Introduction
The prevalence of atrial fibrillation (AF) is increasing owing to the demographics of aging Western societies. In patients below the age of 55 years the prevalence of AF is 0.1%, but this increases to 9% in patients over 80 years of age [
1]. Stroke is the most devastating complication of AF and occurs at an annual rate of 5% in nonanticoagulated patients [
2]. Hence, stroke prevention is an essential part of the management of AF. Oral anticoagulation (OAC) with either warfarin or non-vitamin K anticoagulants (novel oral anticoagulants: NOACs) reduces the risk of stroke by 65%, but it comes at the price of an annual rate of major bleeding complications of up to 5% [
3].
In over 90% of AF patients, thrombi can be detected in the left atrial appendage (LAA), which is the the rationale for LAA occlusion (LAAO) [
4] as an alternative therapy for stroke prevention in AF patients. Several devices have been used (
Figure 1).
The most commonly used percutaneous devices for LAAO are the Amplatzer (St. Jude Medical, St. Paul, MN) and the Watchman (Boston Scientific, Marlborough, MA) devices. Historically, the PLAATO device (ev3 Inc, Plymouth, MN) was the first available percutaneous device for LAAO, but was removed from the market because of economic considerations [
5,
6]. The gap was filled by off-label use of nondedicated Amplatzer devices used primarily for the occlusion of patent foramen ovale (PFO), atrial septal defects (ASDs) or ventricular septal defect (VSDs) [
7]. These nondedicated devices served the purpose of stroke prevention, but came along with a relatively high embolisation rate (12%) [
8]. In 2008, a dedicated Amplatzer device became available (AMPLATZER Cardiac Plug [ACP]), which reduced device embolisations to 2%. Still, given the intricacy of the procedure, the rate of procedural complications remains relatively high (9.8%) and the procedure has a relatively flat learning curve [
8,
9,
10]. The Watchman device is another widely used dedicated LAA occluder. In a landmark randomised study with this device (PROTECT-AF) [
2], patients were randomised to a vitamin K antagonist (VKA) or LAAO. At 1 year, LAAO proved to be noninferior to VKAs for the primary endpoint of stroke, cardiovascular or unexpected death and systemic embolism. In the intervention group, adverse safety events were more frequent, mostly procedure-related pericardial tamponade and periprocedural strokes, again underlining the intricacy of the procedure. With increasing operator experience, procedural adverse safety events were considerably reduced [
11]. However, it took almost 4 years of follow-up to neutralise the initial procedure-related events by fewer adverse thrombotic events thereafter. This translated into superiority of LAAO over VKAs for stroke, systemic embolism and cardiovascular death. Of note, mortality was significantly reduced by 40% in the LAAO group as compared with VKAs [
12]. It can therefore be speculated that with longer follow-up survival curves might further diverge, resulting in an even more pronounced benefit of LAAO. Besides offering an effective treatment option, LAAO proved to be more cost effective than VKAs and even more so than NOACs [
13].
We report our institutional experience with LAAO at the University Heart Centre Zurich, Switzerland over a period of more than 5-years, including different operators and different approaches to LAAO.
Methods
Patients
This was a retrospective single-centre registry study of all patients undergoing LAAO at the University Heart Centre Zurich between June 2010 and November 2015. Indications for LAAO included AF with CHA2DS2-VASc ≥1 and contraindication for OAC, high bleeding risk or patient preference. Written informed consent was obtained from all patients. Data of clinically indicated follow-up procedures and outpatient consultations were collected. From 2015 on, patients who gave a general consent to data collection were additionally included in a prospective registry. Patients who did not give such a general consent were excluded from the registry and from the current analysis. This study was conducted according to the stipulations of the local ethics committee (Ethikkommission des Kantons Zürich, Switzerland).
Procedure
Interventions took place (a) in the catheterisation laboratory, if the procedure was performed under local anaesthesia or with intracardiac echocardiographic (ICE) guidance, or (b) in the hybrid operating suite, if general anaesthesia and transoesophageal echocardiography (TEE) guidance was used, or if LAAO was performed in conjunction with transcatheter aortic valve implantation or transcatheter mitral interventions.
LAAO was either a stand-alone procedure or was combined with selective coronary angiography, percutaneous coronary intervention (PCI), transcatheter aortic valve implantation (TAVI), MitraClip implantation, PFO/ASD closure or other interventions.
At the initiation of the procedure a bolus of 5000 units of heparin was given intravenously. If a PFO or ASD had been detected previously, it was used for left atrial access by one operator (FN); transseptal puncture was routine in the remainder. A standard transseptal sheath (e.g., MullinsTM transseptal shealth and Brockenbrough needle, Medtronic Inc., Minneapolis, MN) was used for transseptal puncture. A 0.035 inch support wire (e.g., Meier Backup wire, Boston Scientific, Marlborough, MA) was used to introduce the large bore Amplatzer delivery sheath through the groin and into the left atrium. If a Watchman occluder was selected, a dedicated Watchman sheath was used.
