Abstract
Detailed clinical assessment, the electrocardiogram and cardiac troponin form the three cornerstones of the early diagnosis of myocardial infarction (MI). After the clinical introduction of high-sensitivity cardiac troponin (hs-cTn) assays about 8 years ago, precise quantification of cardiomyocyte injury around the 99th percentile became possible and thereby substantially increased the accuracy of MI diagnosis from blood drawn at presentation to the emergency department. Higher diagnostic accuracy enabled the development and validation of multiple early hs-cTn-based diagnostic algorithms including the ESC 0/1-hour algorithm, which substantially reduced the time required for safe rule-out or rule-in of MI. This algorithm is widely applicable, including in patients with renal dysfunction and in the USA, where hs-cTn approval is slightly different from the rest of the world. Apart from hs-cTn, other emerging cardiac biomarkers might have the potential to further improve the early triage of patients with suspected MI.
High-sensitivity cardiac troponin
Symptoms suggestive of myocardial infarction (MI) are among the most frequent symptoms leading to an emergency department (ED) presentation [1]. Since clinical assessment and electrocardiogram (ECG) alone are not sufficient to diagnose or exclude non-ST-segment-elevation myocardial infarction (NSTEMI) in most patients, the addition of blood tests to serially measure markers of myocardial necrosis form a cornerstone of the early diagnosis of MI. When considering the clinical utility of cardiac biomarkers for the early diagnosis of MI, it is of utmost importance to interpret them only in the appropriate clinical setting of suspected MI and always in conjunction with detailed clinical assessment and thorough interpretation of the ECG (Figure 1).
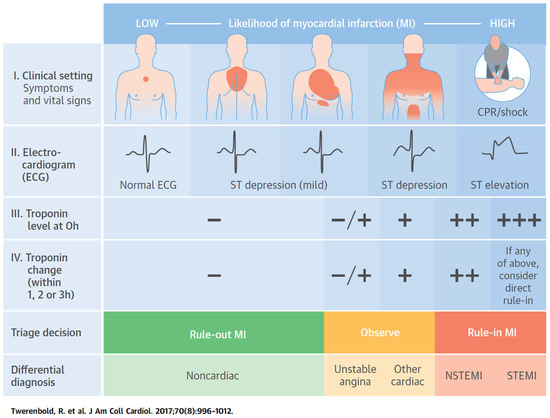
Figure 1.
Clinical assessment of patients presenting with suspected acute coronary syndrome. The initial assessment is based on the integration of low likelihood and/or high likelihood features derived from clinical assessment (symptoms, vital signs), 12-lead ECG, and cardiac troponin determined at presentation to the emergency department and serially thereafter. “Other cardiac” includes, among others, myocarditis, tako-tsubo cardiomyopathy, or congestive heart failure. “Noncardiac” refers to thoracic diseases such as pneumonia or pneumothorax. Cardiac troponin at presentation and its changesduring serial sampling should be interpreted as a quantitative marker: the higher the 0-h level or the absolute change during serial sampling, the higher the likelihood for the presence of myocardial infarction. In patients presenting with cardiac arrest or haemodynamic instability of presumed cardiovascular origin, echocardiography should be performed/interpreted by trained physicians immediately following a 12-lead ECG. If the initial evaluation suggests aortic dissection or pulmonary embolism, D-dimers and multi-detector computed tomography angiography are recommended, according to dedicated algorithms.
Until 1990, aspartate aminotransferase, lactate dehydrogenase, creatine kinase (and its isoforms) or myoglobin were used to detect myocardial necrosis. In the 1990s, cardiac troponin (cTn) T and I assays were clinically introduced, providing higher sensitivity and specificity for myocardial necrosis as compared with the previous markers (Figure 2). Recent improvements in the analytical sensitivity of cTn assay have led to a further refinement in clinical ability to detect and quantify cardiomyocyte injury [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. These assays increased diagnostic accuracy at presentation, substantially reduced the sensitivity deficit of cTn at presentation for MI and the associated “troponin-blind” interval, and allowed the recent development of several novel strategies for the rapid rule-out or early rule-in of NSTEMI [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. These improved assays are labelled “sensitive” if they are able to detect cTn in ~20–50% of healthy individuals, and “high-sensitivity” if they detect cTn in >50% of reference (apparently healthy) subjects and have a coefficient of variation of <10% at the 99th percentile upper reference limit of the assay [8]. High-sensitivity assays have the ability to accurately detect cTn at lower levels than older generation assays, giving them higher sensitivity for the detection of MI at presentation, which means that the time interval to the second measurement of high-sensitivity cTn (hs-cTn) can be significantly shortened [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
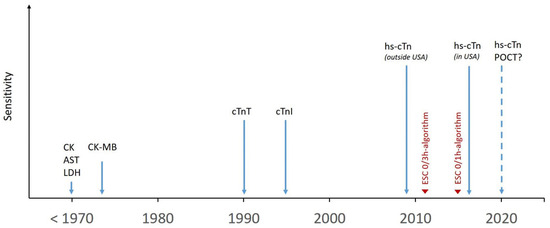
Figure 2.
Historical timeline of myocardial necrosis markers. Clinical introduction of myocardial necrosis markers over time and their respective biochemical and analytical sensitivity. AST = aspartate aminotransferase; CK = Creatine kinase; CK-MB = Creatine kinase muscle/brain; cTn = cardiac troponin; ESC = European Society of Cardiology; hs-cTn = high-sensitivity cardiac troponin; LDH = lactate dehydrogenase; POCT = point of care testing.
