Exercise and Cancer
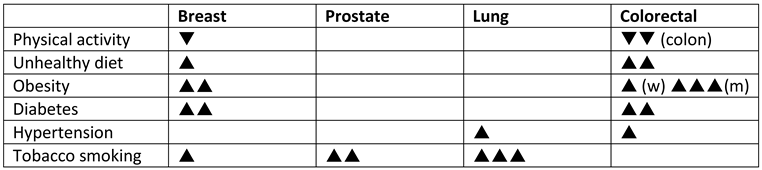
Epidemiology of cancer
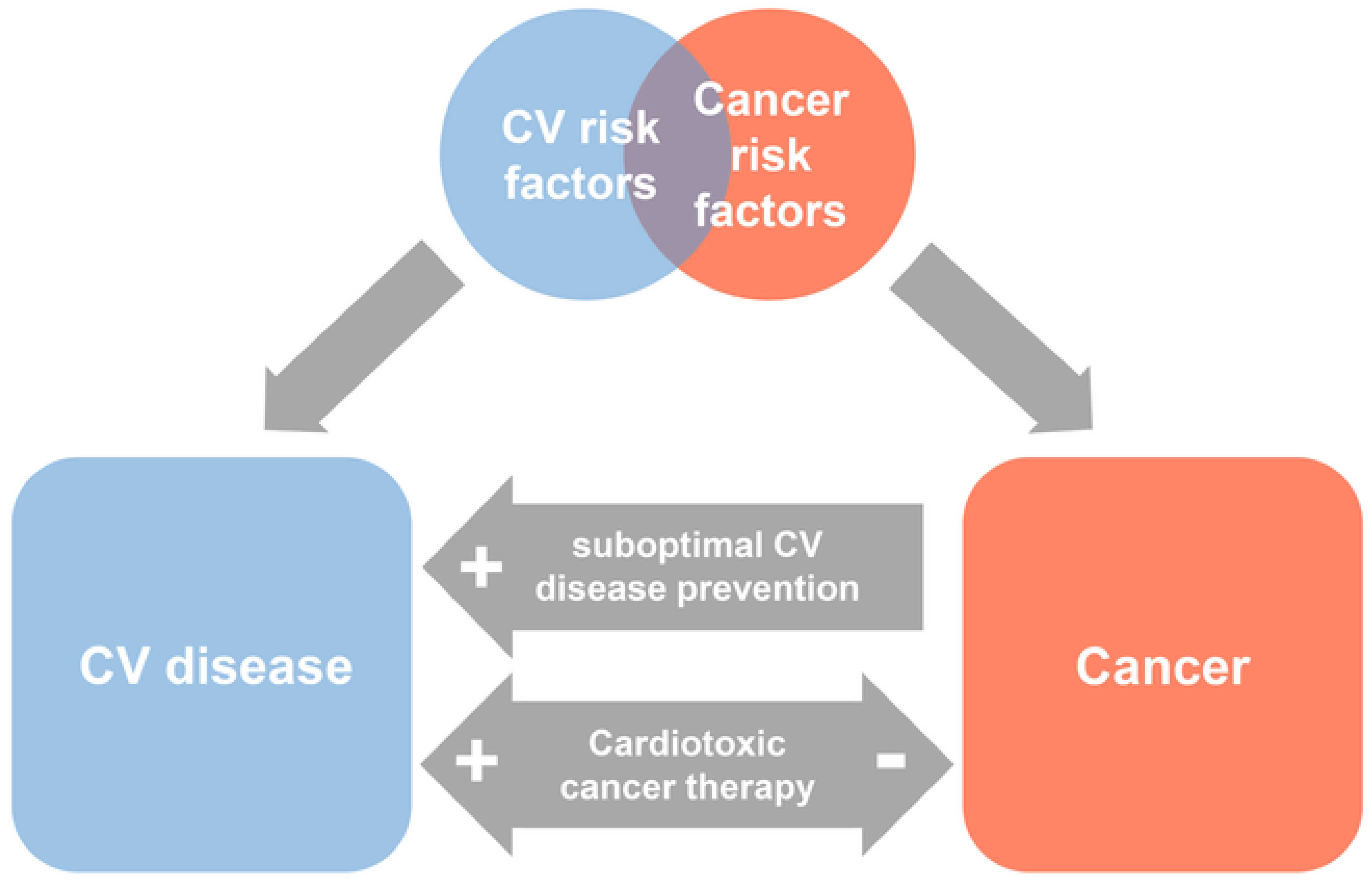
Cardiovascular complications of cancer therapy
Exercise intolerance in cancer patients
Exercise as important adjuvant therapy at all stages of the cancer care pathway
 |
Modulation of cardiotoxicity by aerobic exercise
Exercise recommendations for cancer patients
Implementation of exercise training
Safety of exercise in cancer patients
Gaps in evidence
Key points
Figure legend
References
- Arndt, V.; Feller, A.; Hauri, D.; Heusser, R. Schweizerischer Krebsbericht 2015; Bundesamt für Statistik, 2016. [Google Scholar]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.C.; Amir, E.; Abdel-Qadir, H. Cardiac Outcomes in Survivors of Pediatric and Adult Cancers. Can J Cardiol. 2016, 32, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Eves, N.D.; Haykowsky, M.; Freedland, S.J.; Mackey, J.R. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009, 10, 598–605. [Google Scholar] [CrossRef] [PubMed]
- van Waart, H.; van Harten, W.H.; Buffart, L.M.; Sonke, G.S.; Stuiver, M.M.; Aaronson, N.K. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology 2016, 25, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Conraads, V.; Corra, U.; Dickstein, K.; Francis, D.P.; Jaarsma, T.; et al. Exercise training in heart failure: From theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011, 13, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Stevinson, C.; Campbell, A.; Cavill, N.; Foster, J. Physical activity and cancer. Macmillan Cancer Support 2017.
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Khakoo, A.; Mackey, J.R.; Haykowsky, M.J.; Douglas, P.S.; Jones, L.W. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: Current evidence and underlying mechanisms. Circulation 2011, 124, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; et al. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.R.; Loblaw, A.; Petrella, T.; et al. Exercise for people with cancer: A systematic review. Curr Oncol. 2017, 24, e290–e315. [Google Scholar] [CrossRef] [PubMed]
