Impact of Sex and Gender on Heart Failure
Abstract
Introduction
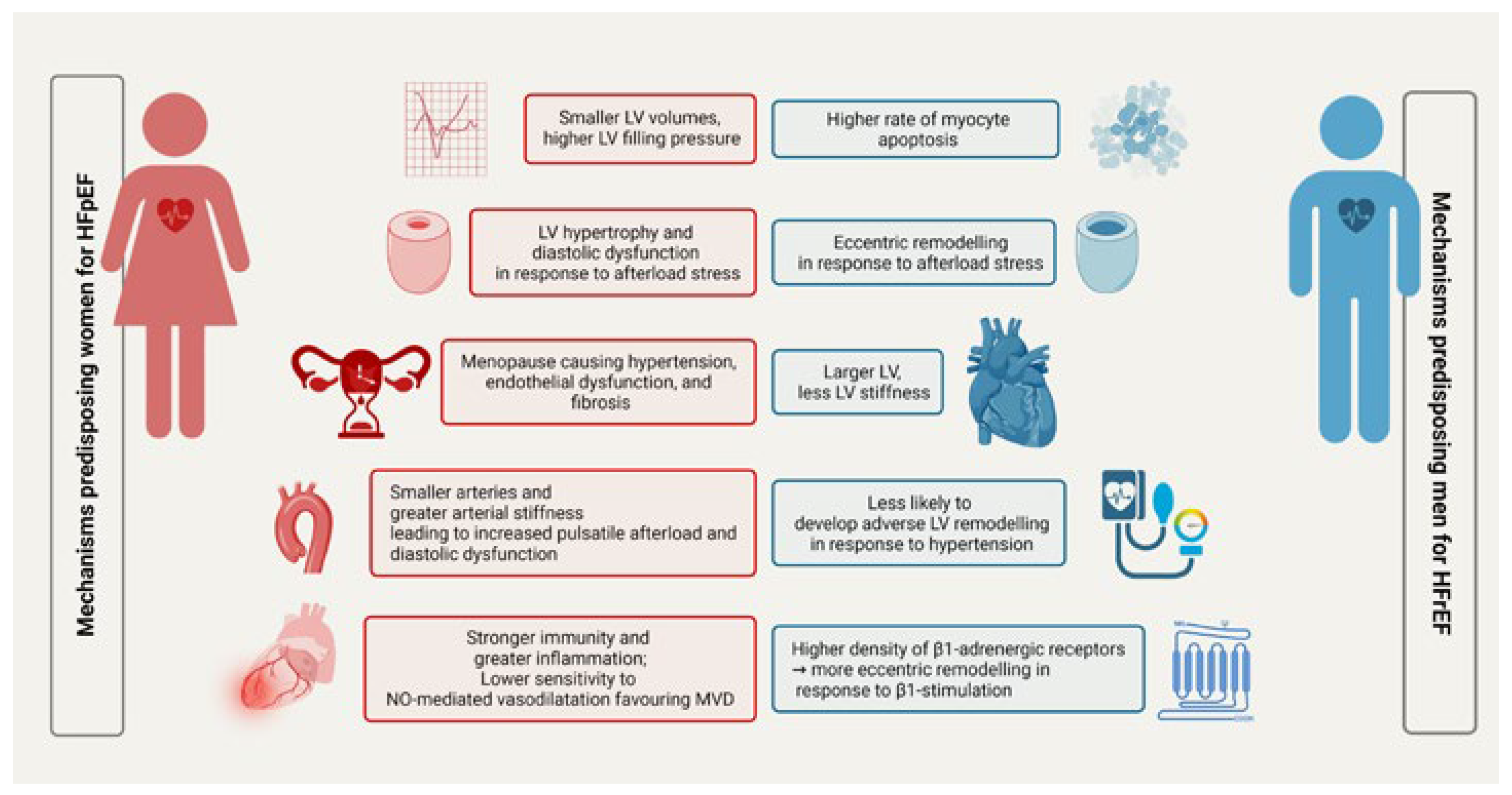
Sex differences in heart failure pathophysiology
Sex and gender differences in heart failure risk factors
Sex and gender differences in heart failure outcomes
Impact of sex and gender on heart failure treatment
Pharmacological therapies
Non-pharmacological therapies and palliative care
Devices and advanced heart failure therapies
Implantable cardioverter-defibrillators and cardiac resynchronisation therapy
Mechanical circulatory support devices
Heart transplantation
Female sex-specific conditions
Conclusions
Funding statement
Disclosure statement
References
- Bragazzi, N.L.; Zhong, W.; Shu, J.; Abu Much, A.; Lotan, D.; Grupper, A.; et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur. J. Prev. Cardiol. 2021, 28, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Komorita, Y.; Peters, S.A.; Woodward, M. Diabetes as a risk factor for heart failure in women and men: A systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019, 62, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; et al. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Pandey, A.; Omar, W.; Ayers, C.; LaMonte, M.; Klein, L.; Allen, N.B.; et al. Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Circulation 2018, 137, 1814–1823. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Zhao, X.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Cannon, C.P.; et al. Get With the Gueidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012, 126, 65–75. [Google Scholar] [CrossRef]
- Lopatin, Y. Heart Failure with Mid-Range Ejection Fraction and How to Treat It. Card. Fail. Rev. 2018, 4, 9–13. [Google Scholar] [CrossRef]
- Bhambhani, V.; Kizer, J.R.; Lima, J.A.; van der Harst, P.; Bahrami, H.; Nayor, M.; et al. Predictors and outcomes of heart failure with mid-range ejection fraction. Eur. J. Heart Fail. 2018, 20, 651–659. [Google Scholar] [CrossRef]
- Burnett, H.; Earley, A.; Voors, A.A.; Senni, M.; McMurray, J.J.; Deschaseaux, C.; et al. Thirty Years of Evidence on the Efficacy of Drug Treatments for Chronic Heart Failure With Reduced Ejection Fraction: A Network Meta-Analysis. Circ. Heart Fail. 2017, 10, e003529. [Google Scholar] [CrossRef]
- Lawson, C.A.; Zaccardi, F.; Squire, I.; Ling, S.; Davies, M.J.; Lam, C.S.; et al. 20-year trends in cause-specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: A population-based study. Lancet Public Health 2019, 4, e406–e20. [Google Scholar] [CrossRef]
- Chung, A.K.; Das, S.R.; Leonard, D.; Peshock, R.M.; Kazi, F.; Abdullah, S.M.; et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: The Dallas Heart Study. Circulation 2006, 113, 1597–1604. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Borlaug, B.A.; Rodeheffer, R.J.; Kass, D.A. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation 2005, 112, 2254–2262. [Google Scholar]
- Taki, M.; Ishiyama, Y.; Mizuno, H.; Komori, T.; Kono, K.; Hoshide, S.; et al. Sex Differences in the Prognostic Power of Brain Natriuretic Peptide and N-Terminal Pro-Brain Natriuretic Peptide for Cardiovascular Events—The Japan Morning Surge-Home Blood Pressure Study. Circ. J. Off. J. Jpn. Circ. Soc. 2018, 82, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Aurigemma, G.P.; Gaasch, W.H. Gender differences in older patients with pressure-overload hypertrophy of the left ventricle. Cardiology 1995, 86, 310–317. [Google Scholar] [CrossRef]
- Kuch, B.; Muscholl, M.; Luchner, A.; Döring, A.; Riegger, G.A.; Schunkert, H.; et al. Gender specific differences in left ventricular adaptation to obesity and hypertension. J. Hum. Hypertens. 1998, 12, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.S.; Magubane, M.; Mokotedi, L.; Norton, G.R.; Woodiwiss, A.J. Sex-Specific Effects of Adrenergic-Induced Left Ventricular Remodeling in Spontaneously Hypertensive Rats. J. Card. Fail. 2017, 23, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; Denardo, S.J.; Davidson, J.B.; Huo, T.; Bairey Merz, C.N.; Pepine, C.J. Association of aortic stiffness and wave reflections with coronary flow reserve in women without obstructive coronary artery disease: An ancillary study from the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2015, 170, 1243–1254. [Google Scholar]
- Dart, A.M.; Kingwell, B.A.; Gatzka, C.D.; Willson, K.; Liang, Y.L.; Berry, K.L.; et al. Smaller aortic dimensions do not fully account for the greater pulse pressure in elderly female hypertensives. Hypertension 2008, 51, 1129–1134. [Google Scholar] [CrossRef]
