Takotsubo cardiomyopathy (TTC), first described in 1990 by Sato et al. [
1], has been increasingly recognised in recent years as a condition clinically mimicking acute myocardial infarction. Coronary artery disease coexists in 15% of patients with TTC and therefore cannot be used to rule out the disease [
2]. It is characterised by apical ballooning with transient left ventricular dysfunction. The left ventricle has the shape of a Japanese octopus fishing pot with a narrow neck and round bottom, called a takotsubo. In two thirds of patients the condition is triggered by physical or emotional stress while in one third of patients no trigger is evident. Common physical triggers include acute respiratory failure, operations, fractures, central nervous system conditions and infections. The disease is most commonly seen in postmenopausal women. Left ventricular function usually recovers within a couple of weeks [
2,
3]. Nevertheless, newer data show that it is a life-threatening condition in the acute phase and associated with substantial long-term morbidity and mortality [
2]. We present here a rare case of TTC in the setting of Graves’ hyperthyroidism without any of the known usual triggers.
Case report
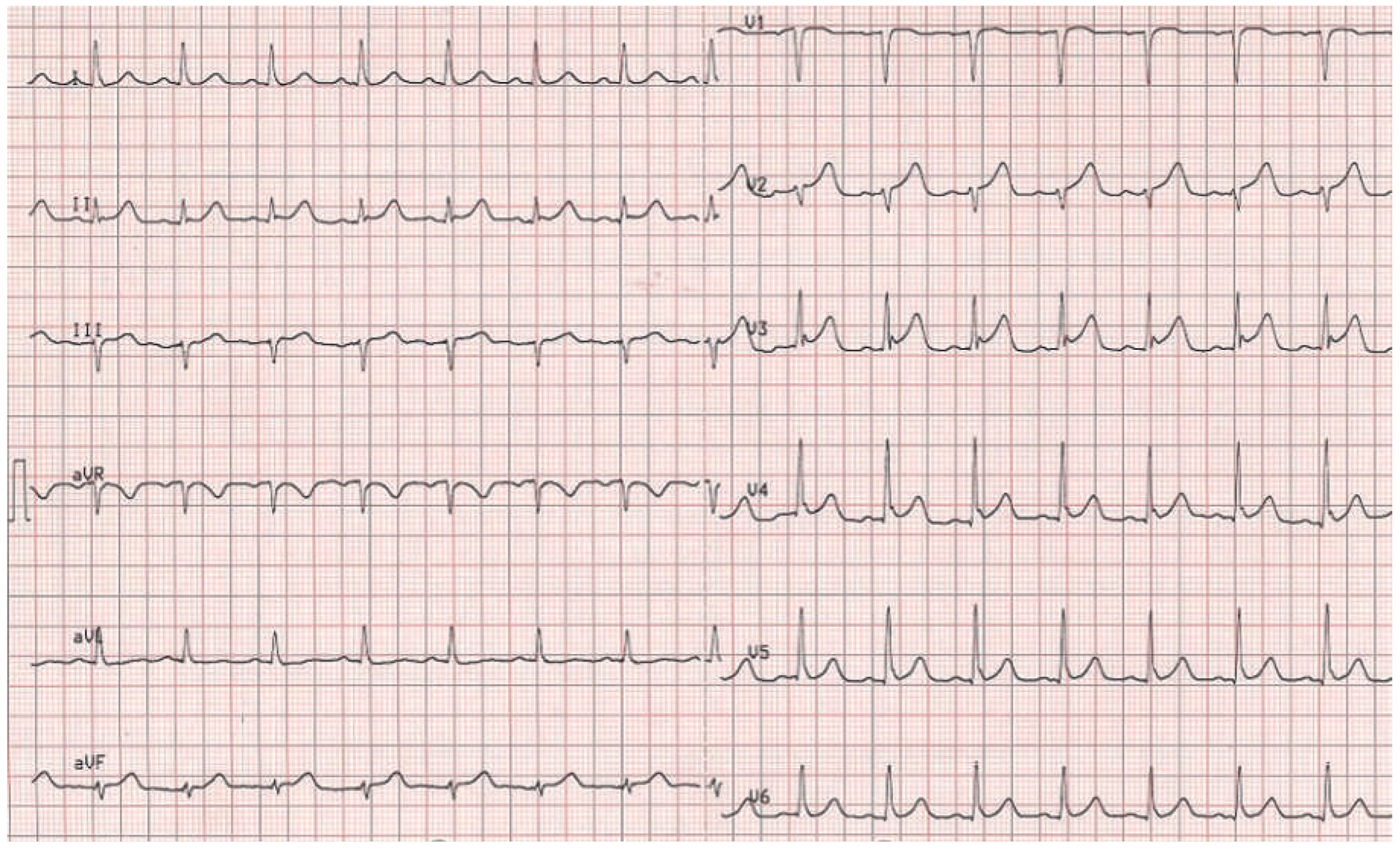
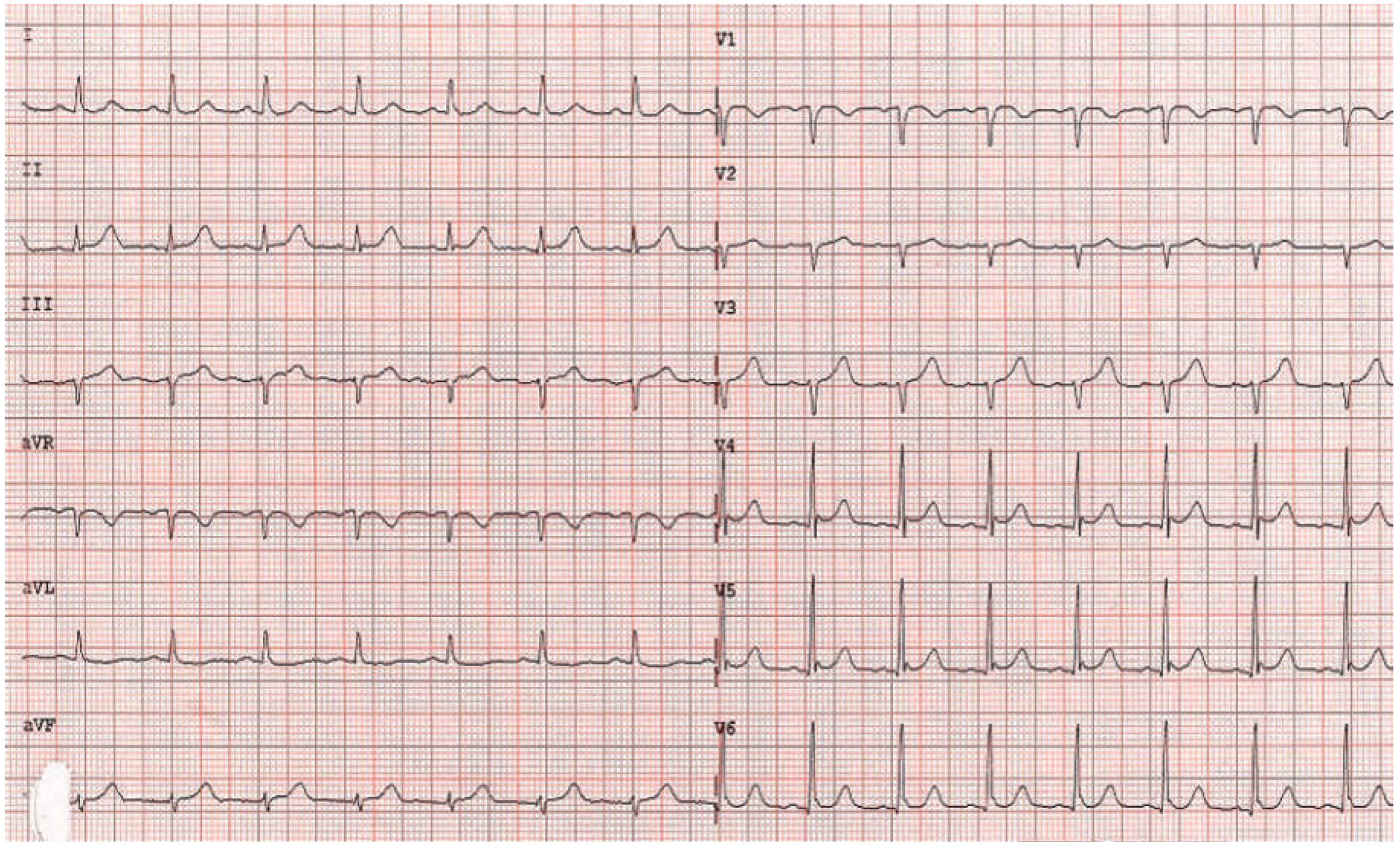