Both cTnT and I are structural proteins unique to the heart. Thus, cTnT and I are organ-specific, but not disease-specific markers. High-sensitivity and sensitive cTnT and I assays exactly quantify the amount of cardiomyocyte injury [3,35]. They should be interpreted as quantitative variables and not in a binary fashion (negative/positive) like a pregnancy test. From a diagnostic perspective, it is highly inappropriate to label a patient as “cTn-positive”, as this would lump together patients with only mildly elevated cTn levels barely above the 99th percentile and an associated positive predictive value (PPV) for NSTEMI of only about 40–50%, and patients with markedly elevated cTn levels (e.g., about five times above the 99th percentile) and an associated PPV of 90%. The higher the cTn level, the higher is the likelihood for the presence of MI.
Fourth universal definition of myocardial infarction
In August 2018, the fourth universal definition of myocardial infarction was published [2]. This document officially introduced the term “myocardial injury”, describing any cTn concentration above the 99th percentile irrespective of its underlying cause (e.g., MI, congestive heart failure, chronic kidney disease). The absence or presence of dynamic changes in cTn concentrations during serial sampling differentiates acute myocardial injury (e.g., MI, myocarditis, Tako-Tsubo cardiomyopathy, congestive heart failure, cardiac ablation) from chronic types (e.g., chronic kidney disease, structural heart disease). Acute myocardial injury is only considered an MI if there is clear clinical evidence of acute myocardial ischaemia (e.g., symptoms of myocardial ischaemia, new ischaemic ECG changes, development of pathological Q waves in the ECG and/or imaging evidence of new loss of viable myocardium or new regional wall motion abnormalities).
To differentiate MI from other causes of chest pain, absolute rather than relative hs-cTn changes seem to be the best metric [12,19,20,21]. The larger the absolute cTn change within 1, 2, or 3 hours, the higher the likelihood for the presence of MI. Continuous medical education and training of physicians in these concepts is essential to avoid inappropriate interpretation of the chronic mild elevations of cTn associated with, for example, heart failure or other structural cardiac disorders as signs of MI.
About false-positive hs-cTn measurements, troponinaemia, troponinitis and other fairy tales
In the absence of overt myocardial ischaemia, elevated cTn levels are often labelled as “false positive” hs-cTn results, troponinaemia or troponinitis. These terms should be avoided, as most of these unexpected hs-cTn elevations are “true-positive” for myocardial injury (rather than MI) and reflect previously undetected or underestimated cardiac disease, including valvular heart disease, heart failure and chronic coronary artery disease. Many cardiac and noncardiac disorders may lead to substantial amounts of cardiomyocyte injury and thereby hs-cTn elevations (Table 1) [3,8]. It is important to note that cTn elevations universally portend a worse prognosis than for otherwise similar patients without a cTn elevation, irrespective of the underlying disease. This is true regardless of whether the patient has heart failure, renal dysfunction, gastrointestinal bleeding, sepsis, respiratory disease, pulmonary embolism, subarachnoid haemorrhage, or stroke or whether the patient is asymptomatic without known cardiovascular disease [36]. Obviously, the medical consequences of cardiomyocyte injury as quantified by cTn elevations will be highly individualised and different from those in patients with MI.

Table 1.
Conditions other than myocardial infarction associated with cardiac troponin elevations.
Rapid triage algorithms using hs-cTn
The most important clinical advantage of the new, moresensitive cTn assays is their ability to substantially reduce the “troponin-blind” interval in the first hours of an MI and thereby to allow novel strategies to rule-out or rule-in NSTEMI early. Multiple troponin-based strategies rely on serial hs-cTn testing. Two of them, the 0/1-hour algorithm and a 0/3-hour algorithm, are currently recommended by the European Society of Cardiology (ESC) with a class I recommendation [3].
It is important to consider five points when applying troponin-based strategies in clinical practice. First, they should be used only in conjunction with full clinical assessment. Second, these strategies should be considered triage strategies rather than definite diagnostic strategies, as additional imaging tests such as invasive coronary angiography, stress testing, echocardiography or computed tomography (CT) angiography may be necessary for a definite diagnosis. Third, the percentage of patients eligible for rule-out or rule-in varies widely from ≈9.8% to 77% depending on the underlying algorithm, the cTn assay used and the clinical setting, including the prevalence of NSTEMI [20,23]. Fourth, these strategies should only be applied after the initial ECG has excluded ST-segment-elevation myocardial infarction (STEMI) since these high-risk patients need prompt identification based on the ECG and immediate reperfusion therapy without the need for cTn testing [2]. Fifth, any triage strategy should be embedded in the local standard operating procedures of the ED.
The main performance metrics of early triage strategies are safety of rule-out (quantified as the negative predictive value [NPV] and sensitivity for NSTEMI), overall efficacy (quantified as the percentage of patients triaged either towards rule-out or rule-in), and accuracy of rule-in (quantified as the PPV and specificity for NSTEMI) if the respective algorithms provides a rule-in strategy.