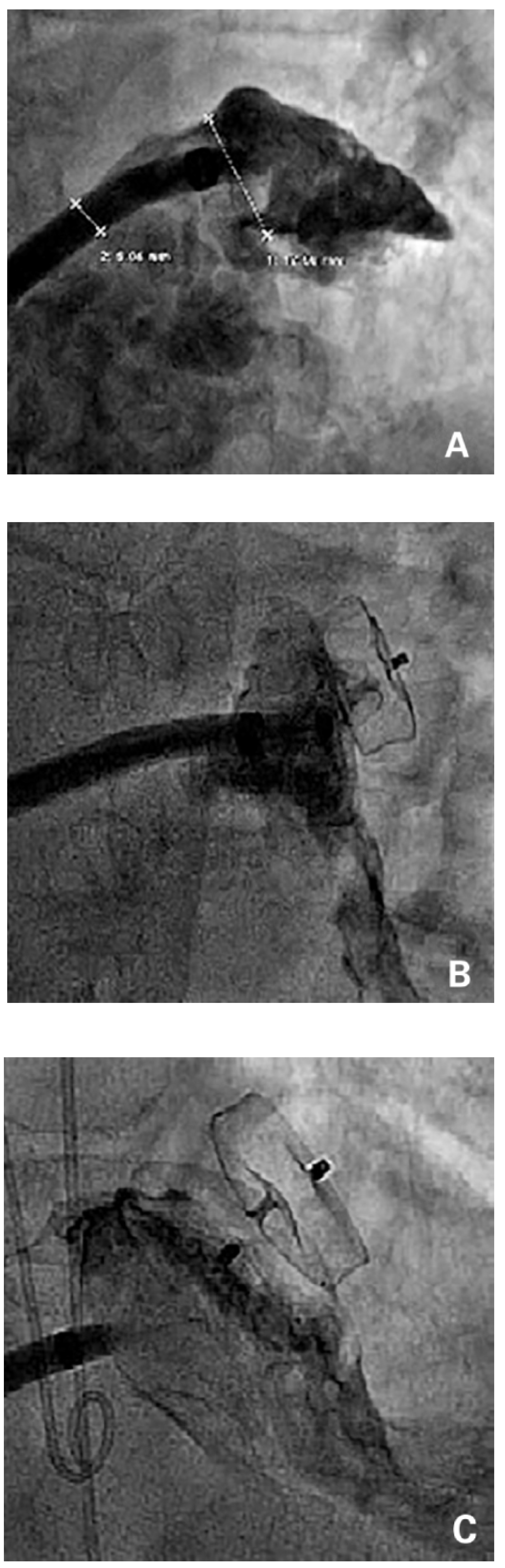
The landing zone, as well as the orifice and the length of the LAA were visualised and the size was estimated with use of contrast injections through the sheath in two angles (typically right anterior oblique [RAO] caudal and RAO cranial). If available, TEE or ICE was used for sizing. An at least 20% oversized device was selected accordingly or a device that best fitted the individual anatomy was used. After deployment of the device, a tug test and contrast injections were performed to confirm a stable device position [
14] (
Figure 2). Transthoracic echocardiography (TTE) was performed on the day of the procedure or the next morning to confirm a correct device position in the LAA. Patients were discharged on acetylsalicylic acid (ASA) 100 mg and clopidogrel 75 mg for 1–6 months. Oral anticoagulation was permanently stopped the day of the procedure.
Follow-up
After 3–6 months, a follow-up TEE was performed to verify correct device position and to exclude relevant residual leaks into the LAA or thrombi on the device. If TEE was not feasible, follow-up TTE or computed tomography (CT) was used instead.
Endpoints
Procedural success was defined as a correct device position upon termination of the procedure. Periprocedural adverse events included a composite endpoint of procedure-related death, stroke, tamponade (need for urgent drainage or surgical bailout), Valve Academic Research Consortium-2 (VARC) major or life-threatening bleeding, VARC kidney injury grade 3 and device embolisation.
Clinical follow-up data included deaths (cardiovascular, other or unknown), cerebrovascular accidents and major bleeding events.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Between-group comparisons were made using one-way analysis of variance or student’s t-test, as appropriate. Categorical data are presented as frequency (percentages) and were compared with use of the Fisher exact or the chi-square test. All probability values and confidence intervals were two-sided. A probability value of <0.05 was considered significant, and all tests were two-tailed. All analyses were performed with SPSS version 21.0 software (SPSS Inc., Chicago, IL).
Results
Patient characteristics
From June 2010 until November 2015 LAAO was performed in 284 patients suffering from AF, of whom 247 patients (164 [86%] males, age 77 ± 8.9 years), were included in this study. In 2015, 132 LAAOs were performed at the University Heart Centre Zurich, of which 95 (72%) were included; the remaining patients did not give signed informed consent for general data collection and participation in the study.
AF was persistent or permanent in 40.1% (99 patients), and paroxysmal in 56.3% (139 patients); 7.9% (15 patients) suffered from (concomitant) atrial flutter. Mean CHA2DS2-Vasc score was 4.5 ± 1.4; mean HAS-BLED score was 3.6 ± 1.1. Patient baseline characteristics and risk factors are listed in
Table 1.
Indications for LAAO
The indication for LAAO was high risk of bleeding in 66.4% (164 patients) or previous relevant bleeding in 42.5% (105 patients). Specifically, 23% (44 patients) had a history of intracranial and 19.9% (38 patients) of gastrointestinal (GI) bleeding, 14.2% (35 patients) had a high risk for falls or prior falls, in 14.2% (34 patients) it was difficult to maintain a therapeutic international normalised ratio (INR) and in 19.8% (49 patients) LAAO was performed to avoid triple anticoagulation with adjunctive antiplatelet therapy.