A 63-year-old female patient was admitted to our emergency department with chest discomfort radiating to her left arm. She had arterial hypertension, which had been newly diagnosed 3 months earlier and was treated with perindopril 5 mg o.d. Additional cardiovascular risk factors were a family history of coronary artery disease and the patient was an active smoker. She reported shortness of breath and dizziness, excessive sweating and a history of palpitations in the past 2 months. Recent stressful emotional or physical events were denied by the patient. She was afebrile and in sinus rhythm with a regular heart rate of 98 beats/min, blood pressure was 143/88 mm Hg and there was a loud systolic murmur. There was no exophthalmos, lid lag or pretibial myxoedema. The initial ECG showed ST elevation in the precordial leads V2–V5 (
Figure 1 and
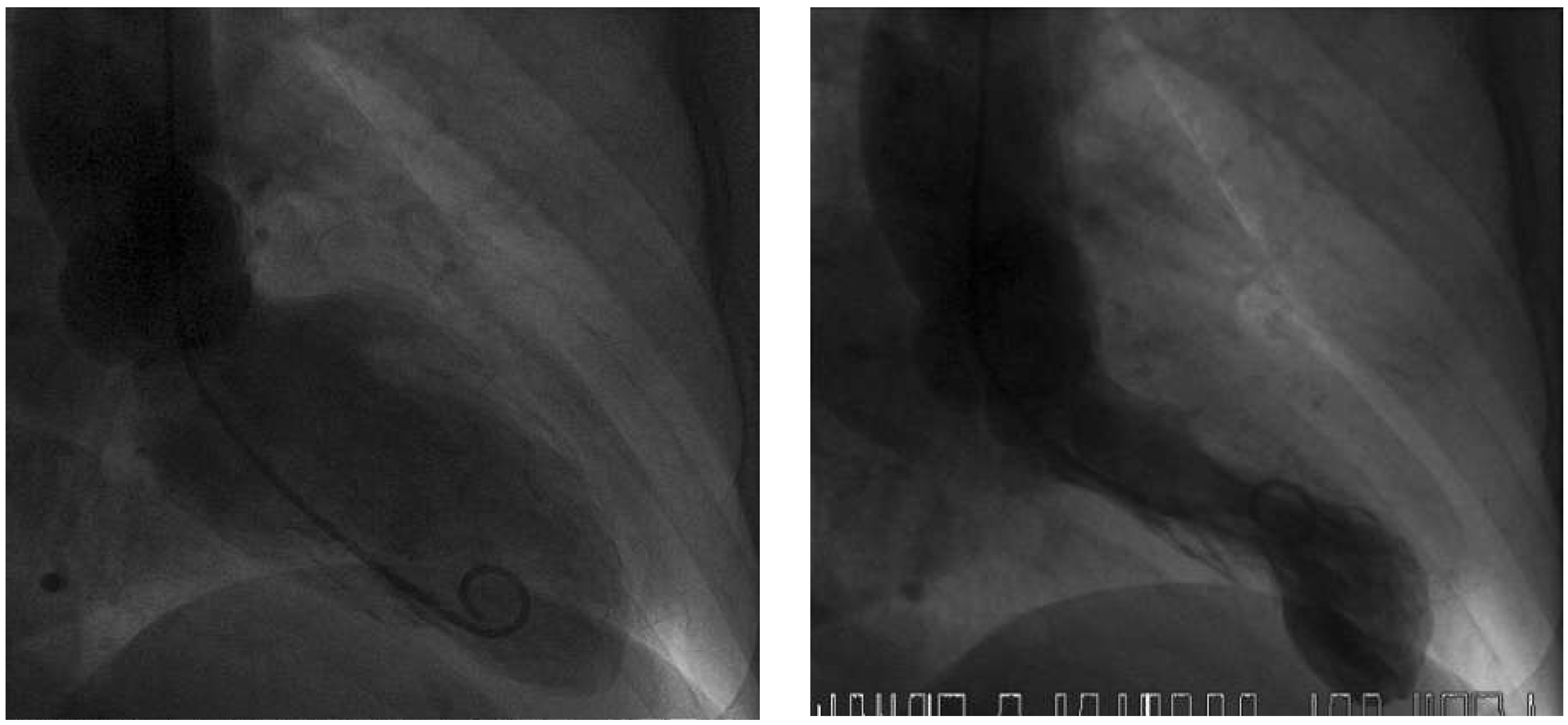
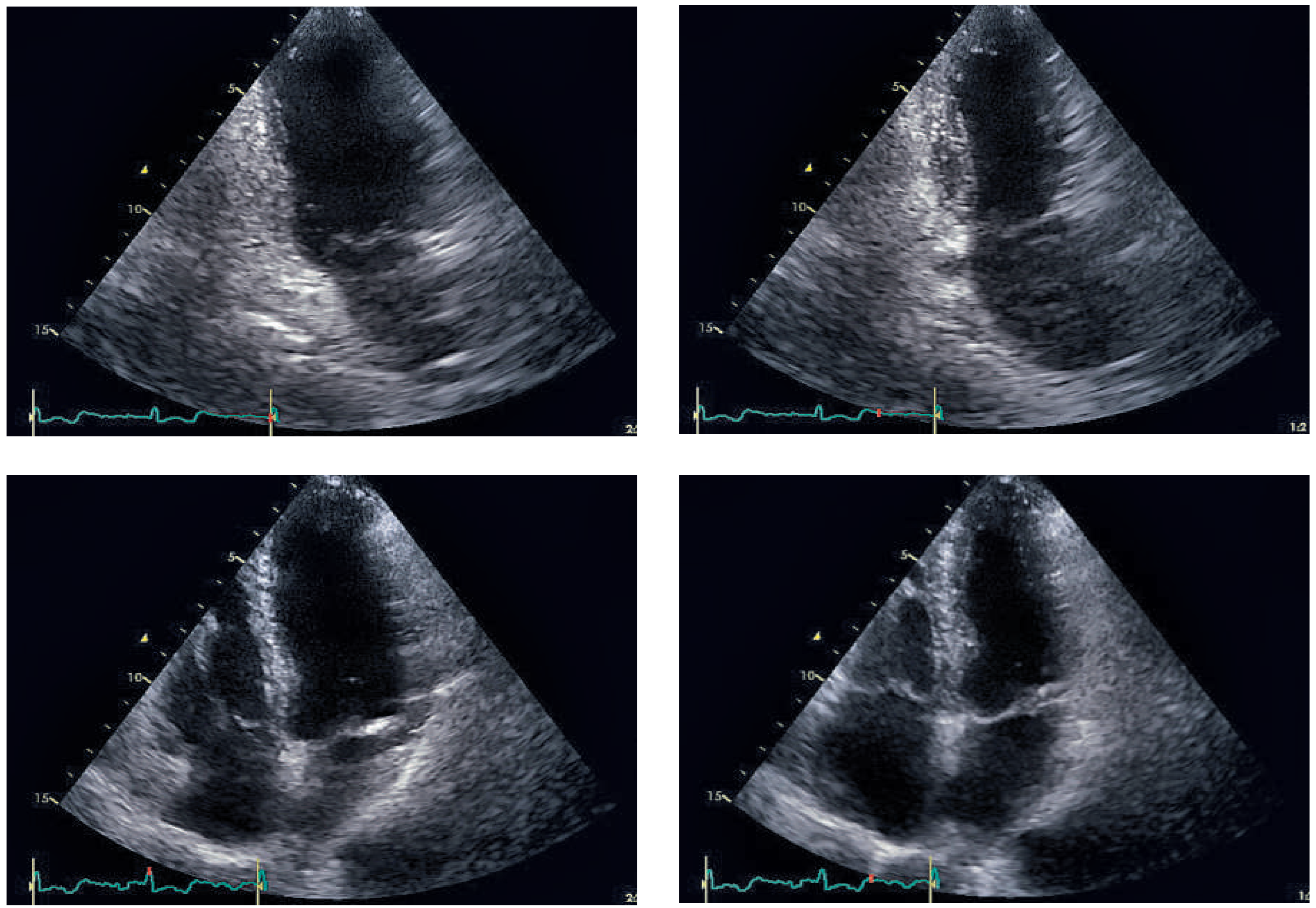
Figure 2); creatine kinase (CK 250 IU/l; normal <190 IU/l) and high-sensitive troponin T (0.337 µg/l; normal <0.014 µg/l) were elevated. Acute myocardial infarction was suspected and the patient underwent emergen cy cardiac catheterisation. The coronary angiogram showed normal coronary arteries, but the ventriculogram revealed apical ballooning suggesting TTC (
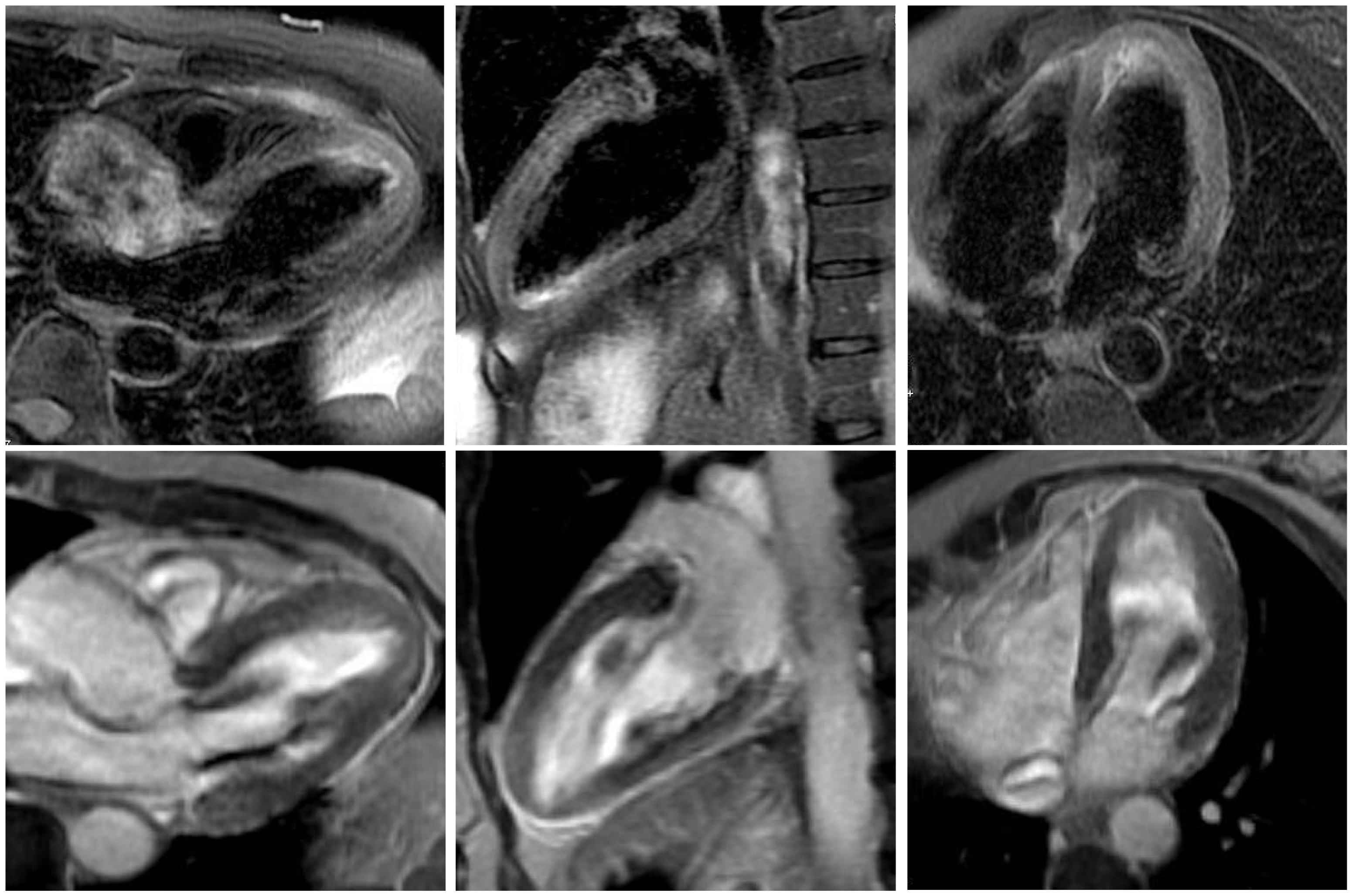
