Abstract
Takotsubo cardiomyopathy (TSC) is characterised by a transient left ventricular dysfunction typically induced by stressful events, such as invasive procedures, in the absence of obstructive coronary artery disease. However, to date, no cases of TSC developing during an interventional cardiology procedure have been described. In this article, we report a case of TSC occurring during the intraprocedural phase of a percutaneous patent foramen ovale closure, performed under general anaesthesia.
Case description
A 53-year-old woman was scheduled for percutaneous closure of a patent foramen ovale (PFO) after a diving accident. Her medical history was otherwise unremarkable and she was not taking any medication. The patient refused preoperative transoesophageal echocardiography (TEE) and opted for a general anaesthesia with intraoperative TEE. The preoperative electrocardiogram (ECG) was normal without any repolarisation issue or QTc interval anomaly.
Neither anxiety nor psychological stress related to the planned procedure was observed in the preoperative period. Preoperative antibiotic prophylaxis was administered (a single intravenous dose of cefazolin 2 g). The endotracheal tube and TEE probe were placed easily after induction of general anaesthesia with propofol, sufentanil and rocuronium. The first TEE pictures showed a normally shaped and nondilated left ventricle with normal ejection fraction (LVEF) (Figure 1; supplementary data online, videos S1 and S2 (Videos S1 and S2: Basal assessment of left ventricle systolic function by intraprocedural transoesophageal echocardiography. The videos are available online at https://cardiovascmed. ch/en/online-only-content)). After femoral venous puncture, a guiding catheter was advanced into the right atrium. At this stage of the procedure and before any contrast medium injection, the patient suddenly developed severe hypotension. TEE showed sudden akinesia of medioapical segments and hypokinesia of basal segments with ventricular ballooning.
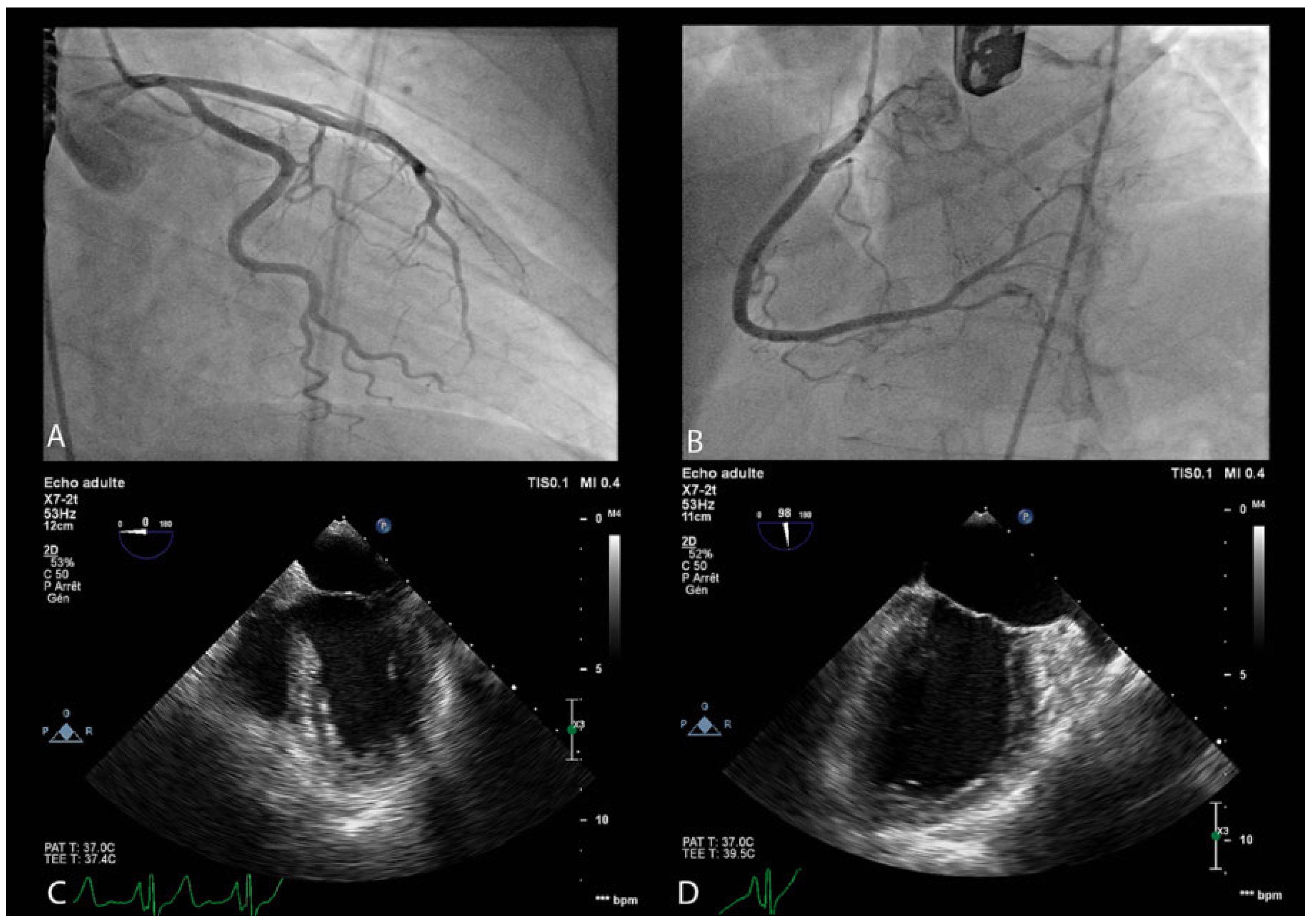
Figure 1.
Panels A and B. Coronary angiography showing a normal coronary tree without any significant stenosis. Panel C and D. Transoesophageal echocardiography: end-systolic left ventricle morphology before and after systolic dysfunction onset. In panel D ventricular ballooning due to medioapical akinesia and basal hypokinesia can be observed.
