Extracorporeal Life Support Use in Cardiac and Circulatory Failure: A Summary of Recently Published S3 Guidelines
Introduction
Guidelines
Staffing issues
Staff training and continuing education
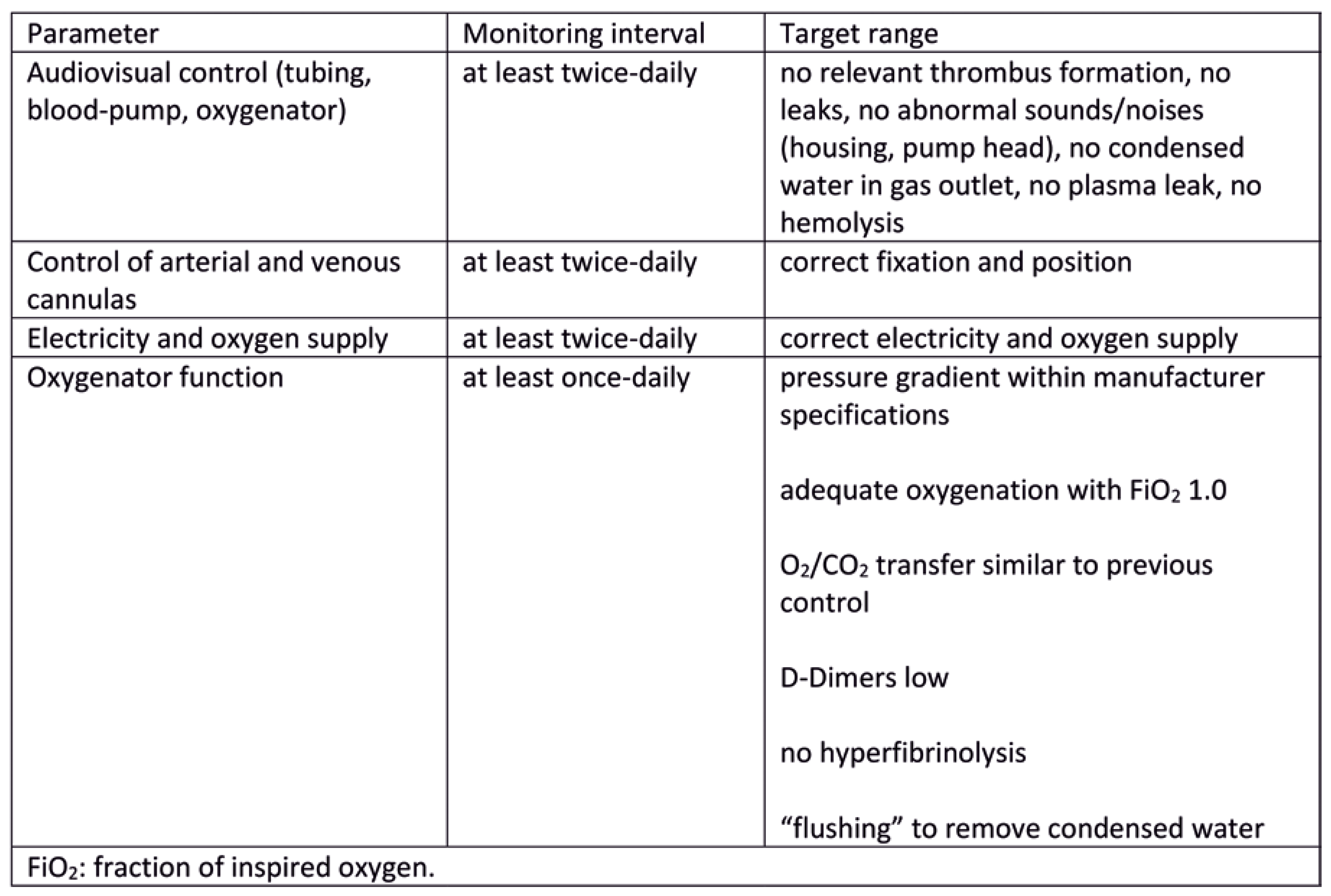
ECLS circuit and cannulation site
Patient ICU care and monitoring
Management of ventricular distension and central hypoxia
- Arterial cannulation of right axillary artery.