Figure 3). Moderate mitral regurgitation was seen on angiography and was later confirmed with transoesophageal echocardiography. Cardiac magnetic resonance imaging (MRI), performed 6 days later, showed no scar on late enhancement and myocardial oedema in T2 imaging of the left ventricular apex (
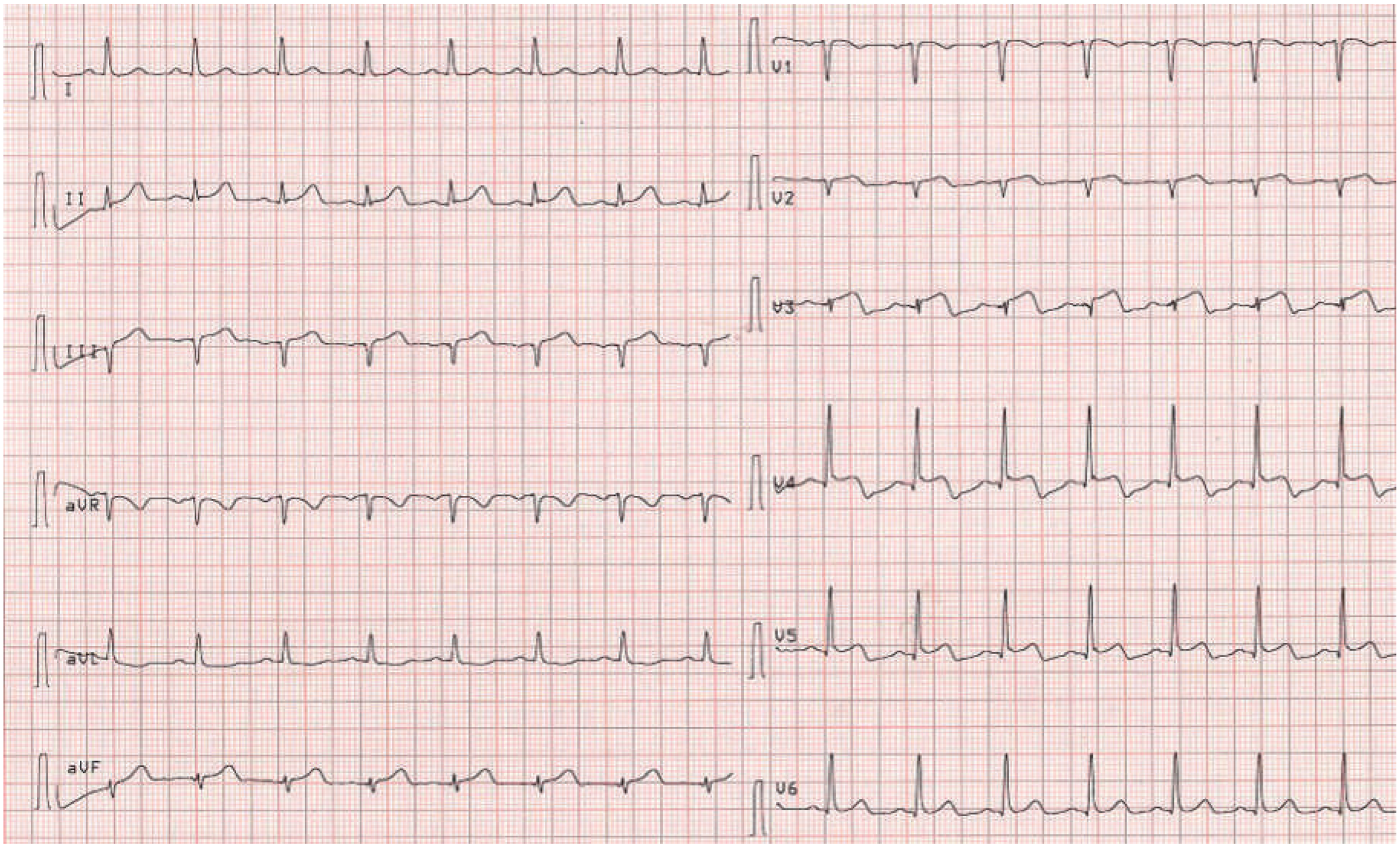
Figure 4). The thyroid-stimulating hormone (TSH) level was suppressed (<0.01 mU/l; normal 0.3–4.2 mU/l) and fT4 and fT3 hormone levels were elevated (40.5 pmol/l and 14.4 pmol/l, respectively; normal 3.1–6.8 pmol/l for fT3 and 12–22 pmol/l for fT4). TSH receptor antibodies (TRAb; specific for Graves’ disease) were elevated as well, confirming the diagnosis of Graves’ disease. Remarkably, a medical check-up 3 months earlier had shown normal thyroid hormone and TSH levels. Therapy with carbimazole 15 mg b.i.d., propranolol 10 mg t.i.d. and perindopril 2.5 mg o.d. was started. The patient’s condition improved and she was discharged 4 days later. On the follow-up ECG before discharge no residual repolarisation abnormalities could be seen (
Figure 5). Echocardiographic follow-up 1 month later showed normal left and right ventricular function and no residual mitral regurgitation (
Figure 6). Thyroid hormone levels were still elevated, carbimazole was continued and 6 months later she underwent radioiodine therapy to control her hyperthyroidism.
