A Patient with Arrhythmias and Infective Cardiac Disease
Abstract
Introduction
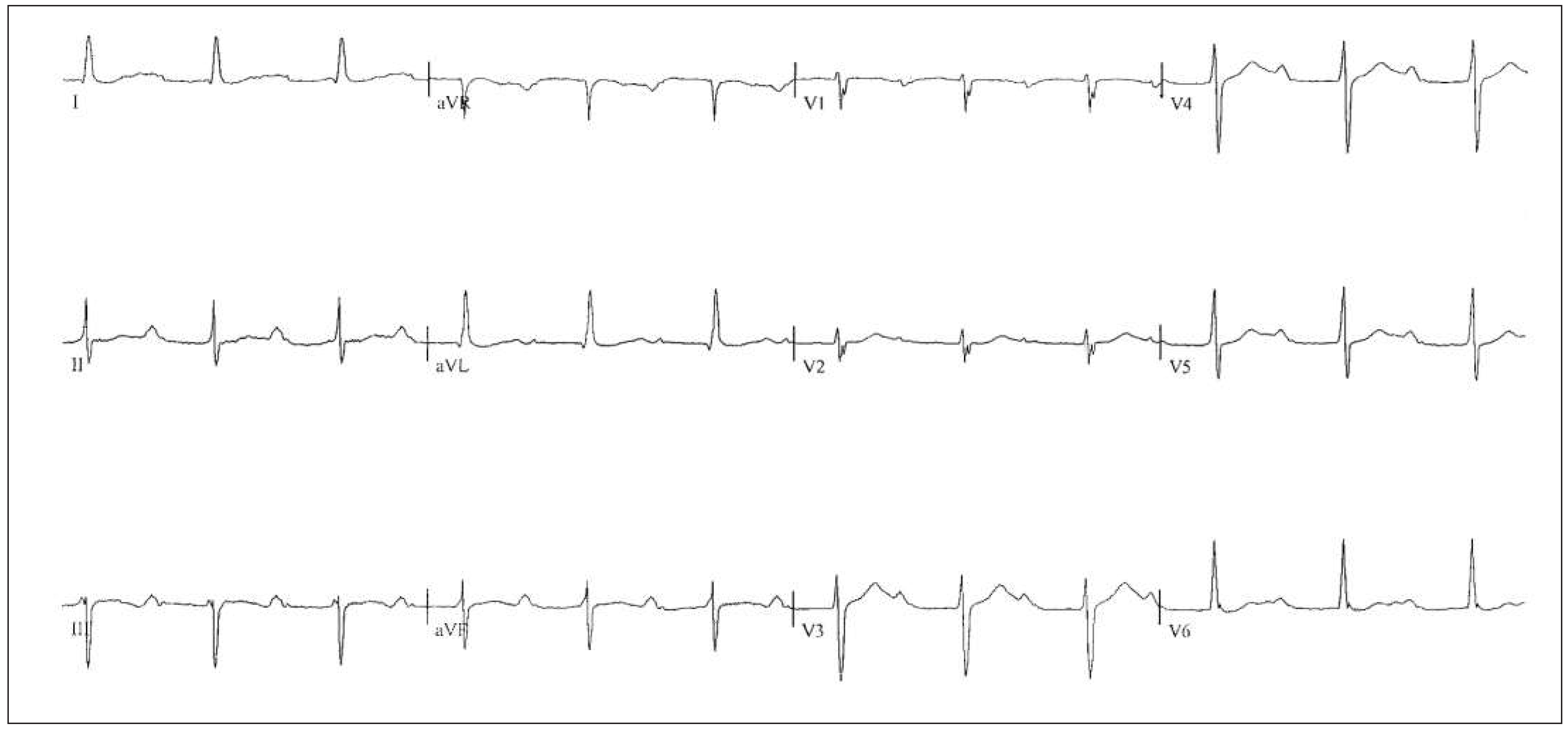
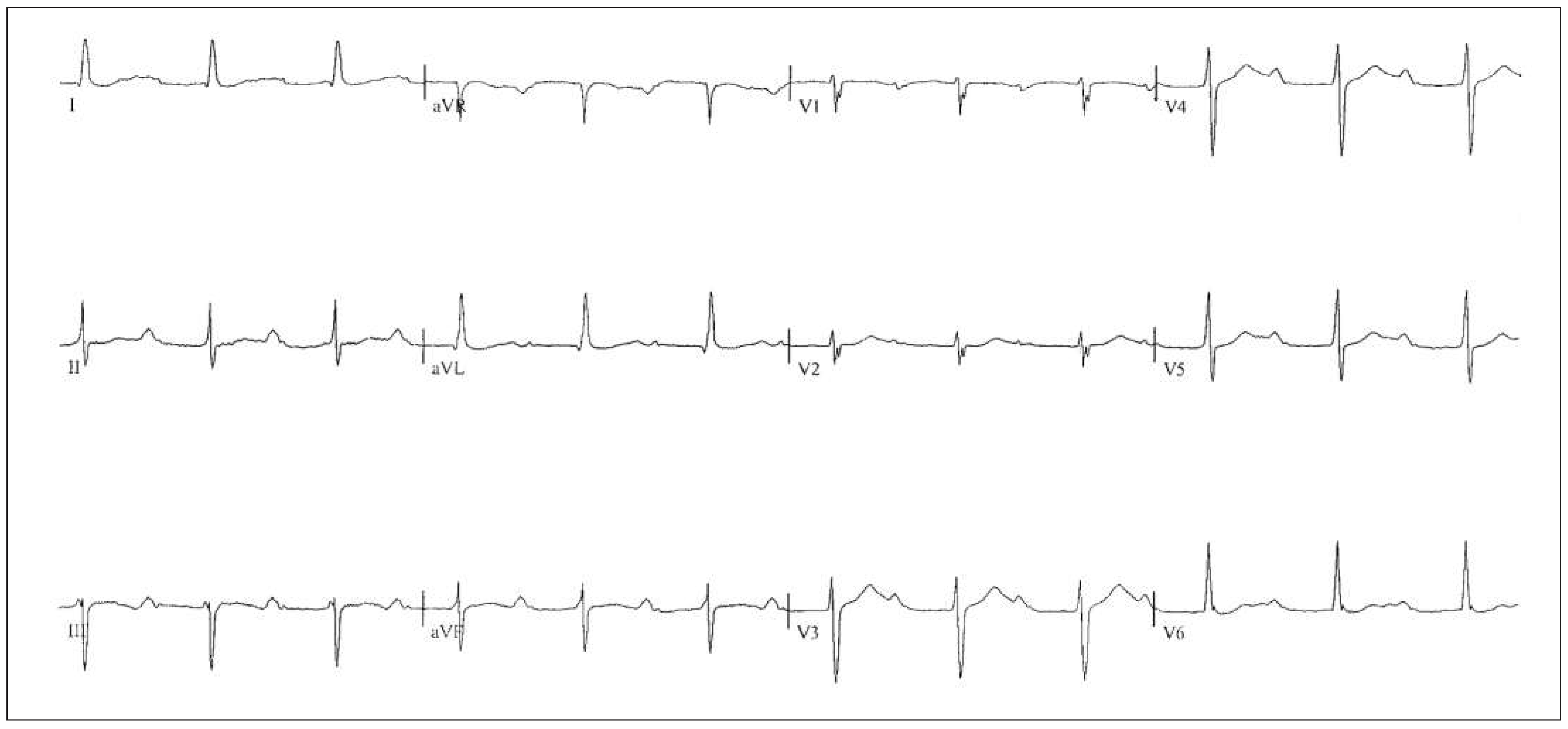
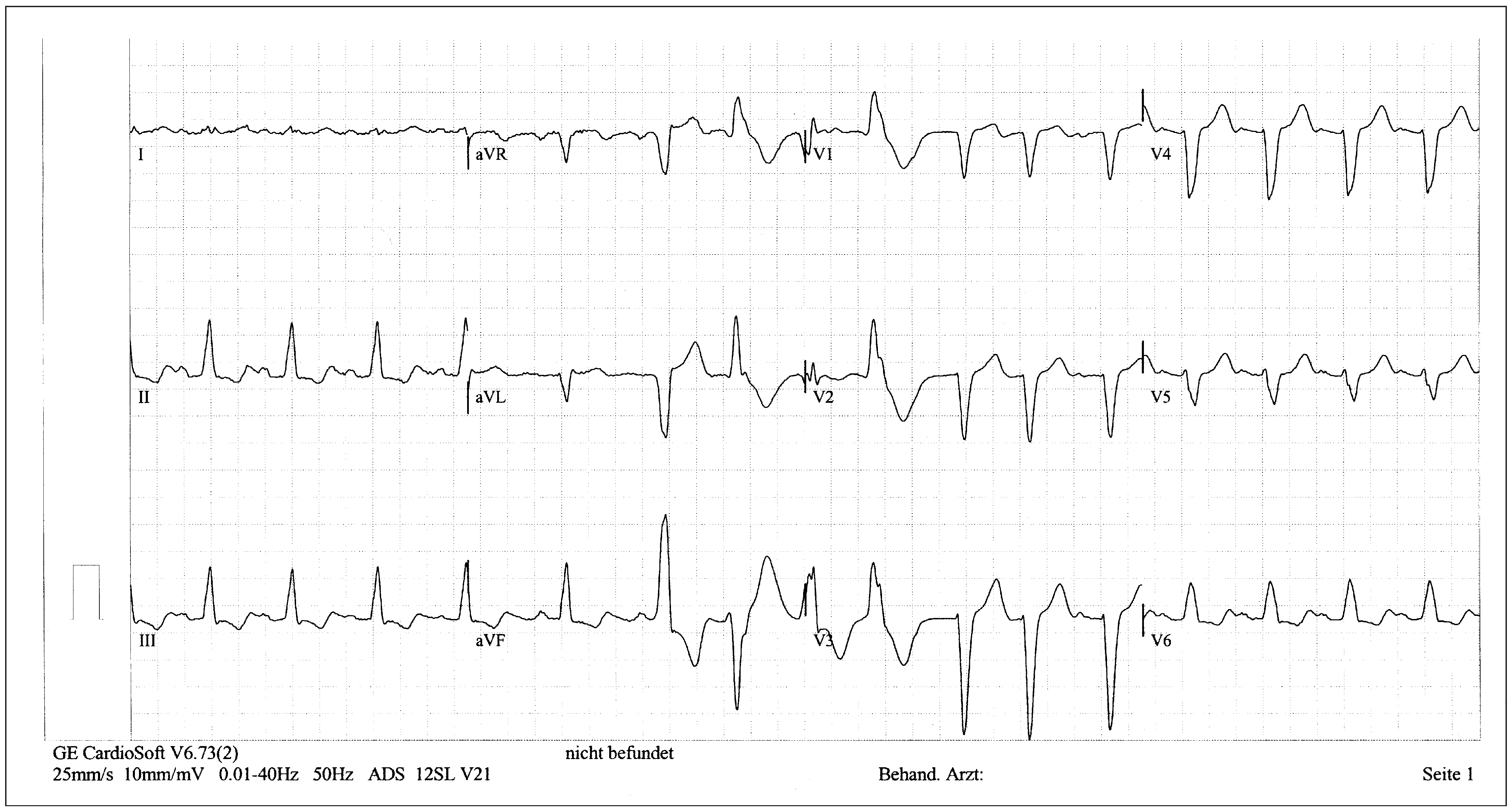
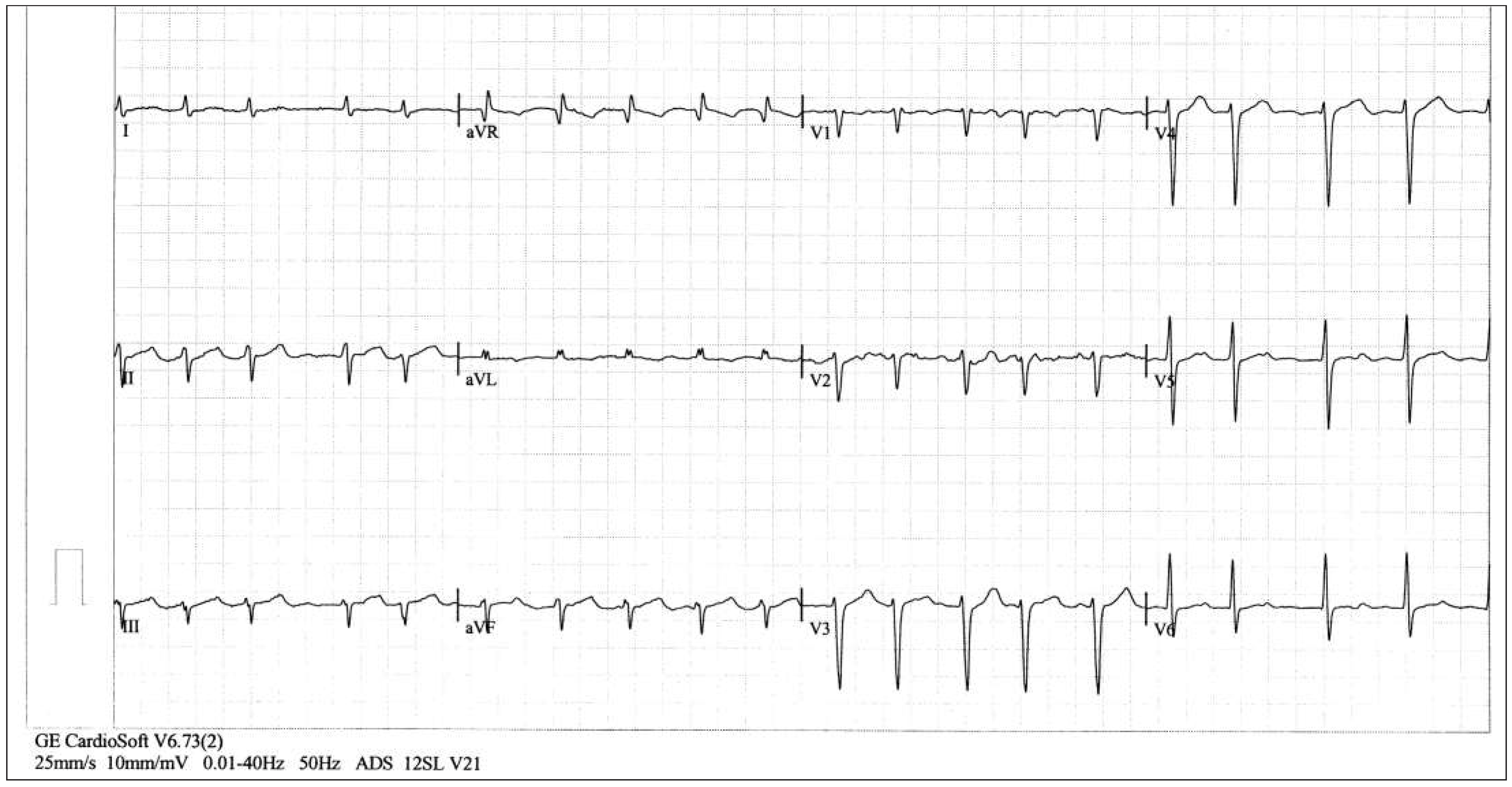
Patient
Six-month follow-up
Sixteen-month follow-up
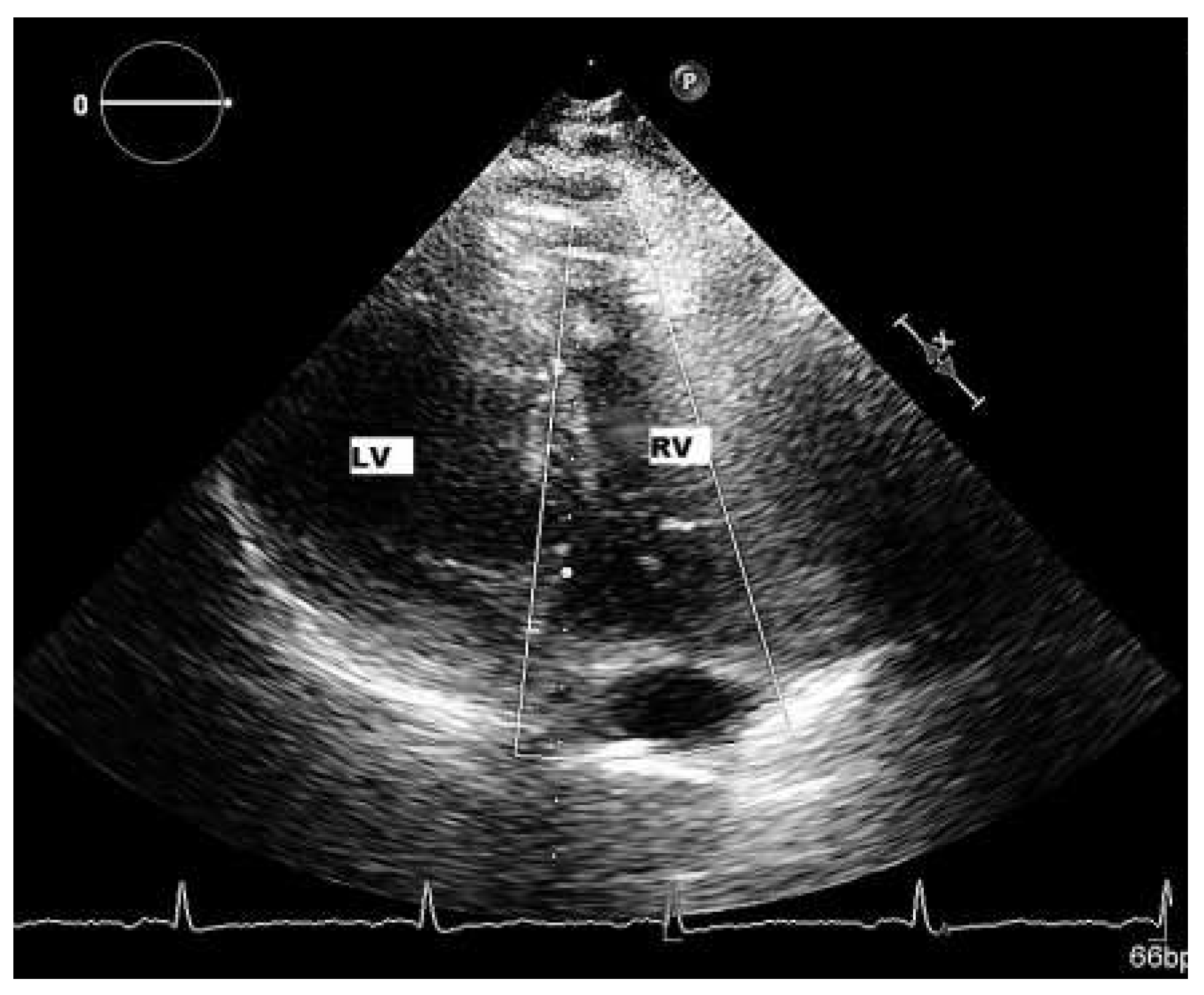
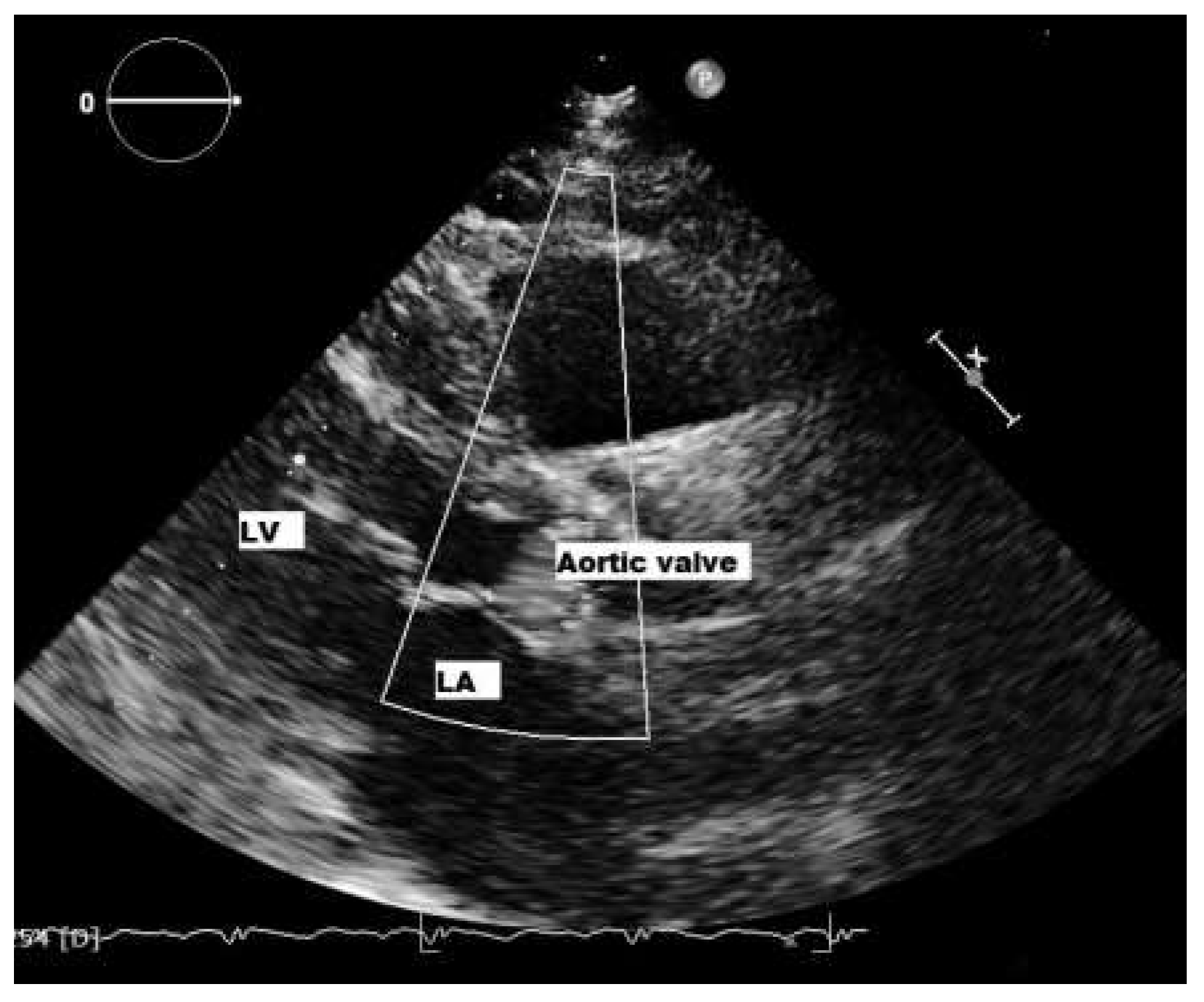
Infected European patients
Diagnosis of Chagas disease
Aetiology
Complications
Pathogenesis
Therapy
Acknowledgments
Disclosure statement
References
- Fox, M.C.; Lakdawala, N.; Miller, A.L.; Loscalzo, J. A Patient with Syncope. New Engl. J. Med. 2013, 369, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. New Engl. J. Med. 2015, 373, 1295–06. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Little, W.C.; et al. Development and Validation of a risk Score for Predicting Death in Chagas’ Heart Disease. New Engl. J. Med. 2006, 355, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Acquatella, H. Heart Disease in Latin America. Echocardiography in Chagas Heart Disease. Circulation 2007, 115, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Sabino, E.C.; Ribeiro, A.L.; Salemi, V.M.; et al. Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi-seropositive former blood donors. Circulation 2013, 127, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, M.J.; Cañas, E.; Elizalde, J.I.; et al. Diagnosis, management and treatment of chronic Chagas’ gastrointestinal disease in areas where Trypanosoma cruzi infection is not endemic. Gastroenterol. Hepatol. 2010, 33, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Pinto Dias, J.C. Natural history of Chagas disease. Arq. Bras. Cardiol. 1995, 65, 359–366. [Google Scholar] [PubMed]
- Rassi AJr Dias, J.C.; Marin-Neto, J.A.; Rassi, A. Challenges and opportunities for primary, secondary, and tertiary prevention of Chagas’ disease. Heart 2009, 95, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Rassi AJr Rassi, A.; Marcondes de Rezende, J. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. North. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Cedillos, R.A.; Póvoa, M.M.; et al. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas’ disease? Lancet 1981, 1, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- del Puerto, R.; Nishizawa, J.E.; Kikuchi, M.; et al. Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia. PLoS Negl. Trop. Dis. 2010, 4, e687. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, L. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 2001, 31, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.S.; Dutra, W.O.; Esper, L.; et al. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin. Immunopathol. 