- Aronow, W.S.; Fleg, J.L.; Pepine, C.J.; Artinian, N.T.; Bakris, G.; Brown, A.S.; et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J. Am. Coll. Cardiol. 2011, 57, 2037–2114. [Google Scholar]
- Gerdts, E.; Sudano, I.; Brouwers, S.; Borghi, C.; Bruno, R.M.; Ceconi, C.; et al. Sex differences in arterial hypertension. Eur. Heart J. 2022, 43, 4777–4788. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Bairey Merz, C.N.; Beltrame, J.F.; Kaski, J.C.; Ogawa, H.; Ong, P.; et al. Coronary Vasomotion Disorders International Study Group (COVADIS). The parallel tales of microvascular angina and heart failure with preserved ejection fraction: A paradigm shift. Eur. Heart J. 2017, 38, 473–477. [Google Scholar]
- Sickinghe, A.A.; Korporaal, S.J.; den Ruijter, H.M.; Kessler, E.L. Estrogen Contributions to Microvascular Dysfunction Evolving to Heart Failure With Preserved Ejection Fraction. Front. Endocrinol Lausanne 2019, 10, 442. [Google Scholar] [CrossRef]
- Loyer, X.; Oliviero, P.; Damy, T.; Robidel, E.; Marotte, F.; Heymes, C.; et al. Effects of sex differences on constitutive nitric oxide synthase expression and activity in response to pressure overload in rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2650–8. [Google Scholar] [CrossRef]
- Loyer, X.; Damy, T.; Chvojkova, Z.; Robidel, E.; Marotte, F.; Oliviero, P.; et al. 17beta-estradiol regulates constitutive nitric oxide synthase expression differentially in the myocardium in response to pressure overload. Endocrinology 2007, 148, 4579–4584. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- InanlooRahatloo, K.; Liang, G.; Vo, D.; Ebert, A.; Nguyen, I.; Nguyen, P.K. Sex-based differences in myocardial gene expression in recently deceased organ donors with no prior cardiovascular disease. PLoS ONE 2017, 12, e0183874. [Google Scholar] [CrossRef] [PubMed]
- Aslam, F.; Bandeali, S.J.; Khan, N.A.; Alam, M. Diastolic dysfunction in rheumatoid arthritis: A meta-analysis and systematic review. Arthritis Care Res. Hoboken 2013, 65, 534–543. [Google Scholar] [CrossRef]
- Chandra, A.; Skali, H.; Claggett, B.; Solomon, S.D.; Rossi, J.S.; Russell, S.D.; et al. Race- and Gender-Based Differences in Cardiac Structure and Function and Risk of Heart Failure. J. Am. Coll. Cardiol. 2022, 79, 355–368. [Google Scholar] [CrossRef]
- Núñez, J.; Lorenzo, M.; Miñana, G.; Palau, P.; Monmeneu, J.V.; López-Lereu, M.P.; et al. Sex differences on new-onset heart failure in patients with known or suspected coronary artery disease. Eur. J. Prev. Cardiol. 2021, 28, 1711–1719. [Google Scholar] [CrossRef]
- Clemens, K.K.; Woodward, M.; Neal, B.; Zinman, B. Sex Disparities in Cardiovascular Outcome Trials of Populations With Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1157–1163. [Google Scholar] [CrossRef]
- Galderisi, M.; Anderson, K.M.; Wilson, P.W.; Levy, D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am. J. Cardiol. 1991, 68, 85–89. [Google Scholar] [CrossRef]
- Goyal, P.; Paul, T.; Almarzooq, Z.I.; Peterson, J.C.; Krishnan, U.; Swaminathan, R.V.; et al. Sex- and Race-Related Differences in Characteristics and Outcomes of Hospitalizations for Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e003330. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e50. [Google Scholar] [CrossRef] [PubMed]
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709. [Google Scholar] [PubMed]
- Sorimachi, H.; Omote, K.; Omar, M.; Popovic, D.; Verbrugge, F.H.; Reddy, Y.N.; et al. Sex and central obesity in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2022, 24, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Sorimachi, H.; Obokata, M.; Takahashi, N.; Reddy, Y.N.; Jain, C.C.; Verbrugge, F.H.; et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure with preserved ejection fraction. Eur. Heart J. 2021, 42, 1595–1605. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Ding, J.; Carr, J.J.; Allison, M.A.; Budoff, M.J.; Tracy, R.P.; et al. Pericardial Fat and the Risk of Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2638–2652. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 996–1002. [Google Scholar] [CrossRef]
- Haghikia, A.; Podewski, E.; Libhaber, E.; Labidi, S.; Fischer, D.; Roentgen, P.; et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic. Res. Cardiol. 2013, 108, 366. [Google Scholar] [CrossRef]