ESC 0/3-hour algorithm
NSTEMI is ruled-out if hs-cTn concentrations remain in the normal range (below the respective assay-specific 99th percentile) in the blood samples drawn at presentation and 3 h after presentation, and if the patient fulfils two additional requirements: to be pain-free and to be at low-risk of in-hospital mortality as quantified by a Global Registry of Acute Coronary Events (GRACE) score below 140 (Figure 3A) [3]. In patients presenting more than 6 h after chest pain onset, in whom chest pain onset can be reliably quantified, one single blood draw at presentation is considered to be sufficient. Patients are ruled-in if they have a clearly elevated hs-cTn blood concentration at presentation, or if the 3-hour sample shows a relevant change. This approach has been recommended by the ESC Guidelines since 2011 and is still the most-widely used algorithm in Europe [3,8]. Its use regarding rule-out of MI seems to be acceptably effective and safe for all hs-cTn assays and possibly also some sensitive cTn assays [37]. The exact performance for rule-in cannot be quantified, as no precise definitions of its rule-in cut-off levels are given. Given the average turnaround time for hs-cTn of about 1 h, the hs-cTn measurement performed at 3 h after ED presentation would become available about 4 h after ED presentation and would allow clinical decision making regarding hospitalisation versus outpatient management about 4 h after ED presentation in the majority of patients. In a recent study, this strategy enabled outpatient management in 56% of patients, with a median time in the ED of about 5 h in the overall population, and 4½ h in those patients managed as out-patients [38].
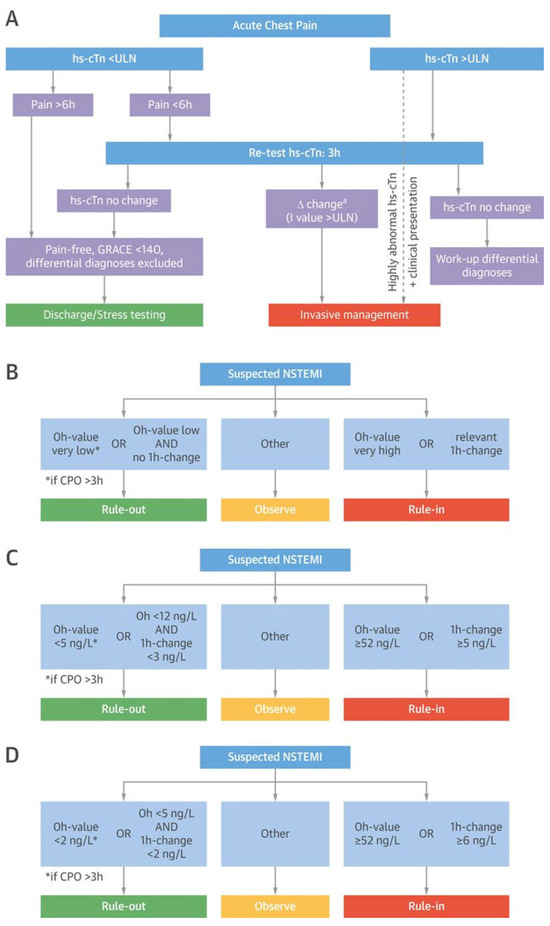
Figure 3.
European Society of Cardiology rapid triage algorithms. (A) The European Society of Cardiology (ESC) 0/3-h rule-out and rule-in algorithm of non–ST-segment–elevation myocardial infarction (NSTEMI) using high-sensitivity cardiac troponin (hs-cTn) assays. (B) The concept of the ESC 0/1-h rule-out and rule-in algorithm using hs-cTn assays in patients presenting with suspected NSTEMI to the emergency department. (C) The ESC 0/1-h ruleout and rule-in algorithm with assay-specific cutoff values for the Elecsys hs-cTnT assay by Roche®. (D) The ESC 0/1-h rule-out and rule-in algorithm with assay-specific cutoff values for the Architect hs-cTnI assay by Architect Abbott®. Adapted from Twerenbold et al. [4] with permission from the publisher. CPO = chest pain onset.
ESC 0/1-hour algorithm
The concept of the ESC 0/1-hour algorithm is exclusively based on information provided by hs-cTn concentrations at ED presentation and their absolute change within 1 hour, using assay-specific cutoffs (concept depicted in Figure 3B; assay-specific cutoffs for hs-cTnT by Roche®, Figure 3C; assay-specific cutoffs for hs-cTnI by Abbott®, Figure 3D) [3,20,29,30,39,40]. The 0/1-hour ESC algorithm results in safe rule-out of NSTEMI (NPV 99–100%) and allows an accurate early triage in about 75% of patients: 60% towards rule-out and in 15% towards rule-in of NSTEMI [40]. The application of the ESC 0/1-hour algorithm is also possible in institutions with a median turn-around time of more than 1 h, since the 1 h only refers to the timing of the serial sample. In these institutions, the second blood draw would simply need to be performed while still awaiting the results from the first blood draw.
Direct rule-out based strategy on undetectable/very low baseline hs-cTn concentrations
Undetectable or very low blood concentrations of hs-cTn at presentation to the ED have a very high (98.6–100%) NPV for NSTEMI. This approach has a unique simplicity, as it requires only a single blood draw for an inexpensive and widely available biomarker test. As the lower limit of detection is assay dependent and varies among the clinically available hs-cTn assays, “very low concentrations, e.g., below the 30th percentile of healthy individuals” may be the preferred metrics to identify biological-equivalent values. Four large studies and three recent meta-analyses have provided consistent results for hs-cTnT and hs-cTnI assays [31,32,41,42,43,44,45,46]. As the release of cTn is a time-dependent phenomenon, this approach should only be used in patients with a chest pain onset at least 2–3 h prior to ED presentation, as safety was found to be reduced in very early presenters in a recent observation [45]. In the 2015 ESC guidelines, this approach is recommended in combination with the 0/1-hour algorithm as the preferred rule-out strategies owing to their excellent balance between speed and accuracy [3].
