Prenatal Consequences of Congenital Heart Disease on Brain Development †
Abstract
Introduction
Altered brain development—encephalopathy of CHD
Neuropathological findings
Prenatal cerebral imaging
Postnatal cerebral imaging
Neurodevelopmental outcome
- CHD patients may develop impaired brain development even before birth.
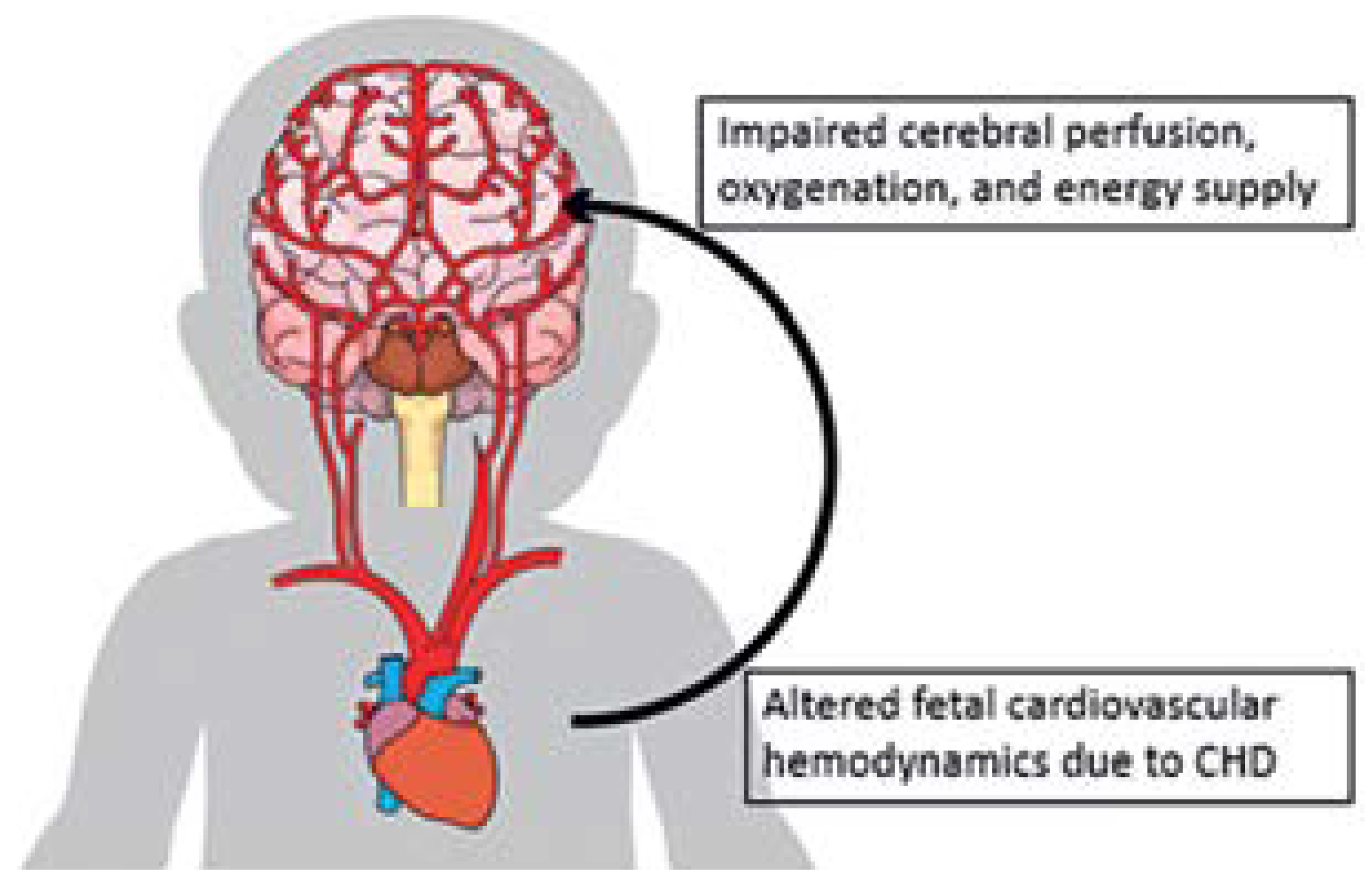
- A reason for impaired brain development is the altered fetal cardiovascular hemodynamics due to complex CHD affecting oxygen delivery and brain nutrition by impaired cerebral perfusion.
- Prenatal to postnatal brain growth trajectories might be different from healthy controls. The brain volume reduction affects global as well as regional brain segments.
- Structural brain lesions are frequently found after birth including small white matter lesions, cerebral bleeding and small cerebral strokes.
- New structural brain lesions may be detected after neonatal cardiopulmonary bypass surgery in a time window when the brain vulnerability is increased due to impaired brain development.