- Goyal, A.; Norton, C.R.; Thomas, T.N.; Davis, R.L.; Butler, J.; Ashok, V.; et al. Predictors of incident heart failure in a large insured population: A one million person-year follow-up study. Circ. Heart Fail. 2010, 3, 698–705. [Google Scholar] [CrossRef]
- Hung, C.L.; Chao, T.F.; Su, C.H.; Liao, J.N.; Sung, K.T.; Yeh, H.I.; et al. Income level and outcomes in patients with heart failure with universal health coverage. Heart 2021, 107, 208–216. [Google Scholar] [CrossRef]
- Christensen, S.; Mogelvang, R.; Heitmann, M.; Prescott, E. Level of education and risk of heart failure: A prospective cohort study with echocardiography evaluation. Eur. Heart J. 2011, 32, 450–458. [Google Scholar] [CrossRef]
- Bennett, S.J.; Perkins, S.M.; Lane, K.A.; Deer, M.; Brater, D.C.; Murray, M.D. Social support and health-related quality of life in chronic heart failure patients. Qual Life Res. 2001, 10, 671–682. [Google Scholar]
- Zhu, W.; Wu, Y.; Zhou, Y.; Liang, W.; Xue, R.; Wu, Z.; et al. Living Alone and Clinical Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. Psychosom. Med. 2021, 83, 470–476. [Google Scholar] [CrossRef]
- Stolfo, D.; Uijl, A.; Vedin, O.; Strömberg, A.; Faxén, U.L.; Rosano, G.M.; et al. Sex-Based Differences in Heart Failure Across the Ejection Fraction Spectrum: Phenotyping, and Prognostic and Therapeutic Implications. JACC Heart Fail. 2019, 7, 505–515. [Google Scholar] [CrossRef]
- Dewan, P.; Rørth, R.; Jhund, P.S.; Shen, L.; Raparelli, V.; Petrie, M.C.; et al. Differential Impact of Heart Failure With Reduced Ejection Fraction on Men and Women. J. Am. Coll. Cardiol. 2019, 73, 29–40. [Google Scholar] [CrossRef]
- Belkin, M.; Wussler, D.; Mueller, C. Health-Related Quality of Life in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2020, 8, 245. [Google Scholar] [CrossRef]
- Arndt, V.; Merx, H.; Stegmaier, C.; Ziegler, H.; Brenner, H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. J. Clin. Oncol. 2004, 22, 4829–4836. [Google Scholar] [CrossRef]
- Phillips Bute, B.; Mathew, J.; Blumenthal, J.A.; Welsh-Bohmer, K.; White, W.D.; Mark, D.; et al. Female gender is associated with impaired quality of life 1 year after coronary artery bypass surgery. Psychosom Med. 2003, 65, 944–951. [Google Scholar] [CrossRef]
- Coelho, R.; Amorim, I.; Prata, J. Coping styles and quality of life in patients with non-insulin-dependent diabetes mellitus. Psychosomatics 2003, 44, 312–318. [Google Scholar] [CrossRef]
- Lam, C.S.; Carson, P.E.; Anand, I.S.; Rector, T.S.; Kuskowski, M.; Komajda, M.; et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failureand preserved ejection fraction: The Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Heart Fail. 2012, 5, 571–578. [Google Scholar] [CrossRef]
- Vanderpool, R.R.; Saul, M.; Nouraie, M.; Gladwin, M.T.; Simon, M.A. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2018, 3, 298–306. [Google Scholar] [CrossRef]
- Lau, E.S.; Panah, L.G.; Zern, E.K.; Liu, E.E.; Farrell, R.; Schoenike, M.W.; et al. Arterial Stiffness and Vascular Load in HFpEF: Differences Among Women and Men. J. Card. Fail. 2022, 28, 202–211. [Google Scholar] [CrossRef]
- Lahm, T.; Patel, K.M.; Crisostomo, P.R.; Markel, T.A.; Wang, M.; Herring, C.; et al. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: The effects of sex and menstrual cycle. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E865–71. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; et al. DELIVER Trial Committees and Investigators.Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 85, 1451–1461. [Google Scholar] [CrossRef]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: A patient-level, pooled meta-analysis of DAPA-H F and DELIVER. Nat. Med. 2022, 28, 1956–1964. [Google Scholar] [CrossRef]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.C.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef]
- Rathore, S.S.; Wang, Y.; Krumholz, H.M. Sex-based differences in the effect of digoxin for the treatment of heart failure. N. Engl. J. Med. 2002, 347, 1403–1411. [Google Scholar] [CrossRef]
- Tamargo, J.; Caballero, R.; Delpón, E. Sex-related differences in the pharmacological treatment of heart failure. Pharmacol. Ther. 2022, 229, 107891. [Google Scholar] [CrossRef]
- D’Amario, D.; Rodolico, D.; Rosano, G.M.; Dahlström, U.; Crea, F.; Lund, L.H.; et al. Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2022, 24, 871–884. [Google Scholar] [CrossRef]