Procedural outcome
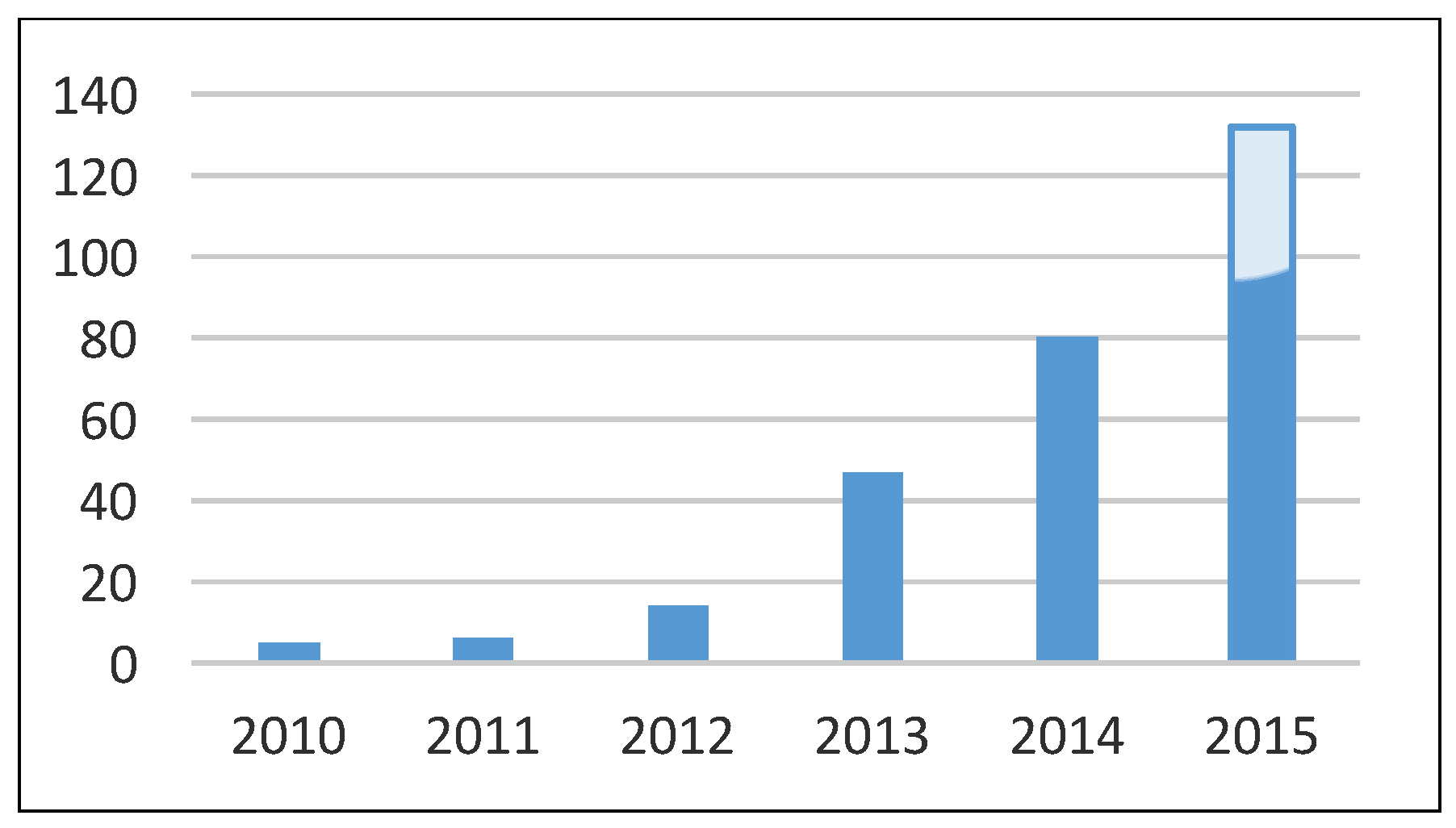
Since 2010, a significant increase in the number of procedures was noted, with 5 procedures in 2010 and 132 (95 included in this study) procedures in the first 11 months of 2015 (p = 0.003;
Figure 3). The exact numbers of interventions each year are shown in
Table 2.
Immediate procedural success was achieved in 98.4% (243 patients). Two of the four unsuccessful interventions were aborted owing to an oversized LAA, one patient had a difficult LAA anatomy not suitable for the scheduled Watchman implantation (chicken-wing) and one intervention was unsuccessful owing to LAAperforation (for details see below).
Periprocedural adverse events occurred in 4.9% (12 patients), and consisted of one (0.4%) periprocedural death and seven tamponades (five requiring urgent percutaneous drainage and two requiring surgical bailout), two life-threatening major bleeds (one TEE-induced upper GI bleeding and one pericardial perforation), three VARC kidney injuries grade 3 and four (1.6%) device embolisations. All embolised devices were snared and a second device was implanted successfully, except in one patient where the embolised device was detected only during follow-up and the patient refused implantation of a second device [
15].
The one patient with periprocedural death underwent a combined intervention: MitraClip intervention and LAAO were successful, whereas a subsequent anterior mini-thoracotomy for a single coronary bypass revealed a pericardial effusion with haemodynamic instability. The reason for the pericardial tamponade was found to be a perforation of the right ventricle. The reason remains unclear, particularly since the patient was stable throughout the percutaneous interventions. Potential causes might have been manipulations of the transseptal needle or it might have occurred during surgery. Emergency sternotomy was performed and the right ventricular perforation was sutured. After successful bypass surgery, the patient could not be weaned from the heart lung machine. The further course was complicated by clotting of the extra corporal circulation and recurrent bleeding from the right ventricle. The patient died subsequently on the day of surgery.