Strengths and limitations of the different rapid triage algorithms
There are some important differences between the above listed algorithms that have to be mentioned. First, direct rule-out strategies rule-out patients with a single hs-cTn measurement at ED presentation, whereas the other algorithms require serial sampling at 1 or 3 hours. Whereas the ESC 0/1-hour algorithm incorporates both the hs-cTn concentration at ED presentation and its absolute change during resampling, the ESC 0/3-hour algorithm relies on the assay-specific 99th percentile only. The integration of absolute changes in the ESC 0/1-hour algorithm has the potential to improve safety and efficacy compared with the ESC 0/3-hour algorithm. However, as direct head-to-head comparisons are still lacking, their impact could not be quantified yet. Second, while the 0/1-hour and 0/3-hour algorithms allow for triage towards rule-out and rule-in of NSTEMI, the other described algorithm (direct rule-out strategy) can only be used for early rule-out of NSTEMI. Third, by incorporating the time since chest pain onset, the ESC 0/1-hour algorithm takes advantage of patients presenting very early after chest pain onset, a subgroup of patients requiring particular attention in order not to miss late rises in hs-cTn [3,45].
Early rule-out algorithms are helping to guide clinicians identifying patients at very low risk for NSTEMI and major adverse cardiovascular events (MACE). However, the strategy for rapid triage of suspected MI in clinical practice must be chosen by each institution individually, depending on the locally used cTn assay (sensitive versus high-sensitivity), wish for additional rule-in guidance and individual preferences regarding targeted balance between safety and efficacy.
Additional helpful steps in the observe zone
The ESC 0/1-hour algorithms [3,20,29,30,39,40] provide detailed guidance for rule-in of NSTEMI in addition to a rule-out strategy. In addition, an intermediate-risk group has been created, leaving up to one third of patients in this observe zone [3,18,20,29,30,39,40,47,48]. These patients are typically elderly men with pre-existing coronary artery disease and have been shown to have increased long-term mortality [49]. Detailed clinical assessment, additional hs-cTn measurement at 3 h and cardiac imaging are key for accurate diagnosis in these patients. The clinical interpretation of mildly abnormal hs-cTn levels is crucial for physicians in the ED, since up to one third of patients triaged to the observe zone are finally diagnosed with NSTEMI or unstable angina. Therefore, hs-cTn should be retested at 3 h to better differentiate an acute cardiac disease (such as NSTEMI) associated with a dynamic hs-cTn course from a chronic cardiac disease reflected by stable hs-cTn levels. Coronary angiography (in those with high likelihood for NSTEMI), echocardiography, and functional stress imaging (in those with low likelihood for NSTEMI) seem to be the preferred tests in observe patients [49].
Coronary computed tomography angiography (CCTA) seems a suitable imaging modality in only a minority [50]. A randomised controlled trial recently showed no benefit of routine CCTA over standard optimal care encompassing hs-cTnT in patients with suspected acute coronary syndrome regarding identification of significant coronary artery disease (CAD) requiring revascularisation within 30 days, duration of hospital stay or direct discharge from the ED [51]. Functional rather than anatomical testing is mandatory to differentiate coronary lesions resulting in myocardial ischaemia and acute chest pain at rest from lesions that are innocent bystanders in the acute chest pain episode leading to ED presentation [49].
Overruling a triage recommendation
Hs-cTn-based triage algorithms must always be used in conjunction with detailed clinical assessment and thorough interpretation of the ECG. This synthesis may well result in overruling a “rule-out” recommendation provided by the hs-cTn-based algorithms in some patients perceived to be at high-risk of NSTEMI. Overruling should then lead to the identical process described for patients assigned the observe zone and should always include an additional hs-cTn measurement at 3 h.
Rule-out for MI does not always equal outpatient management: the novel strategies were developed to safely ruleout NSTEMI, but not other disorders that still may require hospital admission, such as unstable angina, pulmonary embolism, aortic dissection or severe sepsis from pneumonia. Accordingly, the percentage of patients who can possibly be managed as out-patients is smaller than the percentage of patients ruled-out for NSTEMI. Standard operating procedures should be in place to ensure appropriate follow-up of patients rapidly discharged from the ED, which often will include outpatient functional cardiac stress testing.
Hs-cTn in patients with renal dysfunction
Patients with suspected NSTEMI and renal dysfunction are at substantially higher risk of NSTEMI as compared to patients with normal renal function [4,52,53,54]. Accurate rule-out and rule-in of NSTEMI is of paramount importance since patients with renal dysfunction are more prone to adverse events related to cardiovascular medication (e.g., anticoagulation), as well as to cardiovascular procedures including coronary angiography and coronary intervention [3,35]. However, rapid and accurate diagnosis of NSTEMI is challenging in this vulnerable patient subgroup, since they often present with chronically elevated troponin levels (10–20% using s-cTn, up to 70% using hs-cTn) even in conditions other than acute myocardial ischaemia, which are still associated with poor prognosis [54,55]. The underlying pathophysiological mechanism is poorly understood and not primarily explained by reduced glomerular filtration rate [56,57,58,59].
In general, the high diagnostic utility of hs-cTn can be maintained in patients with renal dysfunction if adjusted decision levels higher than the assay-specific 99th percentiles are considered [54]. A recent international analysis addressed the question of whether the ESC 0/1-hour algorithm can safely be used in patients with renal dysfunction [60]. High safety of rule-out (sensitivity 100% for hs-cTnT, 98.6% for hs-TnI) was documented, supporting the use of the ESC 0/1-hour algorithm also in patients with renal dysfunction (Figure 4 depicting performance of the 0/1-hour algorithm using hs-cTnT in different stages of renal dysfunction). However, specificity of rule-in and efficacy of rule-out were decreased as fewer patients with renal dysfunction presented with low hs-cTn blood concentrations. Modifications of the rule-in and rule-out thresholds did not improve the specificity or overall efficacy of the 0/1-hour algorithm.
