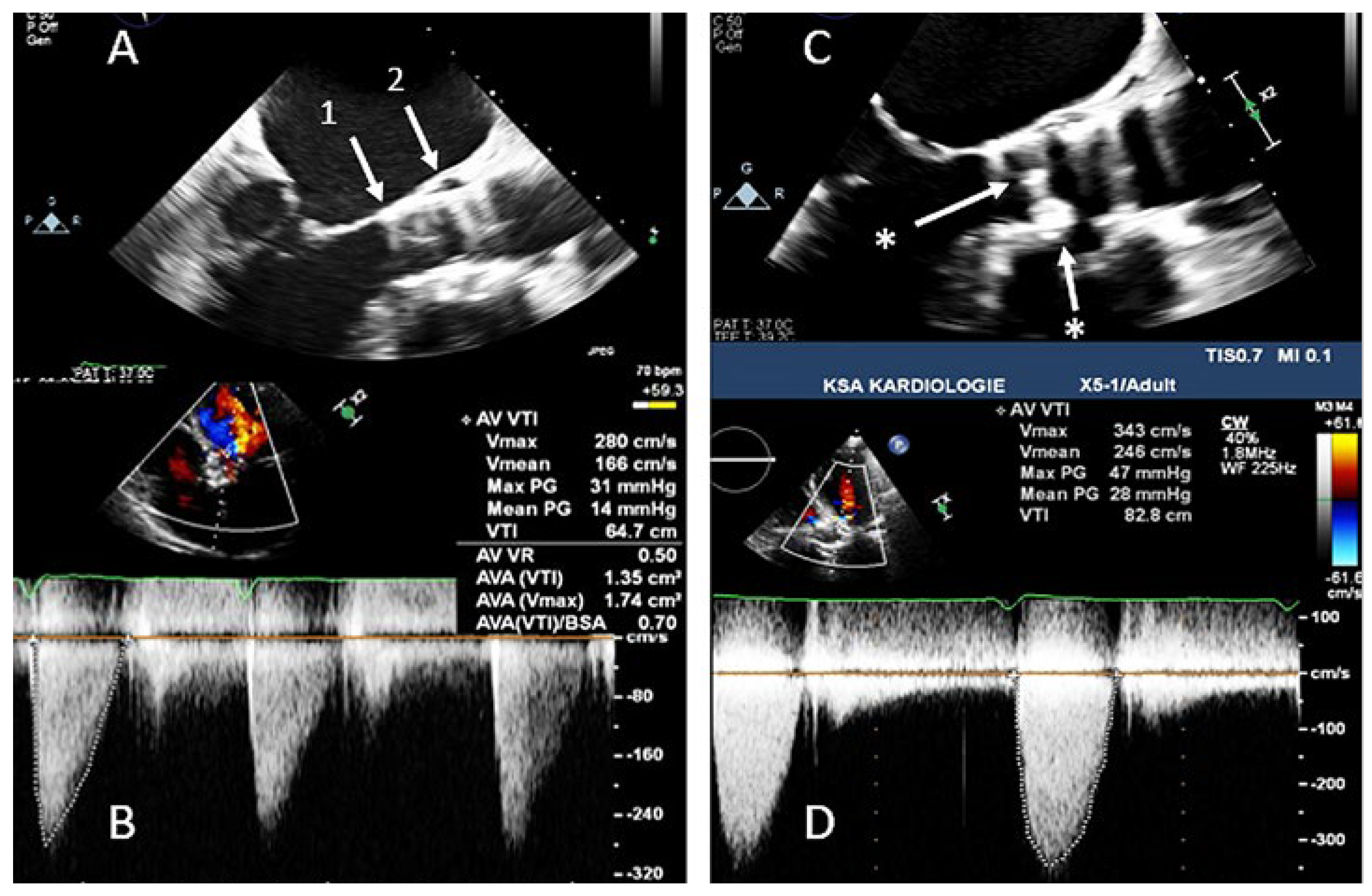
Thrombosis
Infective Endocarditis
Keypoints
- Perform TTE within 24h with / without contrast.
- Perform comprehensive TOE within 48h including assessment of all valves’ morphology and function, screening the LA, LAA and aorta for any thrombi.
- Higher diagnostic sensitivity given by multimodality imaging including cardiac CT and 18F-FDG-PET.
- CT depicts nicely any thrombosis formation in prothesis and any local complication.
- If endocarditis is possible, add 18F-FDG-PET (at the earliest 3 months after implantation).