- Whitelaw, S.; Sullivan, K.; Eliya, Y.; Alruwayeh, M.; Thabane, L.; Yancy, C.W.; et al. Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: A systematic review. Eur. J. Heart Fail. 2021, 23, 15–24. [Google Scholar] [CrossRef]
- Ramirez, F.D.; Motazedian, P.; Jung, R.G.; Di Santo, P.; MacDonald, Z.; Simard, T.; et al. Sex Bias Is Increasingly Prevalent in Preclinical Cardiovascular Research: Implications for Translational Medicine and Health Equity for Women: A Systematic Assessment of Leading Cardiovascular Journals Over a 10-Year Period. Circulation 2017, 135, 625–626. [Google Scholar] [CrossRef]
- Klein, S.L.; Schiebinger, L.; Stefanick, M.L.; Cahill, L.; Danska, J.; Vries, G.J.d.; et al. Sex inclusion in basic research drives discovery. Proc. Natl. Acad. Sci. USA 2015, 112, 5257–5258. [Google Scholar] [CrossRef]
- Santema, B.T.; Ouwerkerk, W.; Tromp, J.; Sama, I.E.; Ravera, A.; Regitz-Zagrosek, V.; et al. ASIAN-HF investigators. Identifying optimal doses of heart failure medications in men compared with women: A prospective, observational, cohort study. Lancet 2019, 394, 1254–1263. [Google Scholar] [CrossRef]
- Konstam, M.A.; Neaton, J.D.; Dickstein, K.; Drexler, H.; Komajda, M.; Martinez, F.A.; et al. HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): A randomised, double-blind trial. Lancet. 2009, 374, 1840–1848. [Google Scholar] [CrossRef]
- Garg, R.; Yusuf, S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995, 273, 1450–1456. [Google Scholar] [CrossRef]
- Shekelle, P.G.; Rich, M.W.; Morton, S.C.; Atkinson, C.S.; Tu, W.; Maglione, M.; et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: A meta-analysis of major clinical trials. J. Am. Coll. Cardiol. 2003, 41, 1529–1538. [Google Scholar] [CrossRef]
- ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: Systematic overview of individual data from 100,000 patients in randomized trials. Circulation 1998, 97, 2202–2212. [Google Scholar] [CrossRef]
- Flather, M.D.; Yusuf, S.; Køber, L.; Pfeffer, M.; Hall, A.; Murray, G.; et al. ACE-Inhibitors Myocardial Infarction Collaborative Group. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: A systematic overview of data from individual patients. Lancet 2000, 355, 1575–1581. [Google Scholar] [CrossRef]
- Seeland, U.; Regitz-Zagrosek, V. Sex and gender differences in cardiovascular drug therapy. Handb. Exp. Pharmacol. 2012, 214, 211–236. [Google Scholar]
- Pitt, B.; Poole-Wilson, P.A.; Segal, R.; Martinez, F.A.; Dickstein, K.; Camm, A.J.; et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial—The Losartan Heart Failure Survival Study ELITE II. Lancet 2000, 355, 1582–1587. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; McMurray, J.J.; Velazquez, E.J.; Rouleau, J.L.; Køber, L.; Maggioni, A.P.; et al. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N. Engl. J. Med. 2003, 349, 1893–1906. [Google Scholar] [CrossRef]
- Majahalme, S.K.; Baruch, L.; Aknay, N.; Goedel-Meinen, L.; Hofmann, M.; Hester, A.; et al. Val-HeFT Study Investigators. Comparison of treatment benefit and outcome in women versus men with chronic heart failure (from the Valsartan Heart Failure Trial). Am. J. Cardiol. 2005, 95, 529–532. [Google Scholar] [CrossRef]
- Young, J.B.; Dunlap, M.E.; Pfeffer, M.A.; Probstfield, J.L.; Cohen-Solal, A.; Dietz, R.; et al. Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: Results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004, 110, 2618–2626. [Google Scholar]
- Lee, V.C.; Rhew, D.C.; Dylan, M.; Badamgarav, E.; Braunstein, G.D.; Weingarten, S.R. Meta-analysis: Angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann. Intern. Med. 2004, 141, 693–704. [Google Scholar] [CrossRef]
- O’Meara, E.; Clayton, T.; McEntegart, M.B.; McMurray, J.J.; Piña, I.L.; Granger, C.B.; et al. CHARM Investigators. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: Results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007, 115, 3111–3120. [Google Scholar] [CrossRef]