- Insertion of another cannula (e.g., via the right internal jugular vein) and change of circuit configuration to veno-arteriovenous (V-AV)
- Insertion of another venous drainage cannula and change of the configuration to VV-A
- Change from peripheral to central cannulation.
ECLS weaning and explantation
- Pulsatile arterial blood pressure and evidence of biventricular contractility on echocardiography
- Mean arterial blood pressure >60 mm Hg
- Mixed venous oxygen saturation (SvO2) ≥ 65% (central venous oxygen saturation [ScvO2] ≥ 60%)
- Lactate values ≤ 2 mmol/l or falling
- Vasopressor/inotropic dosage low or falling
- Sufficient pulmonary oxygenation (Horowitz-index or ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fraction of inspired oxygen (FiO2) > 200 mm Hg) / CO2 elimination performance under lung-protective ventilation
- Compensated end organ functions, especially liver function
Normal ward care
Rehabilitation and follow-up
Disclosure Statement
References
- Michels, G.; Wengenmayer, T.; Hagl, C.; Dohmen, C.; Böttiger, B.W.; Bauersachs, J.; Markewitz, A.; Bauer, A.; Gräsner, J.-T.; Pfister, R.; et al. Empfehlungen zur extrakorporalen kardiopulmonalen Reanimation (eCPR). Med. Klin.-Intensiv. und Notfallmedizin 2018, 113, 478–486. [Google Scholar] [CrossRef]
- Abrams, D.; Garan, A.R.; Abdelbary, A.; Bacchetta, M.; Bartlett, R.H.; Beck, J.; Belohlavek, J.; Chen, Y.-S.; Fan, E.; Ferguson, N.D.; et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensiv. Care Med. 2018, 44, 717–729. [Google Scholar] [CrossRef]
- McCarthy, F.H.; McDermott, K.M.; Spragan, D.; Hoedt, A.; Kini, V.; Atluri, P.; Gaffey, A.; Szeto, W.Y.; Acker, M.A.; Desai, N.D. Unconventional Volume-Outcome Associations in Adult Extracorporeal Membrane Oxygenation in the United States. Ann. Thorac. Surg. 2016, 102, 489–495. [Google Scholar] [CrossRef]
- Barbaro, R.P.; Odetola, F.O.; Kidwell, K.M.; Paden, M.L.; Bartlett, R.H.; Davis, M.M.; Annich, G.M. Association of Hospital-Level Volume of Extracorporeal Membrane Oxygenation Cases and Mortality. Analysis of the Extracorporeal Life Support Organization Registry. Am. J. Respir. Crit. Care Med. 2015, 191, 894–901. [Google Scholar] [CrossRef]
- Huesch, M.D. Volume–Outcome Relationships in Extracorporeal Membrane Oxygenation: Retrospective Analysis of Administrative Data From Pennsylvania, 2007–2015. Asaio J. 2018, 64, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Extracorporeal Life Support Organization. ELSO Guidelines for ECMO Centers. 2014. Available online: https://www.elso.org/Portals/0/IGD/Archive/FileManager/faf3f6a3c7cusersshyerdocumentselsoguidelinesecmocentersv1.8.pdf (accessed on 9 March 2020).