The most severe complication related to LAAO was LAA perforation during repositioning of the device. The device was found to be in the pericardial space. Acute pericardial tamponade was initially treated with pericardial drainage and release of the device and implantation of a second LAA occluder to seal the entrance of the LAA. Ongoing bleeding with haemodynamic instability made emergency surgical bailout necessary. During surgery, the LAA was clipped with an ATR-ICURE 35 mm clip, which terminated the bleeding. The postoperative course was complicated by a prolonged intensive care unit stay with acute renal failure and delirium. The patient was finally discharged to rehabilitation and is currently living independently at home without any long-term sequela (recent follow-up was 2 years after the procedure).
Life-threatening major bleeding occurred in a patient undergoing combined MitraClip implantation and LAAO. During the procedure, acute haemorrhagic shock developed. Emergency gastroscopy revealed TEE-related mid-oesophageal bleeding as the cause. Bleeding stopped spontaneously and the patient fully recovered.
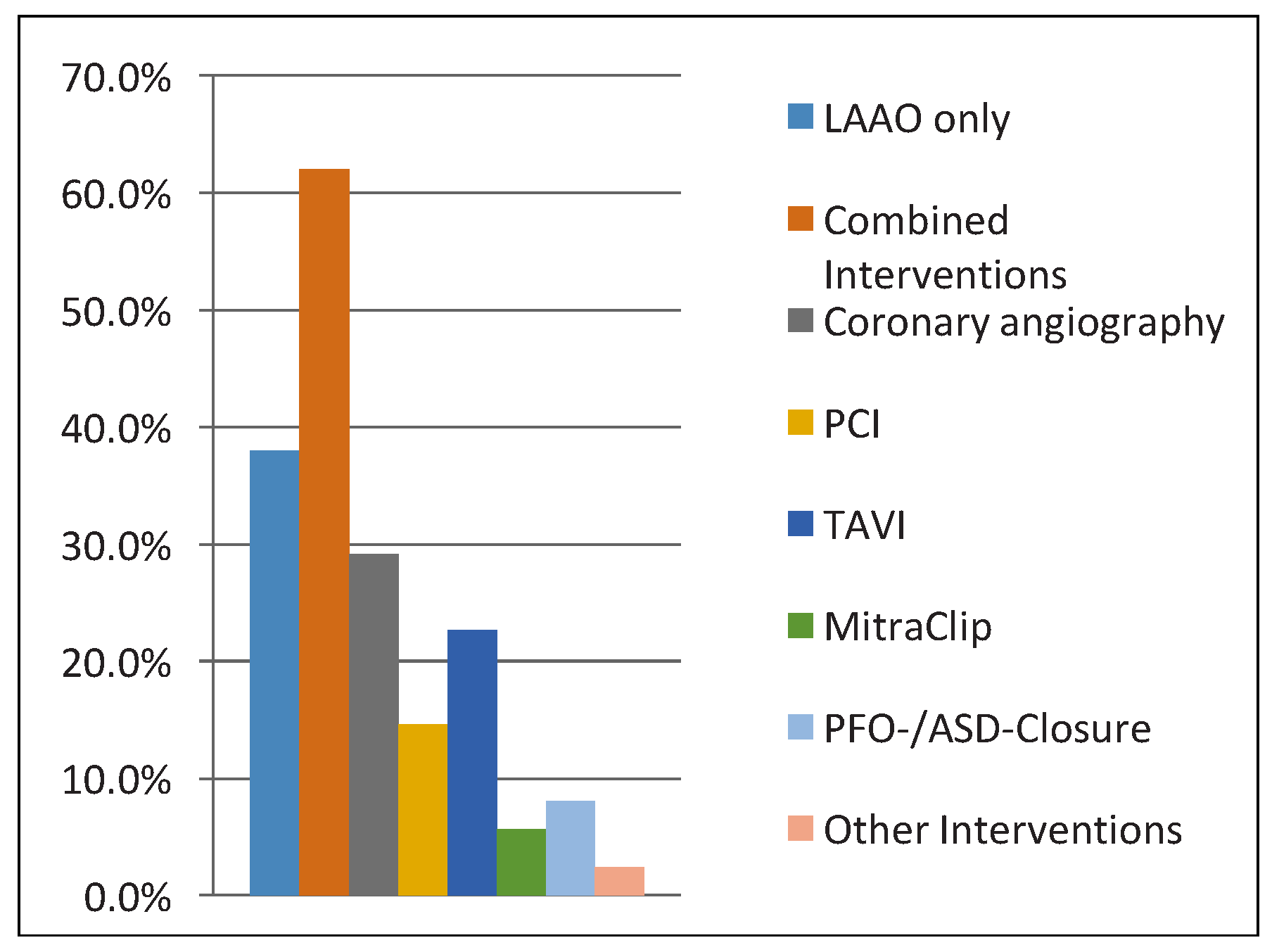
Several of the complications of this series were not directly related to LAAO, and can be explained by the very large number of combined interventions, including high-risk interventions. Overall, there was no statistical difference in complications from 2010 to 2015 (p = 0.6). Indeed, 62% (n = 153) of LAAOs were combined interventions. In 29.1% (72 patients), LAAO was performed after coronary angiography and in 14.6% (36 patients) combined with PCI, in 22.7% (56 patients) with TAVI and in 5.7% (14 patients) with MitraClip. Finally, in 8.1% (20 patients) LAAO was followed by PFO or ASD closure and in 2.4% (6 patients) combined with other interventions (
Figure 4).