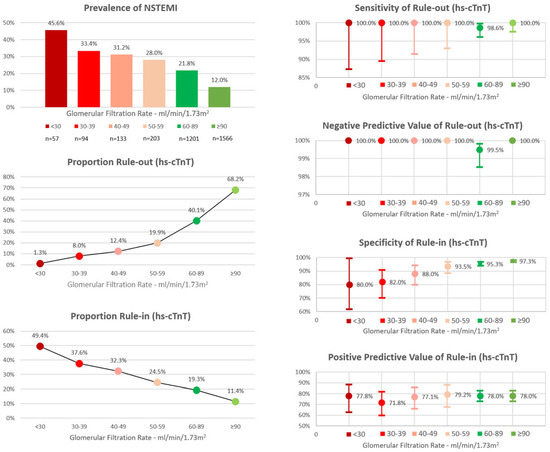
Figure 4.
Performance of the European Society of Cardiology 0/1h-algorithm using high-sensitivity cardiac troponin T in different stages of renal function. hs-cTnT = high-sensitivity cardiac troponin T; NSTEMI = Non-ST-Segment-Elevation Myocardial Infarction. Adapted from Twerenbold et al. [60] with permission from the publisher.
0/1-hour algorithm according to the hs-cTnT approval in the USA
Although hs-cTn assays have been widely used since 2010 in Europe and many other countries outside the USA, the first hs-cTn assay has just received approval by the Food and Drug Administration (FDA) for clinical use in the USA in spring 2017. However, the FDA-approved use of hs-cTnT differs in two important details from its contemporary use in most other countries: First, low concentrations are reported only down to 6 ng/l as compared to 3 ng/ l, and second, a higher age-matched 99th percentile upper reference limit of 19 ng/l is suggested, as compared with 14 ng/l. Both changes could potentially impact the safety and/or efficacy of rapid triage algorithms defined previously in a non-FDA setting. A recent analysis aimed to quantify the impact of the FDA-approved use of hs-cTnT on the safety and efficacy of the ESC 0/1-hour lgorithm [61]. The original ESC 0/1-hour algorithm was minimally adapted to the FDA setting by lifting the direct rule-out cutoff at presentation from <5 ng/l to <6 ng/l, since hs-cTnT levels are only reported down to 6 ng/l in the USA. Rule-out safety as well as rule-in accuracy of the original and the modified algorithm were very high and comparable (NPV 99.8% versus 99.9%, PPV 76.9% versus 76.7%). Both algorithms allowed rapid rule-out and rule-in of NSTEMI in three patients out of four.
Cardiac myosin-binding protein C as a potential alternative to hs-cTn
With the advent of more sensitive cardiac troponin assays, biomarkers previously used to detect myocardial infarction, such as creatine kinase, creatine kinase MB isoform or myoglobin, became less relevant owing to their decreased sensitivity and specificity compared with hs-cTn [2,10]. Hs-cTn assays represent the current gold standard and the other cardiac biomarkers are no longer supported by most current guidelines [2]. Recently, a novel cardiac biomarker indicating myocardial injury has been described: cardiac myosin-binding protein C (cMyC), a cardiac-specific protein of the contractile apparatus of the myocardium (Figure 5). Preliminary preclinical and clinical data suggested a quicker release into blood after myocardial infarction than for hs-cTn [62]. In an European multicentre diagnostic study, 1954 patients presenting to the ED with suspected NSTEMI were analysed. Surprisingly, the diagnostic accuracy of cMyC was similar to that of hs-cTnT and hs-cTnI in the overall population, but statistically superior compared with hs-cTnT in patients presenting early (within the first 2 hours after chest pain onset) [62]. Whether the advantage of this novel biomarker is of sufficient clinical relevance to replace the established hs-cTn assays needs to be examined in further studies.
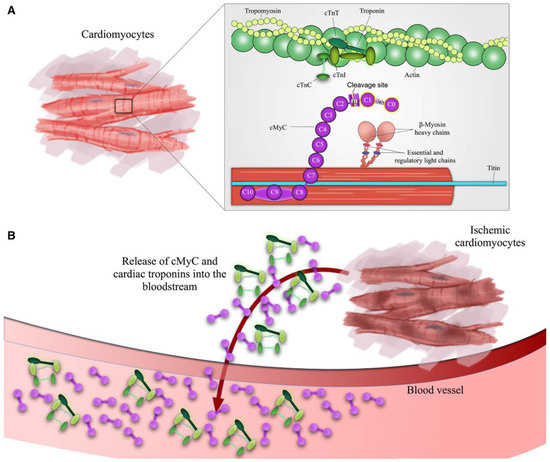
Figure 5.
Depiction of cardiac troponin and cardiac myosin-binding protein C release during myocardial injury. Structure of cardiac myosinbinding protein C and cardiac troponins in (A) healthy cardiomyocytes and (B) ischemia-induced cardiomyocyte damage. The highlighted Nterminal domain C0C1 is the binding site for the previously developed monoclonal antibodies used for detection of the cardiac-specifc isoform of cMyC.16 cMyC indicates cardiac myosin-binding protein C; cTnI, cardiac troponin I; and cTnT, cardiac troponin T. Adapted from Kaier, Twerenbold et al. [62] with permission from the publisher.