2012, 34, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simões, M.V. Pathogenesis of chronic Chagas heart disease. Circulation 2007, 115, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Dutra, W.O.; Menezes, C.A.; Magalhães, L.M.; Gollob, K.J. Immunoregulatory networks in human Chagas disease. Parasite Immunol. 2014, 36, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Global burden of disease estimates for 2000–2012; World Health Organization, 2014; Available online: http://www.who.int/ healthinfo/global_burden_disease/estimates/en/ index2.html.
- McMurray, J.V.; Adamopoulos, S.; Anker, S.D.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2012, 33, 1787–1747. [Google Scholar] [PubMed]

© 2016 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Cocco, G.; Amiet, P. A Patient with Arrhythmias and Infective Cardiac Disease. Cardiovasc. Med. 2016, 19, 28. https://doi.org/10.4414/cvm.2016.00386
Cocco G, Amiet P. A Patient with Arrhythmias and Infective Cardiac Disease. Cardiovascular Medicine. 2016; 19(1):28. https://doi.org/10.4414/cvm.2016.00386
Chicago/Turabian StyleCocco, Giuseppe, and Philipp Amiet. 2016. "A Patient with Arrhythmias and Infective Cardiac Disease" Cardiovascular Medicine 19, no. 1: 28. https://doi.org/10.4414/cvm.2016.00386
APA StyleCocco, G., & Amiet, P. (2016). A Patient with Arrhythmias and Infective Cardiac Disease. Cardiovascular Medicine, 19(1), 28. https://doi.org/10.4414/cvm.2016.00386