- Michels, G.; Wengenmayer, T.; Hagl, C.; Dohmen, C.; Böttiger, B.W.; Bauersachs, J.; Markewitz, A.; Bauer, A.; Gräsner, J.-T.; Pfister, R.; et al. Empfehlungen zur extrakorporalen kardiopulmonalen Reanimation (eCPR). Med. Klin.-Intensiv. und Notfallmedizin 2018, 113, 478–486. [Google Scholar] [CrossRef]
- Combes, A.; Brodie, D.; Bartlett, R.; Brochard, L.; Brower, R.; Conrad, S.; De Backer, D.; Fan, E.; Ferguson, N.; Fortenberry, J.; et al. Position Paper for the Organization of Extracorporeal Membrane Oxygenation Programs for Acute Respiratory Failure in Adult Patients. Am. J. Respir. Crit. Care Med. 2014, 190, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Benk, C.; Beyersdorf, F.; Haimerl, G.; Merkle, F.; Mestres, C.; Pepper, J.; Wahba, A. Position article for the use of extracorporeal life support in adult patients. Eur. J. Cardio-Thoracic Surg. 2011, 40, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Pichler, P.; Antretter, H.; Dünser, M.; Eschertzhuber, S.; Gottardi, R.; Heinz, G.; Pölzl, G.; Pretsch, I.; Rajek, A.; Wasler, A.; et al. Positionspapier der Österreichischen Kardiologischen Gesellschaft zum Einsatz der extrakorporalen Membranoxygenation (ECMO) bei Erwachsenen kardiologischen Patienten. Med. Klin.-Intensiv. und Notfallmedizin 2015, 110, 407–420. [Google Scholar] [CrossRef]
- Richardson, A. (.C.; Schmidt, M.; Bailey, M.; Pellegrino, V.A.; Rycus, P.T.; Pilcher, D.V. ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 2017, 112, 34–40. [Google Scholar] [CrossRef]
- Lorusso, R.; Barili, F.; Di Mauro, M.; Gelsomino, S.; Parise, O.; Rycus, P.T.; Maessen, J.; Mueller, T.; Muellenbach, R.; Belohlavek, J.; et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit. Care Med. 2016, 44, e964–e972. [Google Scholar] [CrossRef]
- Bizzarro, M.J.; Conrad, S.A.; Kaufman, D.A.; Rycus, P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults*. Pediatr. Crit. Care Med. 2011, 12, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.M.; Lew, D.F.; Kao, L.S.; Lally, K.P. Defining risk for infectious complications on extracorporeal life support. J. Pediatr. Surg. 2011, 46, 2260–2264. [Google Scholar] [CrossRef]
- Dalton, H.J.; Garcia-Filion, P.; Holubkov, R.; Moler, F.W.; Shanley, T.; Heidemann, S.; Meert, K.; Berg, R.A.; Berger, J.; Carcillo, J.; et al. Association of Bleeding and Thrombosis With Outcome in Extracorporeal Life Support*. Pediatr. Crit. Care Med. 2015, 16, 167–174. [Google Scholar] [CrossRef]
- Polito, A.; Barrett, C.S.; Wypij, D.; Rycus, P.T.; Netto, R.; Cogo, P.E.; Thiagarajan, R.R. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intensiv. Care Med. 2013, 39, 1594–1601. [Google Scholar] [CrossRef]
- Werho, D.K.; Pasquali, S.K.; Yu, S.; Donohue, J.; Annich, G.M.; Thiagarajan, R.R.; Hirsch-Romano, J.C.; Gaies, M. Epidemiology of Stroke in Pediatric Cardiac Surgical Patients Supported With Extracorporeal Membrane Oxygenation. Ann. Thorac. Surg. 2015, 100, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Wightman, A.M.; Bradford, M.C.; Symons, J.; Brogan, T.V. Impact of Kidney Disease on Survival in Neonatal Extracorporeal Life Support. Pediatr. Crit. Care Med. 2015, 16, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Lipinski, J.; Al-Kindi, S.G.; Patel, T.; Saric, P.; Li, J.; Nadeem, F.; Ladas, T.; Alaiti, A.; Phillips, A.; et al. Simultaneous Venoarterial Extracorporeal Membrane Oxygenation and Percutaneous Left Ventricular Decompression Therapy with Impella Is Associated with Improved Outcomes in Refractory Cardiogenic Shock. Asaio J. 2019, 65, 21–28. [Google Scholar] [CrossRef]
