A Case of Acute Coronary Syndrome Featuring a Forgotten and Disguised Intruder
Abstract
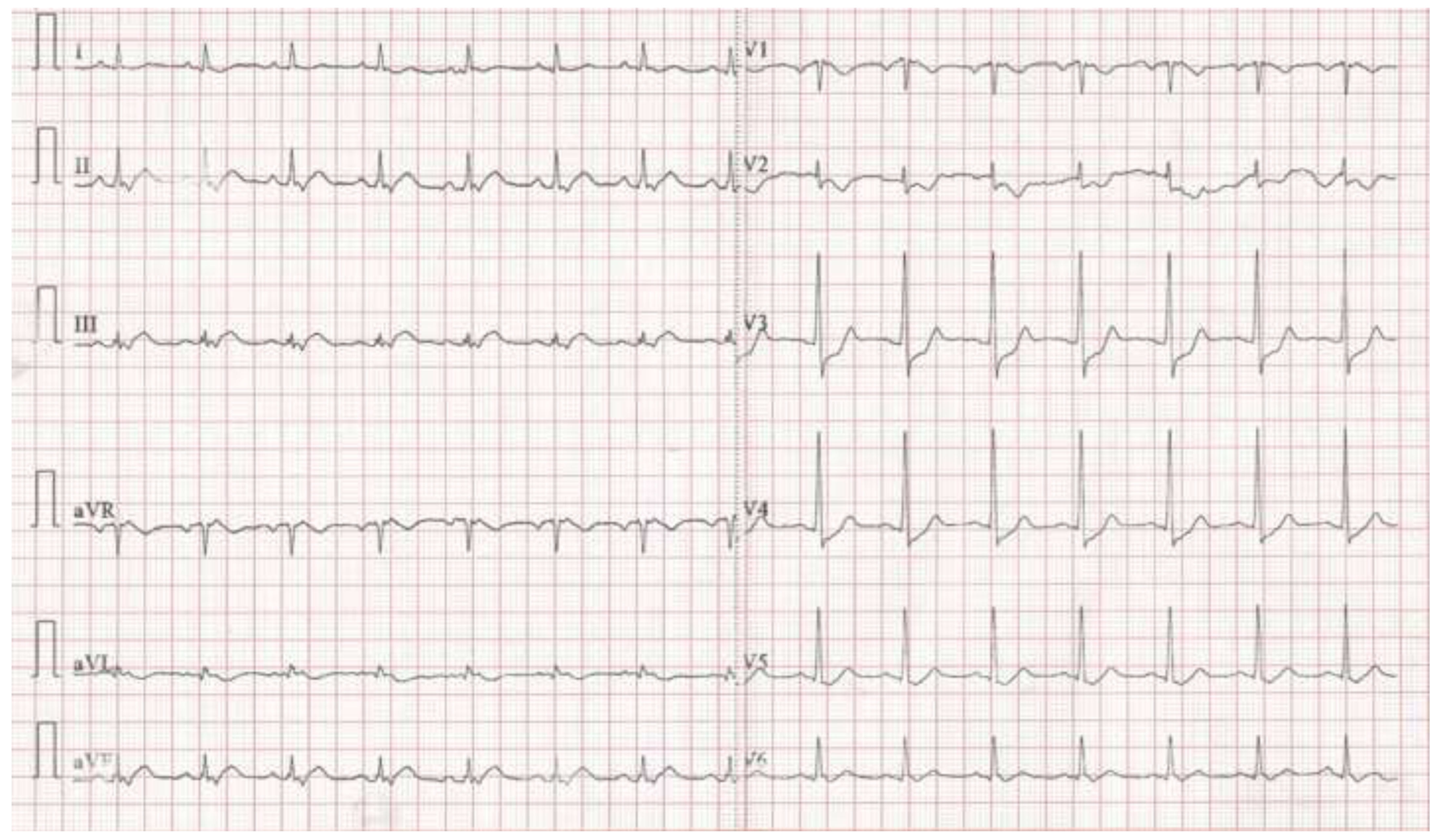
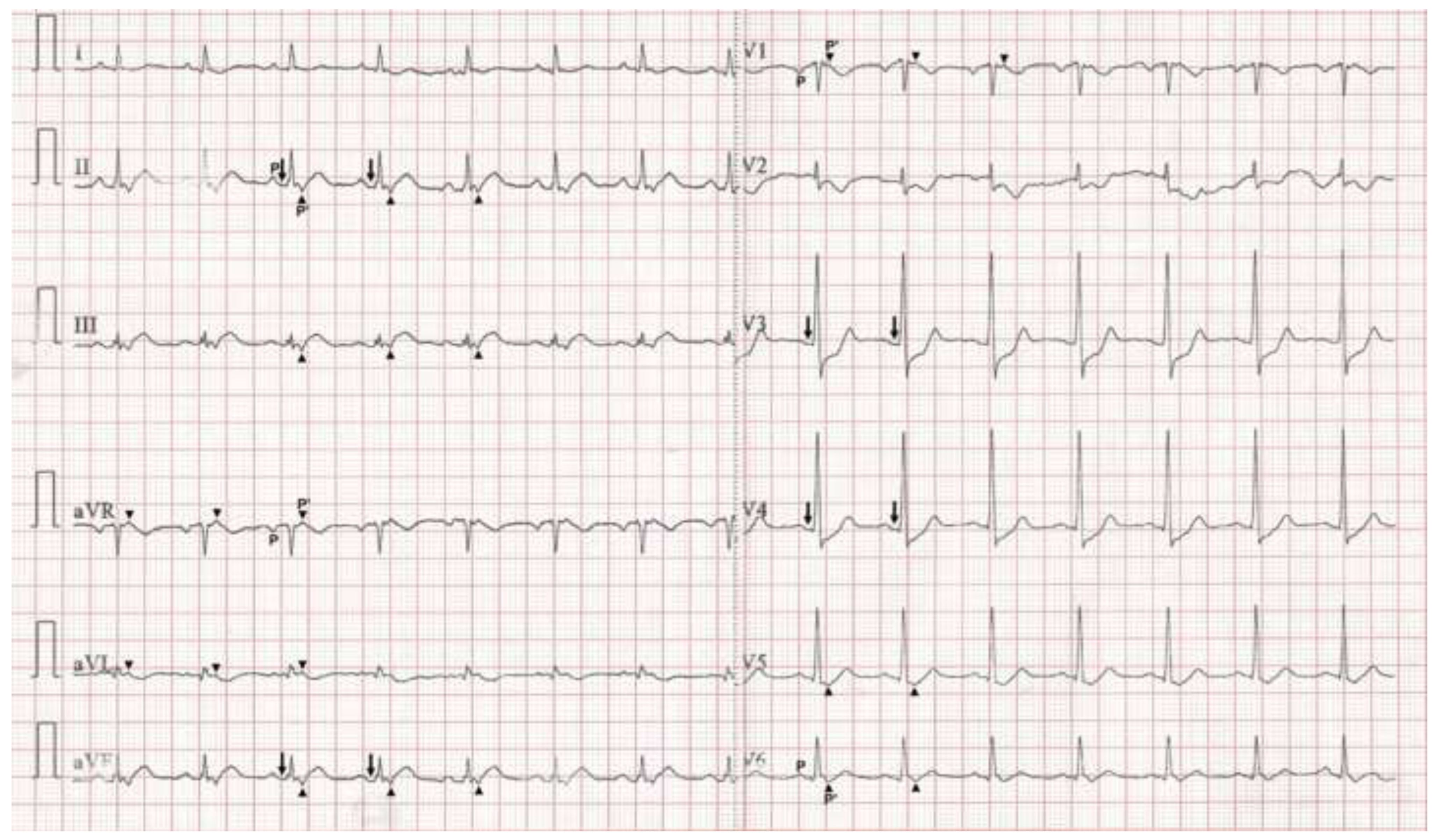
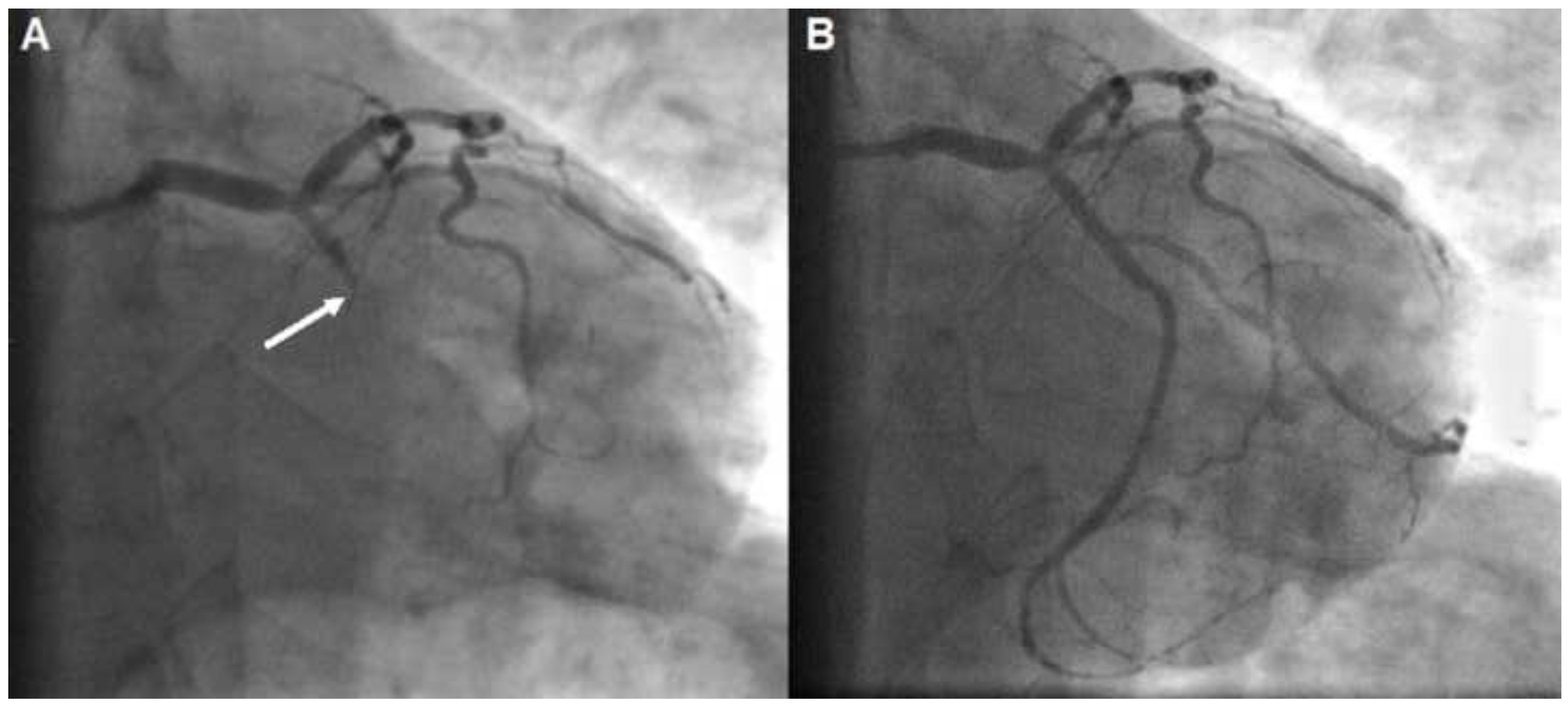
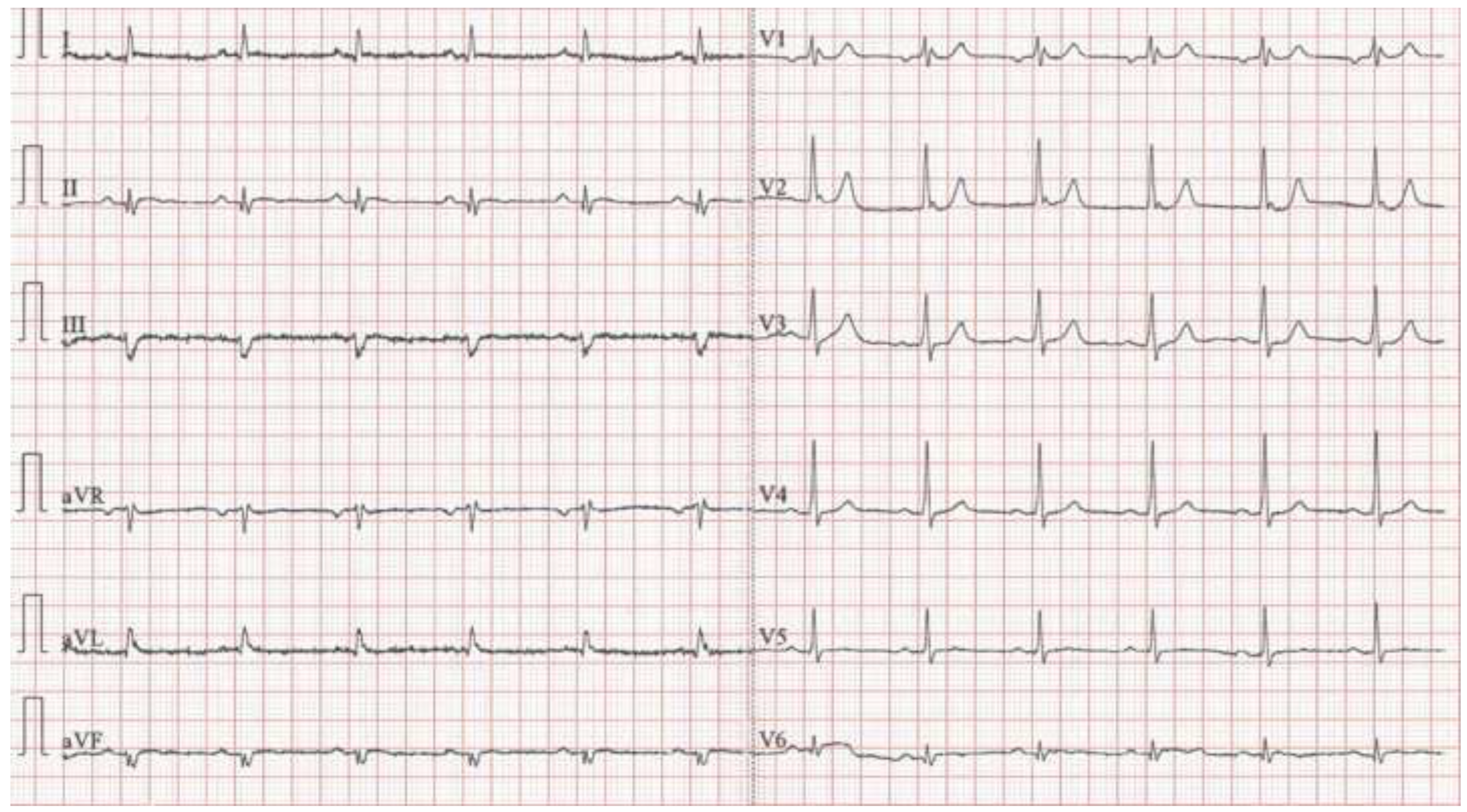
Case presentation
Discussion
Conclusions
References
- Harris, B.C.; Shaver, J.A.; Gray, S., 3rd; Kroetz, F.W.; Leonard, J.J. Left atrial rhythm. Experimental production in man. Circulation 1968, 37, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Mirowski, M. Left atrial rhythm; diagnostic criteria and differentiation from nodal arrhythmias. Am. J. Cardiol. 1966, 17, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.L.; De Venecia, T.; Patnaik, S.; Figueredo, V.M. Atrial myocardial infarction: A tale of the forgotten chamber. Int. J. Cardiol. 2015, 202, 904–909. [Google Scholar] [CrossRef]