LAAO devices
In almost half of the patients (43.3% or 107 patients), ACP devices were used, whereas the Amplatzer Amulet was used in 35.2% (87 patients) and the Watchman Occluders in 21.5% (53 patients) (
Figure 5). The numbers of different devices implanted are listed in
Table 2.
In 6.5% (16 patients), the initial device size was replaced with a different device (2 [3.8%] Watchman, 8 [7.6%] ACP, 6 [6.9%] Amulet). The reasons for replacing the initial device were anatomical (e.g., funnel-shaped landing zone) or mis-sizing. In one patient (0.4%), LAA occlusion could only be achieved after replacing the second device with a third device (ACP).
In 8.2% (20 patients), the LAA was reached using a PFO or ASD, which was subsequently occluded using a PFO or ASD occluder. The PFO and ASD closures were uneventful; the same sheath and delivery system as for the LAA occlusion (TorqVue 45 × 45 sheath) were used.
Mean duration of hospitalisation was 7.4 ± 7.0 days. In patients with isolated LAAO (not combined interventions) median hospitalisation was 3 days; IQR 2–6 days. Seventeen patients (6.9%) were discharged the day of intervention and 57 patients (23.1%) the day after the intervention. For patients referred for isolated LAAO on an elective basis, same day discharge or discharge the day after the procedure is routine at our institution. Patients were discharged on ASA indefinitely (88.3%; 218 patients) and a thienopyridine for either 3 (22.7%; 56 patients), 6 (25.5%; 63 patients) or 12 months (15.8%; 39 patients).
Follow-up
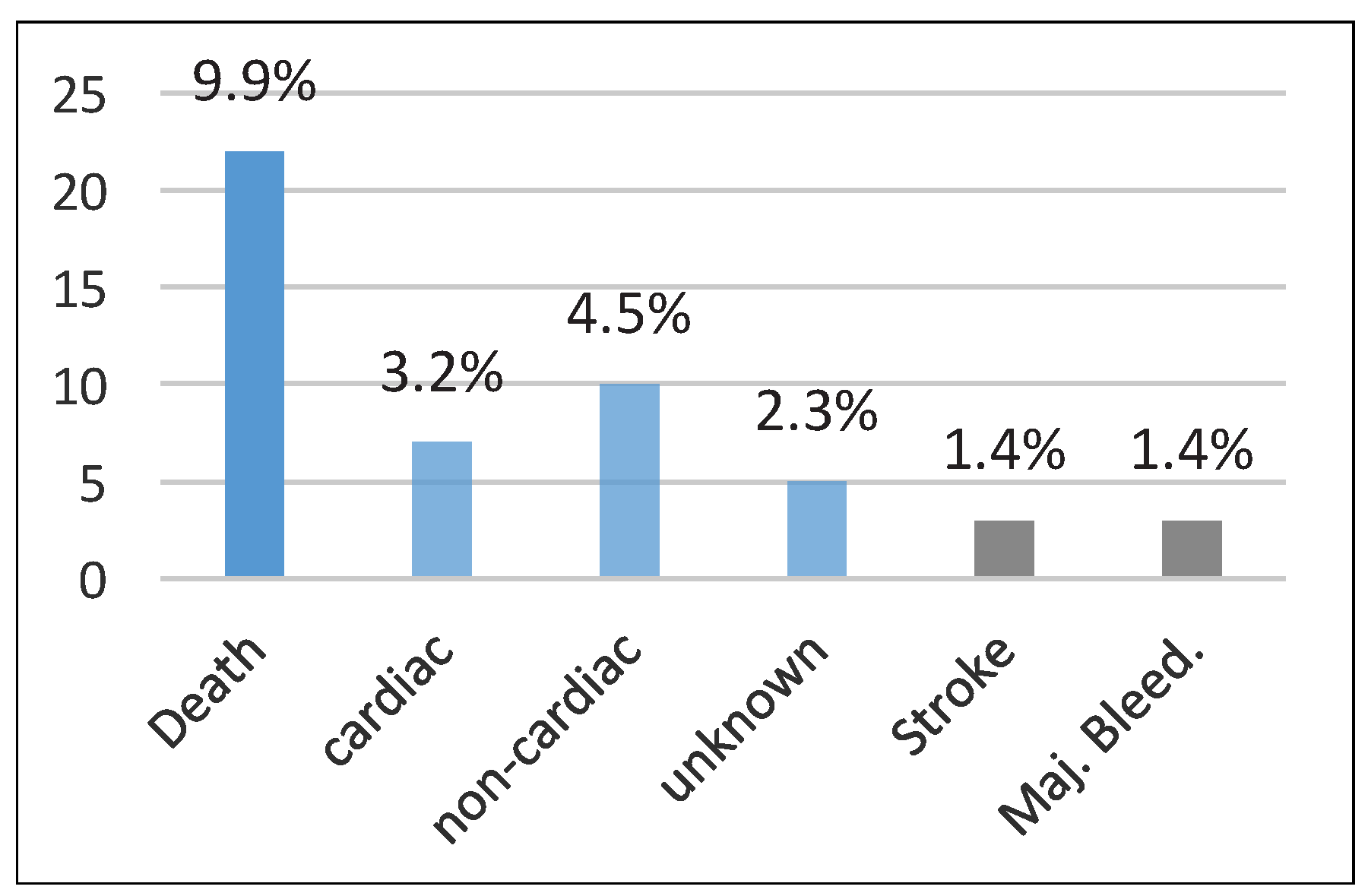
Clinical follow-up was complete in 89.9% (222 patients) after a mean duration of 10 ± 9.6 months. Overall, 9.9% (22 patients) died during follow-up (after a median of 3 months; IQR 1–5 months). The deaths included cardiac deaths of seven patients, which were due to heart failure in three and ventricular tachycardia and/or fibrillation in two, with one each due to stroke or endocarditis (occurring after an
Enterococcus faecalis positive urinary tract infection 7 months after the intervention). Ten deaths were non-cardiac in nature, one due to bleeding and five of unknown cause (
Figure 6).