- Pappalardo, F.; Schulte, C.; Pieri, M.; Schrage, B.; Contri, R.; Soeffker, G.; et al. Concomitant implantation of Impella on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur. J. Heart Fail. 2017. Available online: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/778/CN-01245778/frame.html. [CrossRef]
- Schmack, B.; Seppelt, P.; Weymann, A.; Alt, C.; Farag, M.; Arif, R.; Doesch, A.O.; Raake, P.W.; Kallenbach, K.; Mansur, A.; et al. Extracorporeal life support with left ventricular decompression—improved survival in severe cardiogenic shock: results from a retrospective study. PeerJ 2017, 5, e3813–e3813. [Google Scholar] [CrossRef]
- Distelmaier, K.; Roth, C.; Schrutka, L.; Binder, C.; Steinlechner, B.; Heinz, G.; Lang, I.M.; Maurer, G.; Koinig, H.; Niessner, A.; et al. Beneficial effects of levosimendan on survival in patients undergoing extracorporeal membrane oxygenation after cardiovascular surgery. Br. J. Anaesth. 2016, 117, 52–58. [Google Scholar] [CrossRef]
- Smith, M.; Vukomanovic, A.; Brodie, D.; Thiagarajan, R.; Rycus, P.; Buscher, H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit. Care 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization. Ultrasound Guidance for Extra-corporeal Membrane OxygenationGeneral Guidelines. 2015. [Google Scholar]
- Aissaoui, N.; Luyt, C.-E.; Leprince, P.; Trouillet, J.-L.; Léger, P.; Pavie, A.; Diebold, B.; Chastre, J.; Combes, A. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensiv. Care Med. 2011, 37, 1738–1745. [Google Scholar] [CrossRef]
- Akin, S.; Miranda, D.d.R.; Caliskan, K.; Soliman, O.I.; Guven, G.; Struijs, A.; van Thiel, R.J.; Jewbali, L.S.; Lima, A.; Gommers, D.; et al. Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit. Care 2017, 21, 265–265. [Google Scholar] [CrossRef]
- Cavarocchi, N.C.; Pitcher, H.T.; Yang, Q.; Karbowski, P.; Miessau, J.; Hastings, H.M.; Hirose, H. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J. Thorac. Cardiovasc. Surg. 2013, 146, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Gaies, M.G.; Jeffries, H.E.; Niebler, R.A.; Pasquali, S.K.; Donohue, J.E.; Yu, S.; et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr. Crit. Care Med. 2014, 15, 529–537. [Google Scholar] [CrossRef]
- Ro, S.K.; Kim, J.B.; Jung, S.H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Extracorporeal life support for cardiogenic shock: influence of concomitant intra-aortic balloon counterpulsation. Eur. J. Cardio-Thoracic Surg. 2014, 46, 186–192. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Liao, C.-W.; Wang, C.-H.; Chi, N.-H.; Yu, H.-Y.; Chou, N.-K.; Hwang, J.-J.; Lin, J.-L.; Chiang, F.-T.; Chen, Y.-S. Effects of Additional Intra-aortic Balloon Counter-Pulsation Therapy to Cardiogenic Shock Patients Supported by Extra-corporeal Membranous Oxygenation. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aso, S.; Matsui, H.; Fushimi, K.; Yasunaga, H. The Effect of Intraaortic Balloon Pumping Under Venoarterial Extracorporeal Membrane Oxygenation on Mortality of Cardiogenic Patients: An Analysis Using a Nationwide Inpatient Database. Crit. Care Med. 2016, 44, 1974–1979. [Google Scholar] [CrossRef]
- Pappalardo, F.; Schulte, C.; Pieri, M.; Schrage, B.; Contri, R.; Soeffker, G.; et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur. J. Heart Fail. 2017, 19, 404–412. [Google Scholar] [CrossRef]
- Park, T.K.; Yang, J.H.; Choi, S.-H.; Bin Song, Y.; Hahn, J.-Y.; Choi, J.-H.; Sung, K.; Lee, Y.T.; Gwon, H.-C. Clinical impact of intra-aortic balloon pump during extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock. BMC Anesthesiol. 2014, 14, 27–27. [Google Scholar] [CrossRef] [PubMed]