Discussion
While Graves’ disease is a well-known clinical entity, the awareness of TTC is still low amongst physicians. Despite extensive efforts in the past two decades, the pathophysiology of TTC remains poorly understood, but catecholamines and enhanced sympathetic activity seem to play an important role [2, 3]. This report of a rare case of simultaneous occurrence of both Graves’ disease and TTC in the same patient raises the question of a possible causal relationship between the two conditions. Because of the diverse direct and indirect effects of thyroid hormones on the cardiovascular system, the enhanced sympathetic activity in hyperthyroidism and the hyperadrenergic state it causes, TTC triggered by Graves’ disease seems possible [
4]. As mentioned above, catecholamines seem to play an essential role in the development of TTC. Since the disease usually affects postmenopausal women, low oestro gen levels might also be a significant factor in the pathophysiology of TTC. One possible explanation of TTC is coronary microvascular dysfunction caused by catecholamine excess, either through microvascular spasm or by direct myocardial toxicity [
2]. This theory is supported by the fact that a higher concentration of adrenoreceptors is found in the apical myocardium, the area most frequently affected in TTC. Furthermore, endomyocardial biopsies have shown contraction band necrosis and mononuclear inflammatory infiltrates, findings consistent with catecholamine-mediated cardiotoxicity [
5].
Interestingly, circulating plasma catecholamine levels are usually normal or decreased in hyperthyroidism, which clinically mimics a hyperadrenergic state [
6]. There are conflicting data as to whether elevated levels of thyroid hormones increase the tissue responsiveness to catecholamines or not. Although thyroid hormones upregulate β-adrenergic receptors in the heart, it is unclear if this actually increases tissue responsiveness. Thyroid hormone-induced cellular pathways in the myocardium independent of β-adren ergic stimulation, but very similar to pathways induced by β-adrenergic stimulation, have been proposed as an explanation for the co-occurrence of hyper thyroidism and TTC [4, 6]. Another possible explanation could be the enhanced sympathetic tone. It is known that stress-related cardiomyopathies are associated with an enhanced sympathetic tone. As this is typically seen in Graves’ disease, it could be a possible link between the two diseases. Sympathetically induced intramyocardial electrolyte disturbances can cause myocyte calcium overload and enhanced cellular efflux of potassium ions leading to myonecrosis [
5]. Another hypothesis could be a direct effect of thyroid-stimulating antibodies on the myocardium, since most TTC cases in the setting of hyperthyroidism have been associated with Graves’ hyperthyroidism, where thyroid-stimulating antibodies are elevated.
Our case and previous case reports suggest Graves’ disease as a possible trigger of TTC. Further data from large studies will be needed to support this hypothesis. The largest study to date on TTC by Templin et al. [
2] indicates that roughly one sixth of patients with TTC show thyroid hormone abnormalities. Two thirds of these patients were in a hypothyroid and only one third in a hyperthyroid state, which makes an enhanced sympathetic tone less likely as an explanatory model of TTC in the setting of Graves’ disease. Unfortunately the published data by Templin et al. does not specify how many of these patients with thyroid hormone abnormalities had evident triggering factors or not. Future investigations of this subgroup of patients could be helpful in the evaluation of our hypothesis. All in all we believe Graves’ disease can represent a trigger for TTC even though pathophysiological mechanisms remain unclear. A thyroid hormone-induced effect on the myocardium independent of β-adrenergic stimulation seems most likely.
Disclosure statement
No financial support and no other potential conflict of interest relevant to this article was reported.