Of all deaths, one due to stroke was related to LAAO. In this case the patient died as a result of a cerebrovascular event (major stroke) occurring 2 weeks after successful LAAO in conjunction with transcatheter aortic valve implantation. Upon presentation of the patient with a major stroke, TEE showed mobile thrombus on the disc of the device as the most likely source of embolism. Three cerebrovascular events (1.4%) occurred during follow-up. In two of these patients a control TEE showed no thrombus on the device; the third patient is described above. Major bleeding occurred in 3 (1.4%) patients. Two major bleeds were life-threatening (one traumatic intracerebral bleeding and one GI bleeding with anaemia) and one was not life-threatening (GI bleeding with drop in haemoglobin to 72 g/l). Clinical follow-up data are shown in
Table 3.
Of the 222 patients who had clinical follow-up, 70.4% (174 patients) had echocardiographic imaging, and one patient underwent a CT scan (at his own request), confirming a correct device position in 99.4%. A relevant residual shunt or leak of ≥5 mm was found in only one (0.6%) patient, and thrombus on the device was detected in 3.4% (six patients) in three of whom the thrombus appeared to be mobile (
Table 4). In one patient a leak ≥5 mm was detected between the warfarin ridge and the device, which is considered a harmless form of a leak without perfusion of the distal lobes of the LAA – the patient remains asymptomatic and the leak had closed at 1-year follow-up TEE. If a mobile thrombus was found on the device rivaroxaban therapy was initiated, clopidogrel and ASA were discontinued, and TEE repeated 4–6 weeks later, at which point rivaroxaban was terminated and ASA restarted.
Correct device position could not be confirmed in one patient, where the device was found in the abdominal aorta at follow-up 6 months after implantation. Retrospectively, device embolisation occurred within 24-hours of the procedure, but was missed at discharge. The device was snared and externalised. As the patient did not wish implantation of another device, medical therapy with rivaroxaban was re-initiated [
15].
Discussion
This study, based on a large single centre experience, demonstrated that percutaneous LAAO is technically feasible with a high procedural success rate of over 98%, comparing favourably with previous reports of even larger patient populations (e.g., procedural success: 90.9% in the PROTECT-AF trial [
16], 94.4% in the CAP registry [
14], 95.1% in the PREVAIL trial [
11], 98.5% in the EWOLUTION registry [
17] and 97.3% in the largest European multicentre registry [
18]). However, LAAO is a procedure with a notable complication rate. Indeed, periprocedural adverse events occurred in 4.9% which was comparable to other reports (e.g., 5.0% in the European registry [
18], 7.7% in the PROTECT-AF Trial [
16], 3.7% in the CAP study [
14], 2.2% in the PREVAIL study [
11] and 2.8% in the EWOLUTION registry [
17]).