The LVEF was severely depressed (<20%) (Figure 1; supplementary data online, videos S3 and S4 (Videos S3 and S4: Intra-procedural transoesophageal echocardiography showing left ventricle systolic dysfunction. Akinesia of medioapical segments and hypokinesia of basal segments can be observed. Left ventricle ejection fraction is severely depressed (<20%). The videos are available online at https://cardio-vascmed.ch/en/online-only-content)). An adrenaline infusion was started at a dose of 1.5 μg/min. At the same time, inotropic support with dobutamine infusion was introduced at a dose of 10 μg/kg/min, leading to provisional haemodynamic stabilisation. The ECG was still normal. Coronary angiography showed normal coronary arteries (Figure 1). Intraprocedural Takotsubo cardiomyopathy (TSC) was diagnosed. We decided not to proceed with PFO closure and the patient was transferred to our intensive care unit. During the following hours a new haemodynamic deterioration was observed. Adrenaline infusion was titrated up to 8 μg/ min and an additional infusion of noradrenaline was started and titrated up to 10 μg/min. Her clinical condition slowly improved. Vasopressor support could be stopped during the first 24 hours of stay in the intensive care unit, and dobutamine infusion was stopped within 48 hours from admission. Because there was no ventricular thrombus on serial echocardiographic monitoring and a progressive improvement in LVEF, therapeutic anticoagulation was not introduced. Following haemodynamic stabilisation, cardioprotective therapy with an angiotensin converting enzyme inhibitor (lisinopril 5 mg once daily), but no beta-blocking therapy, was introduced. During the stay in the intensive care unit, serial ECG monitoring showed diffuse T wave inversion, which was more prominent in the anterior leads. Biochemical monitoring showed a slight elevation in myocardial necrosis enzymes with a peak troponin-T of 308 ng/l (upper reference limit of 14 ng/l) and a peak creatine kinase-MB of 6.6 μg/l (upper reference limit of 5 μG/L). Brain-type natriuretic peptide was not assessed because a diagnosis of cardiogenic shock was clinically evident. Complete recovery of the LVEF was observed at day 4 and the patient was subsequently discharged. LVEF recovery was sustained at 1-week follow-up, when cardioprotective therapy with lisinopril was stopped, and at 6 months follow-up. Nevertheless, the patient refused to undergo a second attempt at PFO closure.