Conclusions
Hs-cTn assays improve the safe and rapid triage of patients with suspected NSTEMI and complement assessment of clinical presentation and the ECG. Reduction of the “troponin-blind” interval allows delay in serial hs-cTn re-measurements to be substantially shortened. Many factors other than acute myocardial ischaemia may cause cardiomyocyte injury and therefore mild hs-cTn elevations. Dynamic changes of hs-cTn during serial sampling help to distinguish acute from chronic causes of chest pain and troponin elevations. To maximally profit from hs-cTn assays in clinical practice, they should best be used embedded in an institutional standard operating procedure of the ED and in conjunction with a rapid triage algorithm enabling rapid decision making within a few hours. Such an approach will not only increase patients’ safety as compared with conventional, less sensitive cTn assays, but also substantially reduce duration of stay in the ED and costs. Hs-cTn is the current gold standard biomarker for the detection of myocardial injury. Whether other novel emerging cardiac biomarkers such as cMyC have the potential to replace hs-cTn in the future needs to be explored in further studies.
Disclosure statement
Dr. Twerenbold received research support from the Swiss National Science Foundation (P300PB_167803), the Swiss Heart Foundation, theUniversity Hospital of Basel and the University of Basel as well as speaker honoraria/consulting honoraria from Roche, Abbott, Siemens, Singulex and BRAHMS Thermo Scientific. Dr. Rubini received speaker honoraria from Abbott. Dr. Boeddinghaus received research support from the University Hospital of Basel (Division of Internal Medicine), the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner Foundation, and speaker honoraria/consulting honoraria from Siemens. Dr. Mueller received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the Stiftung für kardiovaskuläre Forschung Basel; Abbott, Alere, Astra Zeneca, Beckman Coulter, Biomerieux, BRAHMS Thermo Scientific, Roche, Siemens, Singulex, Sphingotec, and the Department of Internal Medicine, University Hospital Basel, as well as speaker honoraria/consulting honoraria from Abbott, Alere, Astra Zeneca, Biomerieux, Boehringer Ingelheim, BMS, BRAHMS Thermo Scientific, Cardiorentis, Novartis, Roche, Siemens, and Singulex. All other authors declare no conflicts of interest.
References
- Blomkalns, A.L.; Gibler, W.B. Chest pain unit concept: Rationale and diagnostic strategies. Cardiol Clin. 2005, 23, 411–421. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2018, 39, 3757–3758. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; ESC Scientific Document Group; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Rubini Gimenez, M.; Badertscher, P.; et al. Clinical Use of High-Sensitivity Cardiac Troponin in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol. 2017, 70, 996–1012. [Google Scholar] [CrossRef]
- Twerenbold, R.; Boeddinghaus, J.; Mueller, C. Update on high-sensitivity cardiac troponin in patients with suspected myocardial infarction. Eur Heart J Suppl. 2018, 20, G2–G10. [Google Scholar] [CrossRef]
- Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Rubini Gimenez, M.; Badertscher, P.; et al. How to best use high-sensitivity cardiac troponin in patients with suspected myocardial infarction. Clin Biochem. 2018, 53, 143–155. [Google Scholar] [CrossRef]
- Thygesen, K.; Mair, J.; Katus, H.; Plebani, M.; Venge, P.; Collinson, P.; Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care; et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010, 31, 2197–2204. [Google Scholar] [CrossRef]
- Thygesen, K.; Mair, J.; Giannitsis, E.; Mueller, C.; Lindahl, B.; Blankenberg, S.; Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care; et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012, 33, 2252–2257. [Google Scholar] [CrossRef]
- Reichlin, T.; Hochholzer, W.; Bassetti, S.; Steuer, S.; Stelzig, C.; Hartwiger, S.; et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009, 361, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Zeller, T.; Peetz, D.; Tzikas, S.; Roth, A.; Czyz, E.; et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009, 361, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Giannitsis, E.; Kurz, K.; Hallermayer, K.; Jarausch, J.; Jaffe, A.S.; Katus, H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010, 56, 254–261. [Google Scholar] [CrossRef]
- Haaf, P.; Drexler, B.; Reichlin, T.; Twerenbold, R.; Reiter, M.; Meissner, J.; et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation. 2012, 126, 31–40. [Google Scholar] [CrossRef]
- Reiter, M.; Twerenbold, R.; Reichlin, T.; Haaf, P.; Peter, F.; Meissner, J.; et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011, 32, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Twerenbold, R.; Reichlin, T.; Benz, B.; Haaf, P.; Meissner, J.; et al. Early diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assays. Eur Heart J. 2012, 33, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2009, 55, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Rubini Gimenez, M.; Twerenbold, R.; Reichlin, T.; Wildi, K.; Haaf, P.; Schaefer, M.; et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J. 2014, 35, 2303–2311. [Google Scholar] [CrossRef]