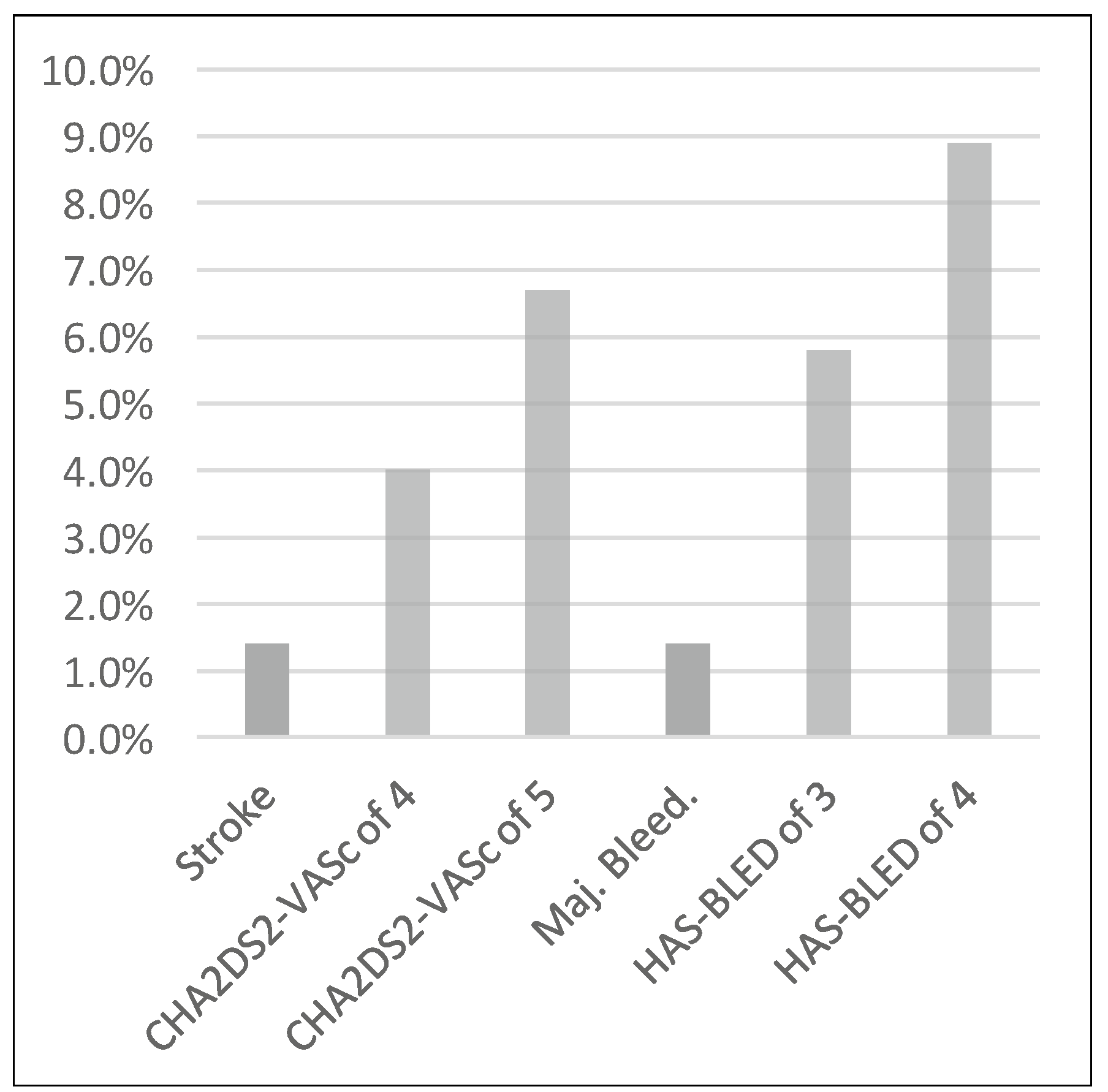
The study population comprised patients at high risk for stroke and bleeding. The average CHA2DS2-Vasc score was 4.5 and a HAS-BLED score of 3.6. Assuming a 65% risk reduction in the occurrence of cerebrovascular accidents with VKAs, the stroke rate after 10 months with VKA therapy would be 1.5%, a value comparable to the observed stroke rate in this registry. On the other hand, major bleeding complications were exceedingly infrequent and were reduced 3.8-fold as compared with the expected rate of 5.5% at 10 months (according to the HAS-BLED score) (
Figure 7).
Combined interventions are routine in our institution and performed in 62% of the patients. As such combined procedures are inherently associated with an increased risk, this fact negatively influenced the overall periprocedural adverse event rate (
Table 5). Indeed, combined interventions accounted for 62% of all procedures and 75% of all adverse events with LAAO in this series. Together with a previous report [
8], this is one of the highest ever reported percentages of combined interventions in an LAAO registry or trial. In the European registry, LAAO was performed as part of a combined intervention in 20.6%, [
18], and no such combined interventions were performed in the PRO-TECT-AF trial. In spite of a higher complication rate, our data support combination of procedures as this tends to be more patient-friendly, and allows a single hospitalisation and early discharge in most cases. However, there is a disincentive in the Swiss healthcare system (due to the design of disease-related groups[DRGs]) to combine procedures and therefore such procedures are staged in most institutions for economic reasons. As under these conditions individual billing of the insurance companies for each procedure is possible, the overall healthcare costs are considerably higher than with combined procedures. Not only does this policy make the overall healthcare system more expensive and less patient-friendly, but it could even result in more adverse events if patients experience bleeding complications while awaiting LAAO. Such disincentives should be removed (e.g., by creating DRGs for combined interventions), such that institutions continuing to perform combined interventions are not punished by the system.