Discussion
TSC is a rare but increasingly reported condition typically characterised by transient left ventricular dysfunction. This syndrome mainly affects elderly female patients and clinically mimics ST-elevation myocardial infarction, in the absence of obstructive coronary artery disease [1]. TSC is usually triggered by stressful events, even though up to 30% of TSC patients do not have any specific trigger [2,3]. The physiopathology of TSC is still not completely understood, but the major pathophysiological phenomenon is thought to be a disproportionate catecholamine discharge in response to stress, with subsequent myocardial stunning. The majority of TSC patients show sympathetic hyperactivity and an increase in circulating levels of catecholamines [4].
Surgical interventions [5,6] represent well-documented triggers of TSC. TSC more frequently manifests itself in the postoperative period but it can also develop during the intraoperative phase with potentially dramatic consequences for the patient. In the literature, interventional cardiology procedures have been sporadically described as TSC triggers. Todaro et al. first reported a case of TSC occurring the day after a percutaneous PFO closure in a 30-year-old woman suffering from a post-traumatic stress disorder [7]. More recently, Harhash et al. described a case of TSC occurring on postoperative day 2 in a 84-year-old woman who had undergone transcatheter aortic valve replacement [8]. However, in both cases TSC manifested in the postoperative period.
In contrast, in our case TSC developed during the intraprocedural phase of a percutaneous PFO closure. This is, to the best of our knowledge, the first reported case of TSC developing during an interventional cardiology procedure. In our patient, the clinical presentation of TSC was particularly severe with haemodynamic instability due to cardiogenic shock. The interest of our case is further increased by the unequivocal TEE documentation of the intraoperative onset of TSC. In many other reports, the intraor perioperative onset of TSC was documented only by means of other imaging techniques, more frequently transthoracic echocardiography, making our striking TEE images of particular importance.
As in others cases of perioperative TSC, it was difficult to clearly identify the aetiological factors triggering the systolic dysfunction. Subclinical psychological stress and anxiety related to the procedure are likely to play an aetiological role in perioperative TSC. Moreover, in our patient, physical stress related to endotracheal tube and TEE probe placement could have led to a catecholamine discharge with subsequent myocardial stunning. Physical stress directly related to the intervention itself is likely to play an additional role in perioperative TSC.
Some specific issues deserve to be discussed in more detail. First of all, an alternative diagnosis of Kounis syndrome should be considered in our case. Kounis syndrome is defined as a hypersensitivity coronary disorder induced by conditions associated with mast-cell and platelet activation [9]. Some of the drugs administered to our patients in the operative setting, such as propofol, rocuronium and cefazolin, have been described as potential Kounis syndrome triggers [9]. However, our patient did not display any other clinical sign suggesting a systemic hypersensitivity reaction. Coronary angiography did not show any epicardial coronary spasm or indirect sign of coronary microvascular dysfunction. Furthermore, the TEE systolic dysfunction pattern was highly indicative of a diagnosis of TSC. Therefore, a diagnosis of Kounis syndrome seems to be unlikely in our patient. Tryptase levels were not assessed because of the low clinical suspicion. One other important issue concerns the pharmacological therapy. Catecholamines seem to have a key role in TSC pathophysiology. There are robust data supporting the role of beta-adrenergic signalling in TSC [10,11]. However, also alpha-adrenoceptors are thought to be implicated in TSC, on the basis of results of more recent studies [12]. Therefore, the use of vasopressors and inotropic drugs could theoretically be detrimental in TSC, owing to their agonist properties on alpha and beta-adrenoceptors.
Cardiogenic shock complicates up to 6.5% of TSC cases [13]. To date, only few data are available regarding the management of this rare and challenging condition and, despite the theoretical limitations previously mentioned, the use of vasoactive amines in this particular subset of patients remains an often life-saving measure, as was the case for our patient. Successful treatment of cardiogenic shock in TSC with other inotropic drugs, such as milrinone or levosimendan, has been sporadically described in case reports but there has been no randomised trial comparing different inotropic therapies in TSC patients [13,14]. Once haemodynamic stabilisation was achieved, we decided to introduce a cardioprotective therapy with lisinopril, but we refrained from introducing a beta-blocking therapy because of the haemodynamic context and the quick recovery of left ventricle systolic function. However, it is worth noting that general management of TSC also remains largely empirical. Even if first studies supported the use of beta-blocking therapy, especially in the acute phase and in the presence of a dynamic midventricular obstruction [15,16], the usefulness of these pharmacological agents to improve short-term outcome and to prevent TSC recurrence is today more and more debated, on the basis of results of more recent studies [17].