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Healey, J.S. Cardioembolic stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Eitel, I.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Kapadia s Agarwal, S.; Miller, D.C.; Webb, J.G.; Mack, M.; Ellis, S.; et al. Insights into Timing, Risk Factors, and Outcomes of Stroke and Transient Ischemic Attack After Transcatheter Aortic Valve Replacement in the PARTNER Trial. Circ. Cardiovasc. Interv. 2016, 9, e002981. [Google Scholar] [CrossRef] [PubMed]
- Spaziano, M.; Francese, D.P.; Leon, M.B.; Généreux, P. Imaging and functional testing to assess clinical and subclinical neurological events after transcatheter or surgical aortic valve replacement: A comprehensive review. J. Am. Coll. Cardiol. 2014, 4, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.L.; Gurvitch, R.; Wijesinghe, N.; Nietlispach, F.; Wood, D.; Cheung, A.; et al. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2011, 4, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Kodali, S.; Makkar, R.; Mehran, R.; Lazar, R.M.; Zivadinov, R.; et al. SENTINEL Trial Investigators. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017, 31, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Del Val, D.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; et al. Stroke Complicating Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 11, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Barros da Silva, P.; Sousa, J.P.; Oliveiros, B.; Donato, H.; Costa, M.; Gonçalves, L.; et al. Stroke after transcatheter edge-to-edge mitral valve repair: A systematic review and meta-analysis. EuroIntervention 2020, 15, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.K.; Wilson, J.S.; Hearne, S.E.; Bashore, T.M. Complications related to percutaneous transvenous mitral commissurotomy. Cathets Cardiovasc. Diagn. 1994, (Suppl. 2), 52–60. [Google Scholar]
- Liu, T.J.; Lai, H.C.; Lee, W.L.; Wang, K.Y.; Wei, H.J.; Ting, C.T.; et al. Percutaneous balloon commissurotomy reduces incidence of ischemic cerebral stroke in patients with symptomatic rheumatic mitral stenosis. Int. J. Cardiol. 2008, 11, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Saad, M.; Girotra, S.; Desai, M.; Elbadawi, A.; Briasoulis, A.; et al. Impact of Pre-Existing and New-Onset Atrial Fibrillation on Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, F.; Benfari, G.; Vanoverschelde, J.L.; Tribouilloy, C.; Avierinos, J.F.; Bursi, F.; MIDA Investigators; et al. Long-Term Implications of Atrial Fibrillation in Patients With Degenerative Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 73, 264–274. [Google Scholar] [CrossRef]
- Roudaut, R.; Serri, K.; Lafitte, S. Thrombosis of prosthetic heart valves: Diagnosis and therapeutic considerations. Heart 2007, 93, 137–142. [Google Scholar] [CrossRef]
- Harkness, A.; Ring, L.; Augustine, D.; Oxborough, D.; Robinson, S.; Sharma, V. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: A guideline from the Briths Society of Echocardiography. Echo Res. Pract. 2020, 7, G1–G18. [Google Scholar] [CrossRef] [PubMed]
- British Society of Echocardiography. EchoCalc for iPhone. London, UK: British Society of Echocardiography. 2015. Available online: https://apps.apple.com/us/app/echocalc/id468166426.
- British Society of Echocardiography. EchoCalc for android. London, UK: British Society of Echocardiography. 2014. Available online: https://play.google.com/store/apps/details?id=bse.echocalc.
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: A report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014, quiz 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2017, 52, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Bax, J.J.; Delgado, V.; Hahn, R.T.; Leipsic, J.; Min, J.K.; Grayburn, P.; et al. Transcatheter Aortic Valve Replacement: Role of Multimodality Imaging in Common and Complex Clinical Scenarios. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Pache, G.; Schoechlin, S.; Blanke, P.; Dorfs, S.; Jander, N.; Arepalli, C.D.; et al. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur. Heart J. 2016, 37, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
| First Author (Year) | N | Prevalece of Thrombosis on MDCT (Time) | Prevalence of Thrombosis on Echo (Time) | Mean Gradient (mm Hg)—EOA (cm2) |
|---|---|---|---|---|
| Latib et al. (2015) | 4266 | NA | 0.61% (median, 181 days) | 40.5 ± 14.0-NA |
| Pache et al. (2016) | 156 | 10.6% (median, 5 days) | NA (median, 5 days) | 8 ± 3.5-NA |
| Leetmaa et al. (2016) | 140 | 4% (1–3 months) | NA (1–3 months) | 19.2–1.44 |
| Del Trigo et al. (2016) | 1521 | NA | 4.5% (4 yrs) | 26.1 ± 11-NA |
| Hansson et al. (2016) | 405 | 7% (1–3 months) | NA (1–3 months) | 10 ± 7–1.5 ± 0.5 |
| Makkar et al. (2016) | 55 | 40% (median, 32 days) | NA (30 days) | 9.2 ± 4.9-NA |
| Makkar et al. (2016) | 132 | 13% (median, 86 days) | NA (30 days) | 8.4 ± 2.9-NA |
| Yanagisawa et al. (2017) | 70 | 14.3% (1 yr) | NA (1 yr) | 8.3 ± 0.8–1.03 ± 0.25 |
| Chakravarty et al. (2017) | 752 | 13% (median, 58 days) | 6% (median, 58 days) | 13.8 ± 10.0-NA |
| Vollema et al. (2017) | 434 | 12% median, 35 days) | 3% (3 yrs) | 9.3 ± 4.7-1.99 ± 0.56 |
| Jose et al. (2017) | 642 | 9/10 (NA) | 2.8% median, 181 days) | 34 ± 14–1.06 ± 0.46 |
| Sondergaard et al. (2017) | 61 | 11% (140 ± 152 days) | NA | 7.0 ± 3.2-NA |
© 2023 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Yakupoglu, H.Y.; Fuchs, T.A. Stroke after Valve Intervention–How to Look For an Embolic Source. Cardiovasc. Med. 2023, 26, 119. https://doi.org/10.4414/cvm.2023.02232
Yakupoglu HY, Fuchs TA. Stroke after Valve Intervention–How to Look For an Embolic Source. Cardiovascular Medicine. 2023; 26(4):119. https://doi.org/10.4414/cvm.2023.02232
Chicago/Turabian StyleYakupoglu, H. Yakup, and Tobias A. Fuchs. 2023. "Stroke after Valve Intervention–How to Look For an Embolic Source" Cardiovascular Medicine 26, no. 4: 119. https://doi.org/10.4414/cvm.2023.02232
APA StyleYakupoglu, H. Y., & Fuchs, T. A. (2023). Stroke after Valve Intervention–How to Look For an Embolic Source. Cardiovascular Medicine, 26(4), 119. https://doi.org/10.4414/cvm.2023.02232