Thrombus formation on the device remains an issue and occurs in 3–4% of patients. If detected early, a short course of VKA, NOAC or low-molecular weight heparin generally resolves the issue [
19,
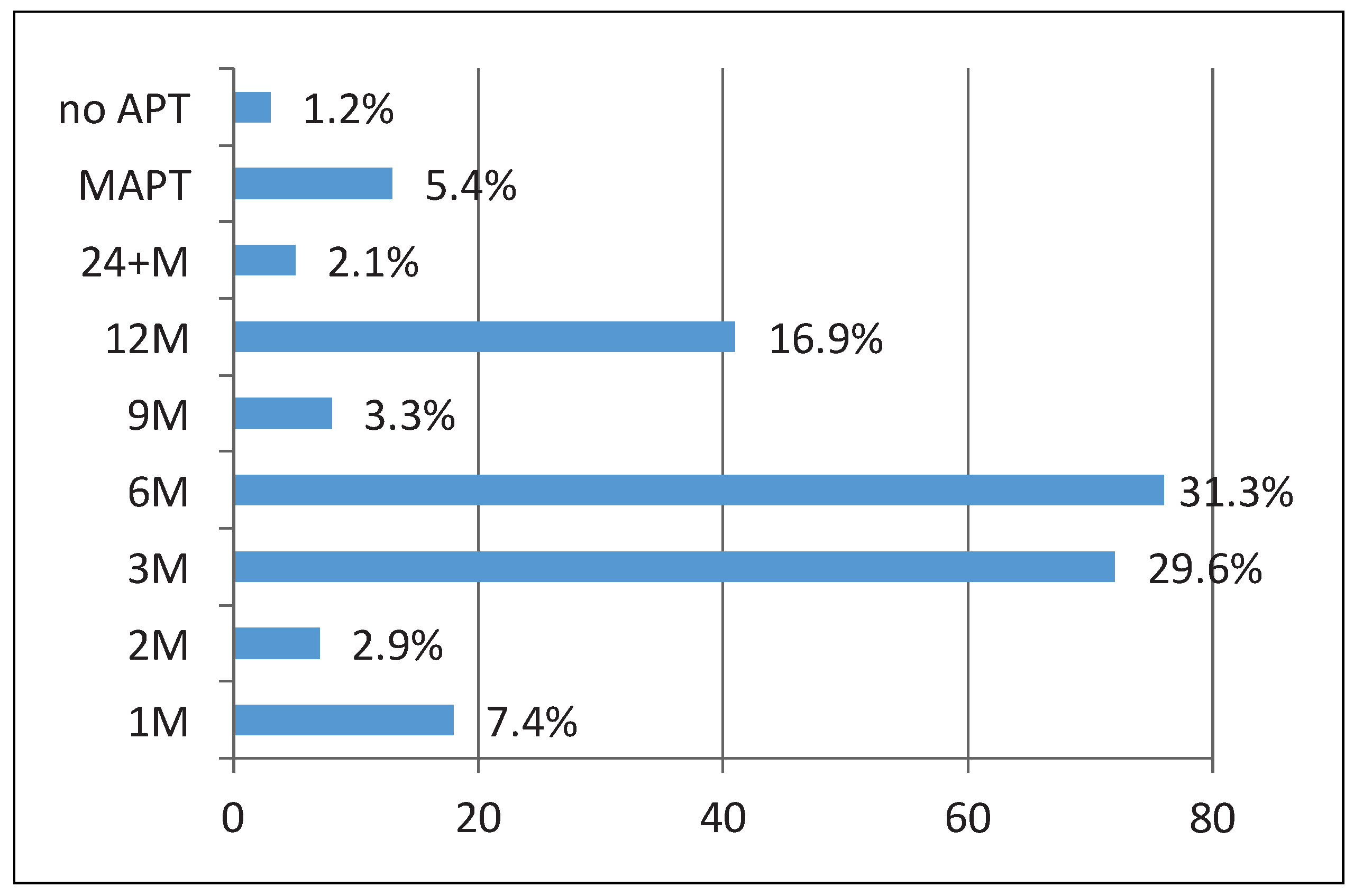
20]. Patients with an absolute contraindication to VKAs may present a dilemma under these conditions, but low-dose oral anticoagulation with an NOAC could be considered. In order to reduce the risk of thrombus formation on the device, a longer course of dual antiplatelet therapy (DAPT) might be preferable, but exposes these fragile patients to a higher bleeding risk. The same is true for the regimen recommended after implantation of the Watchman device: OAC was continued for 6 weeks after device implantation in the PROTECT-AF trial. In patients at very high risk of bleeding, even aspirin monotherapy or no antiplatelet therapy may present an option; otherwise a short course of DAPT of 1 to 3 months depending on the device size is common practice in such patients (
Figure 8). In our cohort a large percentage of combined procedures were performed and often the combined procedure dictated the antiplatelet regimen (e.g. the extension of coronary artery disease). Of note, in none of our patients was OAC continued after LAAO. Based on current experience, protrusion of the disc of the Amplatzer occluder into the neck of the LAA is not considered dangerous and does not seem to require any action [
21,
22]. This supports the theory that it is mostly the trabeculated lobes that are thrombogenic and not the smooth wall of the LAA neck.
As LAAO is a technically demanding intervention, operator experience is crucial. As already shown in the PREVAIL study, procedural success increased and procedural adverse events decreased with increasing experience of the operators [
11]. Therefore, such procedures should preferably be conducted in high volume centres with experienced operators and an experienced team.
Based on the results of recent trials [2, 18], LAAO appears equal or even superior to OAC in preventing stroke, systemic embolism, cardiovascular death, and cardiovascular and overall mortality, at least in experienced hands. In a recent network analysis, LAAO out-performed oral anticoagulants in terms of mortality reduction with a comparable prevention of systemic embolism [
23]. Thus, it can be seen as an alternative to OAC even in patients without contraindications to OAC or at high bleeding risk. Accordingly, all patients suffering from AF should be informed about this treatment option and its procedural success, complication rate and long-term benefit. Trials comparing LAAO and NOACs are currently sparse, with one observational study showing lower rates of cerebrovascular and bleeding events in the LAAO group [
24]. As our institution currently enjoys the largest annual rate of LAAO in Switzerland and hence a large experience with the intervention, LAAO is handled as a potential first-line therapy, comparable to NOACs. In particular, in certain clinical settings, such as patients with an indication for concomitant antiplatelet therapy, LAAO even provides an advantage over NOACs,