- Hudson, M.; Rahme, E.; Behlouli, H.; Sheppard, R.; Pilote, L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure—A population study. Eur. J. Heart Fail. 2007, 9, 602–609. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; et al. I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- McMurray, J.J.; Jackson, A.M.; Lam, C.S.; Redfield, M.M.; Anand, I.S.; Ge, J.; et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation 2020, 141, 338–351. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Piña, I.L.; Camacho, A.; Bapat, D.; Felker, G.M.; Maisel, A.S.; et al. Prospective Study of Biomarkers, Symptom Improvement and Ventricular Remodeling During Entresto Therapy for Heart Failure (PROVE-HF) Study Investigators. Sex-based differences in biomarkers, health status, and reverse cardiac remodelling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Eur. J. Heart Fail. 2020, 22, 2018–2025. [Google Scholar]
- Solomon, S.D.; Vaduganathan, M.; LClaggett, B.; Packer, M.; Zile, M.; Swedberg, K.; et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 2020, 141, 352–361. [Google Scholar] [CrossRef]
- Kostis, W.J.; Shetty, M.; Chowdhury, Y.S.; Kostis, J.B. ACE Inhibitor-Induced Angioedema: A Review. Curr. Hypertens. Rep. 2018, 20, 55. [Google Scholar] [CrossRef]
- Nuechterlein, K.; AlTurki, A.; Ni, J.; Martínez-Sellés, M.; Martens, P.; Russo, V.; et al. Real-World Safety of Sacubitril/Valsartan in Women and Men With Heart Failure and Reduced Ejection Fraction: A Meta-analysis. CJC Open. 2021, 3 (Suppl. 12), S202–S208. [Google Scholar] [CrossRef]
- Rossi, A.; Mikail, N.; Bengs, S.; Haider, A.; Treyer, V.; Buechel, R.R.; et al. Heart-brain interactions in cardiac and brain diseases: Why sex matters. Eur. Heart J. 2022, 43, 3971–3980. [Google Scholar] [CrossRef]
- Jochmann, N.; Stangl, K.; Garbe, E.; Baumann, G.; Stangl, V. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur. Heart J. 2005, 26, 1585–1595. [Google Scholar] [CrossRef]
- Luzier, A.B.; Killian, A.; Wilton, J.H.; Wilson, M.F.; Forrest, A.; Kazierad, D.J. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin. Pharmacol. Ther. 1999, 66, 594–601. [Google Scholar] [CrossRef]
- Packer, M.; Coats, A.J.; Fowler, M.B.; Katus, H.A.; Krum, H.; Mohacsi, P.; et al. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 2001, 344, 1651–1658. [Google Scholar] [CrossRef]
- The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. Circulation 1994, 90, 1765–1773. [Google Scholar] [CrossRef]
- Simon, T.; Mary-Krause, M.; Funck-Brentano, C.; Jaillon, P. Sex differences in the prognosis of congestive heart failure: Results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II). Circulation 2001, 103, 375–380. [Google Scholar]
- Eugene, A.R. Gender based Dosing of Metoprolol in the Elderly using Population Pharmacokinetic Modeling and Simulations. Int. J. Clin. Pharmacol. Toxicol. 2016, 5, 209–215. [Google Scholar] [CrossRef]
- Kanashiro-Takeuchi, R.M.; Heidecker, B.; Lamirault, G.; Dharamsi, J.W.; Hare, J.M. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin. Transl. Sci. 2009, 2, 134–142. [Google Scholar]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; et al. TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar]
- Merrill, M.; Sweitzer, N.K.; Lindenfeld, J.; Kao, D.P. Sex Differences in Outcomes and Responses to Spironolactone in Heart Failure With Preserved Ejection Fraction: A Secondary Analysis of TOPCAT Trial. JACC Heart Fail. 2019, 7, 228–238. [Google Scholar]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; et al. EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am. J. Cardiol. 1996, 78, 902–907. [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Ferreira, J.P.; Pocock, S.J.; McMurray, J.J.; Solomon, S.D.; Lam, C.S.; et al. Sex differences in mineralocorticoid receptor antagonist trials: A pooled analysis of three large clinical trials. Eur. J. Heart Fail. 2020, 22, 834–844. [Google Scholar] [PubMed]
- Rådholm, K.; Zhou, Z.; Clemens, K.; Neal, B.; Woodward, M. Effects of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes in women versus men. Diabetes Obes. Metab. 2020, 22, 263–266. [Google Scholar] [CrossRef]
- Tamargo, J. Sodium–glucose Cotransporter 2 Inhibitors in Heart Failure: Potential Mechanisms of Action, Adverse Effects and Future Developments. Eur. Cardiol. Rev. 2019, 14, 23–32. [Google Scholar] [CrossRef]
- Butt, J.H.; Docherty, K.F.; Petrie, M.C.; Schou, M.; Kosiborod, M.N.; O’Meara, E.; et al. Efficacy and Safety of Dapagliflozin in Men and Women With Heart Failure With Reduced Ejection Fraction: A Prespecified Analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial. JAMA Cardiol. 2021, 6, 678–689. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. M. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Werner, U.; Werner, D.; Heinbüchner, S.; Graf, B.; Ince, H.; Kische, S.; et al. Gender is an important determinant of the disposition of the loop diuretic torasemide. J. Clin. Pharmacol. 2010, 50, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Brandoni, A.; Villar, S.R.; Torres, A.M. Gender-related differences in the pharmacodynamics of furosemide in rats. Pharmacology 2004, 70, 107–112. [Google Scholar] [CrossRef]