- Meune, C.; Reichlin, T.; Irfan, A.; Schaub, N.; Twerenbold, R.; Meissner, J.; et al. How safe is the outpatient management of patients with acute chest pain and mildly increased cardiac troponin concentrations? Clin Chem. 2012, 58, 916–924. [Google Scholar] [CrossRef]
- Reichlin, T.; Cullen, L.; Parsonage, W.A.; Greenslade, J.; Twerenbold, R.; Moehring, B.; et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med. 2015, 128, 369–379.e4. [Google Scholar] [CrossRef]
- Reichlin, T.; Irfan, A.; Twerenbold, R.; Reiter, M.; Hochholzer, W.; Burkhalter, H.; et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011, 124, 136–145. [Google Scholar] [CrossRef]
- Reichlin, T.; Schindler, C.; Drexler, B.; Twerenbold, R.; Reiter, M.; Zellweger, C.; et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012, 172, 1211–1218. [Google Scholar] [CrossRef]
- Mueller, M.; Biener, M.; Vafaie, M.; Doerr, S.; Keller, T.; Blankenberg, S.; et al. Absolute and relative kinetic changes of high-sensitivity cardiac troponin T in acute coronary syndrome and in patients with increased troponin in the absence of acute coronary syndrome. Clin Chem. 2012, 58, 209–218. [Google Scholar] [CrossRef]
- Mueller, C. Biomarkers and acute coronary syndromes: An update. Eur Heart J. 2014, 35, 552–556. [Google Scholar] [CrossRef]
- Than, M.; Cullen, L.; Reid, C.M.; Lim, S.H.; Aldous, S.; Ardagh, M.W.; et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): A prospective observational validation study. Lancet. 2011, 377, 1077–1084. [Google Scholar] [CrossRef]
- Than, M.; Cullen, L.; Aldous, S.; Parsonage, W.A.; Reid, C.M.; Greenslade, J.; et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: The ADAPT trial. J Am Coll Cardiol. 2012, 59, 2091–2098. [Google Scholar] [CrossRef]
- Cullen, L.; Mueller, C.; Parsonage, W.A.; Wildi, K.; Greenslade, J.H.; Twerenbold, R.; et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol. 2013, 62, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Than, M.; Aldous, S.; Lord, S.J.; Goodacre, S.; Frampton, C.M.; Troughton, R.; et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: A randomized clinical trial. JAMA Intern Med. 2014, 174, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Meller, B.; Cullen, L.; Parsonage, W.A.; Greenslade, J.H.; Aldous, S.; Reichlin, T.; et al. Accelerated diagnostic protocol using high-sensitivity cardiac troponin T in acute chest pain patients. Int J Cardiol. 2015, 184, 208–215. [Google Scholar] [CrossRef]
- Hammarsten, O.; Fu, M.L.; Sigurjonsdottir, R.; Petzold, M.; Said, L.; LandinWilhelmsen, K.; et al. Troponin T percentiles from a random population sample, emergency room patients and patients with myocardial infarction. Clin Chem. 2012, 58, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Twerenbold, R.; Wildi, K.; Rubini Gimenez, M.; Bergsma, N.; Haaf, P.; et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ Canadian Medical Association journal = journal de l'Association medicale canadienne. 2015, 187, E243–E252. [Google Scholar] [CrossRef]
- Rubini Gimenez, M.; Twerenbold, R.; Jaeger, C.; Schindler, C.; Puelacher, C.; Wildi, K.; et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med. 2015, 128, 861–870.e4. [Google Scholar] [CrossRef]
- Body, R.; Carley, S.; McDowell, G.; Jaffe, A.S.; France, M.; Cruickshank, K.; et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011, 58, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Rubini Giménez, M.; Hoeller, R.; Reichlin, T.; Zellweger, C.; Twerenbold, R.; Reiter, M.; et al. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitivity cardiac troponin. Int J Cardiol. 2013, 168, 3896–3901. [Google Scholar] [CrossRef]
- Hollander, J.E.; Than, M.; Mueller, C. State-of-the-Art Evaluation of Emergency Department Patients Presenting With Potential Acute Coronary Syndromes. Circulation. 2016, 134, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Zeller, T.; Ojeda, F.; Tzikas, S.; Lillpopp, L.; Sinning, C.; et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011, 306, 2684–2693. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014, 64, e139–228. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.E. Managing Troponin Testing. Ann Emerg Med. 2016, 68, 690–694. [Google Scholar] [CrossRef]
- Wildi, K.; Nelles, B.; Twerenbold, R.; Rubini Giménez, M.; Reichlin, T.; Singeisen, H.; et al. Safety and efficacy of the 0 h/3 h protocol for rapid rule out of myocardial infarction. Am Heart J. 2016, 181, 16–25. [Google Scholar] [CrossRef]
- Twerenbold, R.; Jaeger, C.; Rubini Gimenez, M.; Wildi, K.; Reichlin, T.; Nestelberger, T.; et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J. 2016, 37, 3324–3332. [Google Scholar] [CrossRef]
- Mueller, C.; Giannitsis, E.; Christ, M.; Ordonez-Llanos, J.; deFilippi, C.; McCord, J.; et al. Multicenter Evaluation of a 0-Hour/1-Hour Algorithm in the Diagnosis of Myocardial Infarction With High-Sensitivity Cardiac Troponin T. Ann Emerg Med. 2016, 68, 76–87.e4. [Google Scholar] [CrossRef]
- Twerenbold, R.; Neumann, J.T.; Sörensen, N.A.; Ojeda, F.; Karakas, M.; Boeddinghaus, J.; et al. Prospective Validation of the 0/1-h Algorithm for Early Diagnosis of Myocardial Infarction. J Am Coll Cardiol. 2018, 72, 620–632. [Google Scholar] [CrossRef]