- Kramis, K.; Ruckstuhl, B.; Wyler, M. Nationale Strategie Gegen Krebs 2014–2017; Dialog Nationale Gesundheitspolitik, 2013. [Google Scholar]
- Ture, M.; Barth, J.; Angst, F.; Aeschlimann, A.; Schnyder, U.; Zerkiebel, N.; et al. Use of inpatient rehabilitation for cancer patients in Switzerland: Who undergoes cancer rehabilitation? Swiss Med Wkly. 2015, 145, w14214. [Google Scholar] [CrossRef] [PubMed]
- Kotseva, K.; Wood, D.; De Bacquer, D. Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROASPIRE IV survey. Eur J Prev Cardiol. 2018, 25, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Park, L.G.; Schopfer, D.W.; Zhang, N.; Shen, H.; Whooley, M.A. Participation in Cardiac Rehabilitation Among Patients with Heart Failure. J Card Fail. 2017, 23, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M. [Exercise Training and Physical Activity in Patients with Heart Failure]. Praxis (Bern 1994) 2018, 107, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Bouillet, T.; Bigard, X.; Brami, C.; Chouahnia, K.; Copel, L.; Dauchy, S.; et al. Role of physical activity and sport in oncology: Scientific commission of the National Federation Sport and Cancer CAMI. Crit Rev Oncol Hematol. 2015, 94, 74–86. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Wilhelm, M.; Eser, P.; Wilhelm, M. Exercise and Cancer. Cardiovasc. Med. 2019, 22, 2032. https://doi.org/10.4414/cvm.2019.02032
Wilhelm M, Eser P, Wilhelm M. Exercise and Cancer. Cardiovascular Medicine. 2019; 22(3):2032. https://doi.org/10.4414/cvm.2019.02032
Chicago/Turabian StyleWilhelm, Matthias, Prisca Eser, and Matthias Wilhelm. 2019. "Exercise and Cancer" Cardiovascular Medicine 22, no. 3: 2032. https://doi.org/10.4414/cvm.2019.02032
APA StyleWilhelm, M., Eser, P., & Wilhelm, M. (2019). Exercise and Cancer. Cardiovascular Medicine, 22(3), 2032. https://doi.org/10.4414/cvm.2019.02032