- Verlander, J.W.; Tran, T.M.; Zhang, L.; Kaplan, M.R.; Hebert, S.C. Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J. Clin. Investig. 1998, 101, 1661–1669. [Google Scholar]
- Colbert, J.D.; Martin, B.J.; Haykowsky, M.J.; Hauer, T.L.; Austford, L.D.; Arena, R.A.; et al. Cardiac rehabilitation referral, attendance and mortality in women. Eur. J. Prev. Cardiol. 2015, 22, 979–986. [Google Scholar]
- Supervía, M.; Medina-Inojosa, J.R.; Yeung, C.; Lopez-Jimenez, F.; Squires, R.W.; Pérez-Terzic, C.M.; et al. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clin. Proc. 2017, 92, S0025–6196. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.L.; Seymour, E.M.; Brook, R.D.; Sheth, S.S.; Ghosh, E.; Zhu, S.; et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ. Heart Fail. 2013, 6, 1165–1171. [Google Scholar] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef]
- Mikhalkova, D.; Holman, S.R.; Jiang, H.; Saghir, M.; Novak, E.; Coggan, A.R.; et al. Bariatric Surgery-Induced Cardiac and Lipidomic Changes in Obesity-Related Heart Failure with Preserved Ejection Fraction. Obes. Silver Spring 2018, 26, 284–290. [Google Scholar]
- Van Spall, H.G.; Hill, A.D.; Fu, L.; Ross, H.J.; Fowler, R.A. Temporal Trends and Sex Differences in Intensity of Healthcare at the End of Life in Adults With Heart Failure. J. Am. Heart Assoc. 2021, 10, e018495. [Google Scholar] [CrossRef]
- Zabarovskaja, S.; Gadler, F.; Braunschweig, F.; Ståhlberg, M.; Hörnsten, J.; Linde, C.; et al. Women have better long-term prognosis than men after cardiac resynchronization therapy. Europace 2012, 14, 1148–1155. [Google Scholar]
- Arshad, A.; Moss, A.J.; Foster, E.; Padeletti, L.; Barsheshet, A.; Goldenberg, I.; et al. MADIT-CRT Executive Committee. Cardiac resynchronization therapy is more effective in women than in men: The MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial. J. Am. Coll. Cardiol. 2011, 57, 813–820. [Google Scholar] [PubMed]
- Beela, A.S.; Duchenne, J.; Petrescu, A.; Ünlü, S.; Penicka, M.; Aakhus, S.; et al. Sex-specific difference in outcome after cardiac resynchronization therapy. Eur. Heart, J. Cardiovasc. Imaging 2019, 20, 504–511. [Google Scholar] [CrossRef]
- Linde, C.; Cleland, J.G.; Gold, M.R.; Claude Daubert, J.; Tang, A.S.; Young, J.B.; et al. The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: An individual-patient data meta-analysis. Eur. J. Heart Fail. 2018, 20, 780–791. [Google Scholar]
- Lee, A.W.; O’Regan, D.P.; Gould, J.; Sidhu, B.; Sieniewicz, B.; Plank, G.; et al. Sex-Dependent QRS Guidelines for Cardiac Resynchronization Therapy Using Computer Model Predictions. Biophys. J. 2019, 117, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.O.; Contractor, T.; Zachariah, D.; van Spall, H.G.; Parwani, P.; Minissian, M.B.; et al. Sex Disparities in the Choice of Cardiac Resynchronization Therapy Device: An Analysis of Trends, Predictors, and Outcomes. Can. J. Cardiol. 2021, 37, 86–93. [Google Scholar] [CrossRef]
- Varma, N.; Manne, M.; Nguyen, D.; He, J.; Niebauer, M.; Tchou, P. Probability and magnitude of response to cardiac resynchronization therapy according to QRS duration and gender in nonischemic cardiomyopathy and LBBB. Heart Rhythm 2014, 11, 1139–1147. [Google Scholar] [CrossRef]
- Russo, A.M.; Daugherty, S.L.; Masoudi, F.A.; Wang, Y.; Curtis, J.; Lampert, R. Gender and outcomes after primary prevention implantable cardioverter-defibrillator implantation: Findings from the National Cardiovascular Data Registry (NCDR). Am. Heart J. 2015, 170, 330–338. [Google Scholar] [CrossRef]
- Ghanbari, H.; Dalloul, G.; Hasan, R.; Daccarett, M.; Saba, S.; David, S.; et al. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2009, 169, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Pelargonio, G.; Dello Russo, A.; Casella, M.; Bisceglia, C.; Bartoletti, S.; et al. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: A systematic review and meta-analysis. Heart Rhythm 2010, 7, 876–882. [Google Scholar] [CrossRef]