- Fiol-Sala, M.; Birnbaum, Y.; Nikus, K.; Bayés de Luna, A. Chapter 7: Acute Coronary Syndrome with ST Elevation (STE-ACS). In Electrocardiography in Ischemic Heart Disease: Clinical and Imaging Correlations and Prognostic Implications, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 111–179. [Google Scholar]
- Liu, C.K.; Greenspan, G.; Piccirillo, R.T. Atrial Infarction of the Heart. Circulation 1961, 23, 331–338. [Google Scholar] [CrossRef]
- Burch, G.E. Of the P-R segment depression and atrial infarction. Am. Hear. J. 1976, 91, 129–130. [Google Scholar] [CrossRef]
- Sivertssen, E.; Hoel, B.; Bay, G.; Jörgensen, L. Electrocardiographic atrial complex and acute atrial myocardial infarction. Am. J. Cardiol. 1973, 31, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Lazar, E.J.; Goldberger, J.; Peled, H.; Sherman, M.; Frishman, W.H. Atrial infarction: diagnosis and management. Am. Hear. J. 1988, 116, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Gardin, J.M.; Singer, D.H. Atrial infarction. Importance, diagnosis, and localization. Arch. Intern. Med. 1981, 141, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.L.; Collins, K.A. Left atrial infarction: a case report and review of the literature. Am. J. Forensic Med. Pathol. 2010, 31, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, J.; Vives-Borrás, M.; Gomis, P.; Ordoñez-Llanos, J.; Ferrero-Gregori, A.; Serra-Peñaranda, A.; Cinca, J. Electrophysiological Effects of Selective Atrial Coronary Artery Occlusion in Humans. Circulation 2016, 133, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
© 2022 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Andreou, A.Y. A Case of Acute Coronary Syndrome Featuring a Forgotten and Disguised Intruder. Cardiovasc. Med. 2022, 25, 225. https://doi.org/10.4414/cvm.2022.02225
Andreou AY. A Case of Acute Coronary Syndrome Featuring a Forgotten and Disguised Intruder. Cardiovascular Medicine. 2022; 25(5):225. https://doi.org/10.4414/cvm.2022.02225
Chicago/Turabian StyleAndreou, Andreas Y. 2022. "A Case of Acute Coronary Syndrome Featuring a Forgotten and Disguised Intruder" Cardiovascular Medicine 25, no. 5: 225. https://doi.org/10.4414/cvm.2022.02225
APA StyleAndreou, A. Y. (2022). A Case of Acute Coronary Syndrome Featuring a Forgotten and Disguised Intruder. Cardiovascular Medicine, 25(5), 225. https://doi.org/10.4414/cvm.2022.02225