- Bréchot, N.; Demondion, P.; Santi, F.; Lebreton, G.; Pham, T.; Dalakidis, A.; Gambotti, L.; Luyt, C.-E.; Schmidt, M.; Hekimian, G.; et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur. Hear. Journal. Acute Cardiovasc. Care 2017, 7, 62–69. [Google Scholar] [CrossRef] [PubMed]

| Deutsche Gesellschaft für Thorax-, Herz- und Gefäßchirurgie e.V. (DGTHG) |
| Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin e.V. (DGAI) |
| Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin (DIVI) |
| Deutsche Gesellschaft für Kardiologie - Herz- und Kreislaufforschung e.V. (DGK) |
| Gesellschaft für Neonatologie und pädiatrische Intensivmedizin e.V. (GNPI) |
| Deutsche Gesellschaft für Pädiatrische Kardiologie e.V. (DGPK) |
| Deutsche Gesellschaft für Internistische Intensivmedizin und Notfallmedizin (DGIIN) |
| Deutsche Gesellschaft für Innere Medizin e.V. (DGIM) |
| Deutsche Gesellschaft für Kinder- und Jugendmedizin e.V. (DGKJ) |
| Deutsche Gesellschaft für Kinderchirurgie (DGKCH) |
| Deutsche Gesellschaft für Thoraxchirurgie (DGT) |
| Deutsche Gesellschaft für Fachkrankenpflege und Funktionsdienste e. V. (DGF) |
| Schweizerische Gesellschaft für Herz- und thorakale Gefässchirurgie (SGHC) |
| Österreichische Gesellschaft für Thorax- und Herzchirurgie (ÖGTHC) |
| Deutsche Herzstiftung e.V. |
| Deutscher Verband für Physiotherapie (ZVK) |
| Akademie für Ethik in der Medizin (AEM) |
| Deutsche Gesellschaft für Kardiotechnik e.V. (DGfK) |
| ECLS initiation | ECLS continuation | |
|---|---|---|
| Cardiac surgery | X | X |
| Cardiology | X | X |
| Anaesthesiology | X | X |
| Intensive care medicine | X | X |
| Neurology | X | |
| Neurosurgery | X | |
| General surgery | X | |
| Vascular surgery | X | |
| Angiology | X | |
| Radiology | X | |
| Haematology | X | |
| Gastroenterology | X | |
| Nephrology | X | |
| Pulmonology | X | |
| Ethics committee | X |
© 2022 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Dzemal, O.; Starck, C.; Wessel, L.; Miera, O.; Werdan, K.; Burckhardt, M.; Muellenbach, R.; Jaksties, R.; Schmidt, F.; Wiebe, K.; et al. Extracorporeal Life Support Use in Cardiac and Circulatory Failure: A Summary of Recently Published S3 Guidelines. Cardiovasc. Med. 2022, 25, 165. https://doi.org/10.4414/cvm.2022.02234
Dzemal O, Starck C, Wessel L, Miera O, Werdan K, Burckhardt M, Muellenbach R, Jaksties R, Schmidt F, Wiebe K, et al. Extracorporeal Life Support Use in Cardiac and Circulatory Failure: A Summary of Recently Published S3 Guidelines. Cardiovascular Medicine. 2022; 25(6):165. https://doi.org/10.4414/cvm.2022.02234
Chicago/Turabian StyleDzemal, Omer, Christoph Starck, Lukas Wessel, Oliver Miera, Karl Werdan, Marion Burckhardt, Ralf Muellenbach, Rolf Jaksties, Florian Schmidt, Karsten Wiebe, and et al. 2022. "Extracorporeal Life Support Use in Cardiac and Circulatory Failure: A Summary of Recently Published S3 Guidelines" Cardiovascular Medicine 25, no. 6: 165. https://doi.org/10.4414/cvm.2022.02234
APA StyleDzemal, O., Starck, C., Wessel, L., Miera, O., Werdan, K., Burckhardt, M., Muellenbach, R., Jaksties, R., Schmidt, F., Wiebe, K., Schmid, C., Kluge, S., Pilarczyk, K., Haake, N., Schaible, T., Flemmer, A., Klotz, S., Assmann, A., Janssens, U., ... Boeken, U. (2022). Extracorporeal Life Support Use in Cardiac and Circulatory Failure: A Summary of Recently Published S3 Guidelines. Cardiovascular Medicine, 25(6), 165. https://doi.org/10.4414/cvm.2022.02234