- Reasons for impaired neurodeveopmental outcome in children with complex type of CHD are multifactorial and include cumulative effects with repetitive hypoxemic and ischemic conditions at different time points (fetal, postnatal, intraoperative and postoperative) leading to impaired micro- and macrostructural brain development.
Long-term outcome
Outlook
Acknowledgments
Conflicts of Interest
References
- Latal, B. Neurodevelopmental Outcomes of the Child with Congenital Heart Disease. Clin Perinatol. 2016, 43, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Wernovsky, G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006, 16 (Suppl. S1), 92–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.T.; Seed, M.; Sun, L.; Marini, D. Fetal brain issues in congenital heart disease. Transl Pediatr. 2021, 10, 2182–2196. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.J.; Manlhiot, C.; Porayette, P.; et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.P.; McQuillen, P.S.; Hamrick, S.; Xu, D.; Glidden, D.V.; Charlton, N.; et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007, 357, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Encephalopathy of congenital heart disease- destructive and developmental effects intertwined. J Pediatr. 2014, 164, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.A.; Rorke, L.B.; Weinberg, P.M.; Clancy, R.R. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics 1990, 85, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Kinney, H.C.; Panigrahy, A.; Newburger, J.W.; Jonas, R.A.; Sleeper, L.A. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005, 110, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Alves de Alencar Rocha, A.K.; Allison, B.J.; Yawno, T.; Polglase, G.R.; Sutherland, A.E.; Malhotra, A.; et al. Early- versus Late-Onset Fetal Growth Restriction Differentially Affects the Development of the Fetal Sheep Brain. Dev Neurosci. 2017, 39, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Ayres, N.; Cuneo, B.; Gotteiner, N.; Hornberger, L.; Spevak, P.J.; et al. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J Am Soc Echocardiogr. 2004, 17, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Triulzi, F.; Manganaro, L.; Volpe, P. Fetal magnetic resonance imaging: Indications, study protocols and safety. Radiol Med. 2011, 116, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Kuklisova-Murgasova, M.; Quaghebeur, G.; Rutherford, M.A.; Hajnal, J.V.; Schnabel, J.A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal. 2012, 16, 1550–1564. [Google Scholar] [CrossRef] [PubMed]
- Limperopoulos, C.; Tworetzky, W.; McElhinney, D.B.; Newburger, J.W.; Brown, D.W.; Robertson, R.L., Jr.; et al. Brain volume and metabolism in fetuses with congenital heart disease: Evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010, 121, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Clouchoux, C.; du Plessis, A.J.; Bouyssi-Kobar, M.; Tworetzky, W.; McElhinney, D.B.; Brown, D.W.; et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2013, 23, 2932–2943. [Google Scholar] [CrossRef] [PubMed]
- Ortinau, C.M.; Rollins, C.K.; Gholipour, A.; Yun, H.J.; Marshall, M.; Gagoski, B.; et al. Early-Emerging Sulcal Patterns Are Atypical in Fetuses with Congenital Heart Disease. Cereb Cortex. 2019, 29, 3605–3616. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Scott, J.; Habas, P.A.; Kim, K.; Corbett-Detig, J.; Rousseau, F.; et al. Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J Neurosci. 2011, 31, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Easley, R.B.; Marino, B.S.; Jennings, J.; Cassedy, A.E.; Kibler, K.K.; Brady, K.M.; et al. Impaired cerebral autoregulation and elevation in plasma glial fibrillary acidic protein level during cardiopulmonary bypass surgery for CHD. Cardiol Young. 2018, 28, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.L.; Mir, I.N.; Herrera, C.L.; Sharma, K.; Spong, C.Y.; Twickler, D.M.; et al. Neuroplacentology in congenital heart disease: Placental connections to neurodevelopmental outcomes. Pediatr Res. 2021; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Courtney, J.; Troja, W.; Owens, K.J.; Brockway, H.M.; Hinton, A.C.; Hinton, R.B.; et al. Abnormalities of placental development and function are associated with the different fetal growth patterns of hypoplastic left heart syndrome and transposition of the great arteries. Placenta. 2020, 101, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ortinau, C.M.; Mangin-Heimos, K.; Moen, J.; Alexopoulos, D.; Inder, T.E.; Gholipour, A.; et al. Prenatal to postnatal trajectory of brain growth in complex congenital heart disease. Neuroimage Clin. 2018, 20, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Meuwly, E.; Feldmann, M.; Knirsch, W.; von Rhein, M.; Payette, K.; Dave, H.; Research Group Heart and Brain*; et al. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci Rep. 2019, 9, 10885. [Google Scholar] [CrossRef] [PubMed]
- von Rhein, M.; Buchmann, A.; Hagmann, C.; Dave, H.; Bernet, V.; Scheer, I.; Heart and Brain Research Group; et al. Severe Congenital Heart Defects Are Associated with Global Reduction of Neonatal Brain Volumes. J Pediatr. 2015, 167, 1259–1263.e1. [Google Scholar] [CrossRef] [PubMed]