- Butt, J.H.; Yafasova, A.; Elming, M.B.; Dixen, U.; Nielsen, J.C.; Haarbo, J.; et al. Efficacy of Implantable Cardioverter Defibrillator in Nonischemic Systolic Heart Failure According to Sex: Extended Follow-Up Study of the DANISH Trial. Circ. Heart Fail. 2022, 15, e009669. [Google Scholar] [CrossRef]
- Lampert, R.; McPherson, C.A.; Clancy, J.F.; Caulin-Glaser, T.L.; Rosenfeld, L.E.; Batsford, W.P. Gender differences in ventricular arrhythmia recurrence in patients with coronary artery disease and implantable cardioverter-defibrillators. J. Am. Coll. Cardiol. 2004, 43, 2293–2299. [Google Scholar] [CrossRef]
- Dewidar, O.; Podinic, I.; Barbeau, V.; Patel, D.; Antequera, B.; et al. Integrating sex and gender in studies of cardiac resynchronization therapy: A systematic review. ESC Heart Fail. 2022, 9, 420–427. [Google Scholar] [CrossRef]
- Magnussen, C.; Bernhardt, A.M.; Ojeda, F.M.; Wagner, F.M.; Gummert, J.; de By, T.M.; et al. Gender differences and outcomes in left ventricular assist device support: The European Registry for Patients with Mechanical Circulatory Support. J. Heart Lung Transplant. 2018, 37, 61–70. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Truby, L.K.; Garan, A.R.; Givens, R.C.; Takeda, K.; Takayama, H.; et al. Sex-Related Differences in Use and Outcomes of Left Ventricular Assist Devices as Bridge to Transplantation. JACC Heart Fail. 2019, 7, 250–257. [Google Scholar] [PubMed]
- Kenigsberg, B.B.; Majure, D.T.; Sheikh, F.H.; Afari-Armah, N.; Rodrigo, M.; Hofmeyer, M.; et al. Sex-Associated Differences in Cardiac Reverse Remodeling in Patients Supported by Contemporary Left Ventricular Assist Devices. J. Card. Fail. 2020, 26, 494–504. [Google Scholar] [CrossRef]
- Alasnag, M.; Truesdell, A.G.; Williams, H.; Martinez, S.C.; Qadri, S.K.; Skendelas, J.P.; et al. Mechanical Circulatory Support: A Comprehensive Review With a Focus on Women. Curr. Atheroscler. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Gruen, J.; Caraballo, C.; Miller, P.E.; McCullough, M.; Mezzacappa, C.; Ravindra, N.; et al. Sex Differences in Patients Receiving Left Ventricular Assist Devices for End-Stage Heart Failure. JACC Heart Fail. 2020, 8, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Sherazi, S.; Kutyifa, V.; McNitt, S.; Papernov, A.; Hallinan, W.; et al. Effect of Gender on the Risk of Neurologic Events and Subsequent Outcomes in Patients With Left Ventricular Assist Devices. Am. J. Cardiol. 2017, 119, 297–301. [Google Scholar] [CrossRef]
- Zafar, F.; Villa, C.R.; Morales, D.L.; Blume, E.D.; Rosenthal, D.N.; Kirklin, J.K.; et al. Does Small Size Matter With Continuous Flow Devices?: Analysis of the INTERMACS Database of Adults With BSA ≤1.5 m2. JACC Heart Fail. 2017, 5, 123–131. [Google Scholar] [CrossRef]
- Ahmed, A.; Adegbala, O.; Akintoye, E.; Inampudi, C.; Ajam, M.; Yassin, A.S.; et al. Gender Differences in Outcomes After Implantation of Left Ventricular Assist Devices. Ann. Thorac. Surg. 2020, 109, 780–786. [Google Scholar] [CrossRef]
- Mariani, S.; Li, T.; Bounader, K.; Boethig, D.; Schöde, A.; Hanke, J.S.; et al. Sex differences in outcomes following less-invasive left ventricular assist device implantation. Ann. Cardiothorac. Surg. 2021, 10, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Huckaby, L.V.; Seese, L.M.; Aranda-Michel, E.; Mathier, M.A.; Hickey, G.; Keebler, M.E.; et al. Sex-Based Heart Transplant Outcomes After Bridging With Centrifugal Left Ventricular Assist Devices. Ann. Thorac. Surg. 2020, 110, 2026–2033. [Google Scholar] [CrossRef]
- Nayak, A.; Hu, Y.; Ko, Y.A.; Steinberg, R.; Das, S.; Mehta, A.; et al. Creation and Validation of a Novel Sex-Specific Mortality Risk Score in LVAD Recipients. J. Am. Heart Assoc. 2021, 10, e020019. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; et al. International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation Councils. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar]
- Hsich, E.M. Sex Differences in Advanced Heart Failure Therapies. Circulation 2019, 139, 1080–1093. [Google Scholar] [CrossRef]
- Ayesta, A. Influence of Sex-Mismatch on Prognosis After Heart Transplantation. Front. Cardiovasc. Med. 2021, 8, 617062. [Google Scholar] [CrossRef]
- International Thoracic Organ Transplant (TTX) Registry Data Slides: ISHLT TTX Registry. 2022. Available online: https://ishltregistries.org/registries/slides.Asp.
- Organ Procurement Transplantation Network, U.S. Department of Health & Human Services. 2022. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#.