- Zhelev, Z.; Hyde, C.; Youngman, E.; Rogers, M.; Fleming, S.; Slade, T.; et al. Diagnostic accuracy of single baseline measurement of Elecsys Troponin T high-sensitive assay for diagnosis of acute myocardial infarction in emergency department: Systematic review and meta-analysis. BMJ. 2015, 350, h15. [Google Scholar] [CrossRef]
- Body, R.; Burrows, G.; Carley, S.; Cullen, L.; Than, M.; Jaffe, A.S.; et al. Highsensitivity cardiac troponin t concentrations below the limit of detection to exclude acute myocardial infarction: A prospective evaluation. Clin Chem. 2015, 61, 983–989. [Google Scholar] [CrossRef]
- Bandstein, N.; Ljung, R.; Johansson, M.; Holzmann, M.J. Undetectable highsensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol. 2014, 63, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Anand, A.; Sandoval, Y.; Lee, K.K.; Smith, S.W.; Adamson, P.D.; High-STEACS investigators; et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: A cohort study. Lancet. 2015, 386, 2481–2488. [Google Scholar] [CrossRef]
- Boeddinghaus, J.; Nestelberger, T.; Twerenbold, R.; Wildi, K.; Badertscher, P.; Cupa, J.; et al. Direct Comparison of 4 Very Early Rule-Out Strategies for Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin I. Circulation. 2017, 135, 1597–1611. [Google Scholar] [CrossRef]
- Chapman, A.R.; Lee, K.K.; McAllister, D.A.; Cullen, L.; Greenslade, J.H.; Parsonage, W.; et al. Association of High-Sensitivity Cardiac Troponin I Concentration With Cardiac Outcomes in Patients With Suspected Acute Coronary Syndrome. JAMA. 2017, 318, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Druey, S.; Wildi, K.; Twerenbold, R.; Jaeger, C.; Reichlin, T.; Haaf, P.; et al. Early rule-out and rule-in of myocardial infarction using sensitive cardiac Troponin I. Int J Cardiol. 2015, 195, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Boeddinghaus, J.; Reichlin, T.; Cullen, L.; Greenslade, J.H.; Parsonage, W.A.; Hammett, C.; et al. Two-Hour Algorithm for Triage toward Rule-Out and Rule-In of Acute Myocardial Infarction by Use of High-Sensitivity Cardiac Troponin I. Clin Chem. 2016, 62, 494–504. [Google Scholar] [CrossRef]
- Nestelberger, T.; Wildi, K.; Boeddinghaus, J.; Twerenbold, R.; Reichlin, T.; Giménez, M.R.; et al. Characterization of the observe zone of the ESC 2015 high-sensitivity cardiac troponin 0h/1h-algorithm for the early diagnosis of acute myocardial infarction. Int J Cardiol. 2016, 207, 238–245. [Google Scholar] [CrossRef]
- Schlett, C.L.; Hoffmann, U.; Geisler, T.; Nikolaou, K.; Bamberg, F. Cardiac computed tomography for the evaluation of the acute chest pain syndrome: State of the art. Radiol Clin N Am. 2015, 53, 297–305. [Google Scholar] [CrossRef]
- Dedic, A.; Lubbers, M.M.; Schaap, J.; Lammers, J.; Lamfers, E.J.; Rensing, B.J.; et al. Coronary CT Angiography for Suspected ACS in the Era of HighSensitivity Troponins: Randomized Multicenter Study. J Am Coll Cardiol. 2016, 67, 16–26. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Anavekar, N.S.; McMurray, J.J.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.L.; et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef]
- Twerenbold, R.; Wildi, K.; Jaeger, C.; Gimenez, M.R.; Reiter, M.; Reichlin, T.; et al. Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients With Renal Dysfunction. Circulation. 2015, 131, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- deFilippi, C.R.; Herzog, C.A. Interpreting Cardiac Biomarkers in the Setting of Chronic Kidney Disease. Clin Chem. 2017, 63, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bozbas, H.; Korkmaz, M.E.; Atar, I.; Eroglu, S.; Ozin, B.; Yildirir, A.; et al. Serum levels of cardiac enzymes before and after renal transplantation. Clin Cardiol. 2004, 27, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Fridén, V.; Starnberg, K.; Muslimovic, A.; Ricksten, S.E.; Bjurman, C.; Forsgard, N.; et al. Clearance of cardiac troponin T with and without kidney function. Clin Biochem. 2017, 50, 468–474. [Google Scholar] [CrossRef]
- Irfan, A.; Twerenbold, R.; Reiter, M.; Reichlin, T.; Stelzig, C.; Freese, M.; et al. Determinants of high-sensitivity troponin T among patients with a noncardiac cause of chest pain. Am J Med. 2012, 125, 491–498.e1. [Google Scholar] [CrossRef]
- Mueller, C.; Laule-Kilian, K.; Scholer, A.; Nusbaumer, C.; Zeller, T.; Staub, D.; et al. B-type natriuretic peptide for acute dyspnea in patients with kidney disease: Insights from a randomized comparison. Kidney Int. 2005, 67, 278–284. [Google Scholar] [CrossRef]
- Twerenbold, R.; Badertscher, P.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Puelacher, C.; et al. 0/1-Hour Triage Algorithm for Myocardial Infarction in Patients With Renal Dysfunction. Circulation. 2018, 137, 436–451. [Google Scholar] [CrossRef]
- Twerenbold, R.; Badertscher, P.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Rubini Gimenez, M.; et al. Effect of the FDA Regulatory Approach on the 0/1-h Algorithm for Rapid Diagnosis of MI. J Am Coll Cardiol. 2017, 70, 1532–1534. [Google Scholar] [CrossRef] [PubMed]
- Kaier, T.E.; Twerenbold, R.; Puelacher, C.; Marjot, J.; Imambaccus, N.; Boeddinghaus, J.; et al. Direct Comparison of Cardiac Myosin-Binding Protein C With Cardiac Troponins for the Early Diagnosis of Acute Myocardial Infarction. Circulation. 2017, 136, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.