- Reich, B.; Heye, K.N.; O’Gorman Tuura, R.; Beck, I.; Wetterling, K.; Hahn, A.; et al. Interrelationship Between Hemodynamics, Brain Volumes, and Outcome in Hypoplastic Left Heart Syndrome. Ann Thorac Surg. 2019, 107, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- von Rhein, M.; Buchmann, A.; Hagmann, C.; Huber, R.; Klaver, P.; Knirsch, W.; et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain 2014, 137 Pt 1, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Verrall, C.E.; Yang, J.Y.; Chen, J.; Schembri, A.; d’Udekem, Y.; Zannino, D.; et al. Neurocognitive Dysfunction and Smaller Brain Volumes in Adolescents and Adults With a Fontan Circulation. Circulation. 2021, 143, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, S.; Latal, B.; Miller, S.P.; McQuillen, P.S. The neonatal brain in critical congenital heart disease: Insights and future directions. Neuroimage. 2019, 185, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Gaynor, J.W.; Licht, D.J. Brain Injury During Transition in the Newborn with Congenital Heart Disease: Hazards of the Preoperative Period. Semin Pediatr Neurol. 2018, 28, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Heye, K.N.; Knirsch, W.; Scheer, I.; Beck, I.; Wetterling, K.; Hahn, A.; et al. Health-related quality of life in pre-school age children with single-ventricle CHD. Cardiol Young. 2019, 29, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Rometsch, S.; Greutmann, M.; Latal, B.; Bernaschina, I.; Knirsch, W.; Schaefer, C.; et al. Predictors of quality of life in young adults with congenital heart disease. Eur Heart J Qual Care Clin Outcomes. 2019, 5, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Chavez, T.; O’Neil, S.; Votava-Smith, J.; Miller, D.; delCastillo, S.; et al. Synchronous Aberrant Cerebellar and Opercular Development in Fetuses and Neonates with Congenital Heart Disease: Correlation with Early Communicative Neurodevelopmental Outcomes, Initial Experience. AJP Rep. 2017, 7, e17–e27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jakab, A.; Meuwly, E.; Feldmann, M.; Rhein, M.V.; Kottke, R.; O’Gorman Tuura, R.; Research Group Heart and Brain; et al. Left temporal plane growth predicts language development in newborns with congenital heart disease. Brain. 2019, 142, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Andropoulos, D.B.; Easley, R.B.; Brady, K.; McKenzie, E.D.; Heinle, J.S.; Dickerson, H.A.; et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg. 2012, 94, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Beca, J.; Gunn, J.K.; Coleman, L.; Hope, A.; Reed, P.W.; Hunt, R.W.; et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013, 127, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Andropoulos, D.B.; Ahmad, H.B.; Haq, T.; Brady, K.; Stayer, S.A.; Meador, M.R.; et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: A retrospective cohort study. Paediatr Anaesth. 2014, 24, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Bataillard, C.; Ehrler, M.; Ullrich, C.; Knirsch, W.; Gosteli-Peter, M.A.; et al. Cognitive and Executive Function in Congenital Heart Disease: A Meta-analysis. Pediatrics. 2021, 148, e2021050875. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.S.; Lipkin, P.H.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council; et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation. 2012, 126, 1143–1172. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.B.; Bucher, H.U. Un début précoce dans la vie: qu’apporte un registre national? Forum Med Suisse. 2013, 13, 35–37. [Google Scholar] [CrossRef]
- Martin, G.R.; Jonas, R.A. Surgery for Congenital Heart Disease: Improvements in Outcomes. Am J Perinatol. 2018, 35, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Pickup, L.; Gaffey, T.; Clift, P.; Bowater, S.; Thorne, S.; Hudsmith, L. Employment characteristics of a complex adult congenital heart disease cohort. Occup Med (Lond). 2017, 67, 453–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soufi, A.; Gouton, M.; Metton, O.; Mitchell, J.; Bernard, Y.F.; Bozio, A.; et al. Quality of life of adult Fontan patients. Cardiol Young. 2021, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
© 2022 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Steger, C.; Knirsch, W. Prenatal Consequences of Congenital Heart Disease on Brain Development. Cardiovasc. Med. 2022, 25, 103. https://doi.org/10.4414/cvm.2022.02217
Steger C, Knirsch W. Prenatal Consequences of Congenital Heart Disease on Brain Development. Cardiovascular Medicine. 2022; 25(4):103. https://doi.org/10.4414/cvm.2022.02217
Chicago/Turabian StyleSteger, Céline, and Walter Knirsch. 2022. "Prenatal Consequences of Congenital Heart Disease on Brain Development" Cardiovascular Medicine 25, no. 4: 103. https://doi.org/10.4414/cvm.2022.02217
APA StyleSteger, C., & Knirsch, W. (2022). Prenatal Consequences of Congenital Heart Disease on Brain Development. Cardiovascular Medicine, 25(4), 103. https://doi.org/10.4414/cvm.2022.02217