- Hsich, E.M.; Blackstone, E.H.; Thuita, L.; McNamara, D.M.; Rogers, J.G.; Ishwaran, H.; et al. Sex Differences in Mortality Based on United Network for Organ Sharing Status While Awaiting Heart Transplantation. Circ. Heart Fail. 2017, 10. [Google Scholar] [CrossRef]
- Moayedi, Y.; Fan, C.P.; Cherikh, W.S.; Stehlik, J.; Teuteberg, J.J.; Ross, H.J.; et al. Survival Outcomes After Heart Transplantation: Does Recipient Sex Matter? Circ Heart Fail. 2019, 12, e006218. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Arcari, L.; Núñez Gil, I.J.; Stiermaier, T.; El-Battrawy, I.; Guerra, F.; Novo, G.; et al. Gender Differences in Takotsubo Syndrome. J. Am. Coll. Cardiol. 2022, 79, 2085–2093. [Google Scholar] [CrossRef]
- Komesaroff, P.A.; Esler, M.D.; Sudhir, K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J. Clin. Endocrinol. Metab. 1999, 84, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Sader, M.A.; Celermajer, D.S. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc. Res. 2002, 53, 597–604. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Palaniswamy, C.; Mujib, M.; Ahn, C.; et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: A nationwide population-based study. J. Am. Heart Assoc. 2014, 3, e001056. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J.; König, T.; van der Meer, P.; Petrie, M.C.; Hilfiker-Kleiner, D.; Mbakwem, A.; et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2019, 21, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Kerpen, K.; Koutrolou-Sotiropoulou, P.; Zhu, C.; Yang, J.; Lyon, J.A.; Lima, F.V.; et al. Disparities in death rates in women with peripartum cardiomyopathy between advanced and developing countries: A systematic review and meta-analysis. Arch. Cardiovasc. Dis. 2019, 112, 187–198. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017, 2, 88–93. [Google Scholar] [CrossRef]
- Drafts, B.C.; Twomley, K.M.; D’Agostino, R., Jr.; Lawrence, J.; Avis, N.; Ellis, L.R.; et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc. Imaging. 2013, 6, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, J.B.; Hurria, A.; Owusu, C.; Steingart, R.M.; Gross, C.P. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J. Am. Coll. Cardiol. 2012, 60, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Saiki, H.; Petersen, I.A.; Scott, C.G.; Bailey, K.R.; Dunlay, S.M.; Finley, R.R.; et al. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017, 135, 1388–1396. [Google Scholar] [CrossRef] [PubMed]

| HF phenotype | Knowledge Gap/Problem | Intervention | Benefit |
|---|---|---|---|
| All | Optimal drug doses for women. Information on drug efficacy and safety in women. | Randomised clinical trials need to include participants proportionate to the sex-specific distribution of the disease. An approach targeted at current barriers for female participation (e.g., increasing the number of female trial leaders) alongside an awareness programme on the benefits of the study drug, might increase the participation of women in HF trials. | Increasing female representation in HF clinical trials is essential to decreasing sex disparities in clinical care of all HF patients. |
| Lack of sex-specific criteria for advanced HF therapy/devices: Women are less likely than men to receive a cardiac device in clinical practice, although they show better responses. Women account for a minority of patients on the waiting list for heart transplantation. | Implementation of sex-specific prediction models and identification of barriers impeding advanced HF therapies in women. | Overcoming barriers impeding advanced HF therapies in women alongside technological advances in mechanical circulatory support will likely increase their implantation in women. | |
| Impact of sociocultural gender on access to HF health care. Women with HF are referred for health care services less frequently than men. | An increased understanding how society, family and environment affect health care and prognosis of female and male HF patients is needed. | Studies focusing on sociocultural gender will help clinicians to provide more appropriate levels of care and understand HF as a multifaceted disease. | |
| HFpEF | Sex-specific prevention strategies are lacking. Hypertension, obesity, and type II diabetes, the most common HFpEF antecedents, are less well controlled in women. | Greater efforts in primary prevention of HFpEF are needed through aggressive treatment of risk factors. | Sex-specific prevention strategies will reduce the medical and societal impact of this disorder. |
| Women are more often affected by HFpEF, but outcomes are worse in men. Sex-specific disease mechanisms in HFpEF are unknown. | Suitable female/male preclinical HFpEF models to study HFpEF disease mechanisms are required. Age needs to be incorporated in preclinical HFpEF models. | Exploring mechanisms that predispose men for worse outcomes. Gaining insights into the age-related derangements that predispose women and the elderly to HFpEF will advance the development of new therapies for HFpEF | |
| NT-proBNP levels are higher in women than men across the LVEF spectrum. | Implementation of sex-specific thresholds for natriuretic peptides. | Sex-specific thresholds for natriuretic peptides may improve their diagnostic utility for HFpEF. | |
| HFsnEF | Evidence suggests that the association between LVEF and mortality shows a U-shaped relationship. Patients with an LVEF >70% face a higher mortality than patients with preserved LVEF. Mechanisms are unknown. | More research is necessary to identify clinical relevance and prevention/treatment strategies of HFsnEF. | Identification of prevention/treatment strategies of HFsnEF might particularly benefit women who have a higher LVEF than men. |
| HFmrEF | Lack of sex-specific outcome data. | More research is necessary to identify sex-specific predictors for treatment responses and adverse outcomes. | Sex-specific risk prediction will enable early preventive and therapeutic measures. |
© 2023 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Delco, A.; Portmann, A.; Mikail, N.; Rossi, A.; Haider, A.; Bengs, S.; Gebhard, C. Impact of Sex and Gender on Heart Failure. Cardiovasc. Med. 2023, 26, 88. https://doi.org/10.4414/cvm.2023.02274
Delco A, Portmann A, Mikail N, Rossi A, Haider A, Bengs S, Gebhard C. Impact of Sex and Gender on Heart Failure. Cardiovascular Medicine. 2023; 26(3):88. https://doi.org/10.4414/cvm.2023.02274
Chicago/Turabian StyleDelco, Alessia, Angela Portmann, Nidaa Mikail, Alexia Rossi, Ahmed Haider, Susan Bengs, and Catherine Gebhard. 2023. "Impact of Sex and Gender on Heart Failure" Cardiovascular Medicine 26, no. 3: 88. https://doi.org/10.4414/cvm.2023.02274
APA StyleDelco, A., Portmann, A., Mikail, N., Rossi, A., Haider, A., Bengs, S., & Gebhard, C. (2023). Impact of Sex and Gender on Heart Failure. Cardiovascular Medicine, 26(3), 88. https://doi.org/10.4414/cvm.2023.02274