Conclusion
Surgical interventions and invasive procedures, including interventional cardiology procedures, represent potential triggers to TSC. In the present article, we described the first case of TSC occurring during an interventional cardiology procedure, with striking TEE pictures unequivocally documenting the intraoperative onset of TSC. Anaesthetists and interventional cardiologists should be aware of this dangerous complication, which can even occur during the intraprocedural phase of routine and minimally invasive procedures, with potentially life-threatening consequences, as in our case.
Disclosure statement
No financial support and no other potential conflict of interest relevant to this article was reported.
Abbreviations
| ECG | Electrocardiogram |
| LVEF | Left ventricle ejection fraction |
| PFO | Patent foramen ovale |
| TSC | Takotsubo cardiomyopathy |
| TEE | Transoesophageal echocardiography |
References
- Y-Hassan, S.; De Palma, R. Contemporary review on the pathogenesis of Takotsubo syndrome: The heart shedding tears: Norepinephrine churn and foam at the cardiac nerve terminals. Int J Cardiol 2017, 228, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Vedad, R.; Angeras, O.; Ramunddal, T.; Petursson, P.; Haraldsson, I.; et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction – A report from the SWEDEHEART registry. Int J Cardiol 2015, 185, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schumann, S.P.; Gersternblith, G.; et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Eng J Med 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T.; Kim, J.Y.; Kim, Y.W.; Choi, K.H.; Park, B.H.; Lim, H.K. Stress-induced cardiomyopathy after general anaesthesia for total gastrectomy – A case report. Korean J Anesthesiol 2010, 58, 299–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruiz, S.; Martinez-Marin, M.; Luque, P.; Nassar, N.; Oros, D. Takotsubo cardiomyopathy after cesarean section: A case report and literature review. J Obstet Gynaecol Res 2016, 43, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.C.; Oreto, L.; Zwicke, D.L.; Kay, J.; Bajwa, T.; Khandheria, B.K. Stress-induced cardiomyopathy after patent foramen ovale closure: what role did anaesthesia play? J Cardiothorac Vasc Anesth 2012, 26, e52–e54. [Google Scholar] [CrossRef] [PubMed]
- Harhash, A.; Koulogiannis, K.P.; Marcoff, L.; Kipperman, R. Takotsubo cardiomyopathy after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2016, 9, 1302–1304. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med 2016, 54, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Borchet, T.; Hubscher, D.; Guessoum, C.I.; Lam, T.D.; Ghadri, J.R.; Schellinger, I.N.; et al. Catecholamine-dependent β-adrenergic signaling in a pluripotent stem cell model of takotsubo cardiomyopathy. J Am Coll Cardiol 2017, 70, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; et al. High level of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Shintani-Ishida, K.; Unuma, K.; Yoshida, K. Immobilization stress with α2-adrenergic stimulation induces regional and transient reduction of cardiac contraction through Gi-coupling in rats. Int Heart J 2015, 56, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Doyen, D.; Dellamonica, J.; Moceri, P.; Moschietto, S.; Hyvernat, H.; Ferrari, E.; et al. Tako-Tsubo cardiomyopathy presenting with cardiogenic shock successfully treated with milrinone: A case report. Heart Lung 2014, 43, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, L. Levosimendan: the inotrope of choice in cardiogenic shock secondary to takotsubo cardiomyopathy? Heart Lung Circ 2007, 16, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Hashimoto, A.; Tsuchihashi, K.; Nagao, K.; Kyuma, M.; Ooiwa, H.; et al. Clinical implications of midventricular obstruction and intravenous propranolol use in transient left ventricular apical ballooning (Takotsubo cardiomyopathy). Am Heart J 2008, 155, 526.e1–526.e7. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Okatani, H.; Shiota, M.; Nakao, T.; Ise, R.; Kito, G.; et al. Effects of metoprolol on epinephrine-induced takotsubo-like left ventricular dysfunction in non-human primates. Hypertens Res 2009, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.