A Valve-in-Valve Approach for Ebstein’s Anomaly
Abstract
Introduction: Ebstein’s anomaly
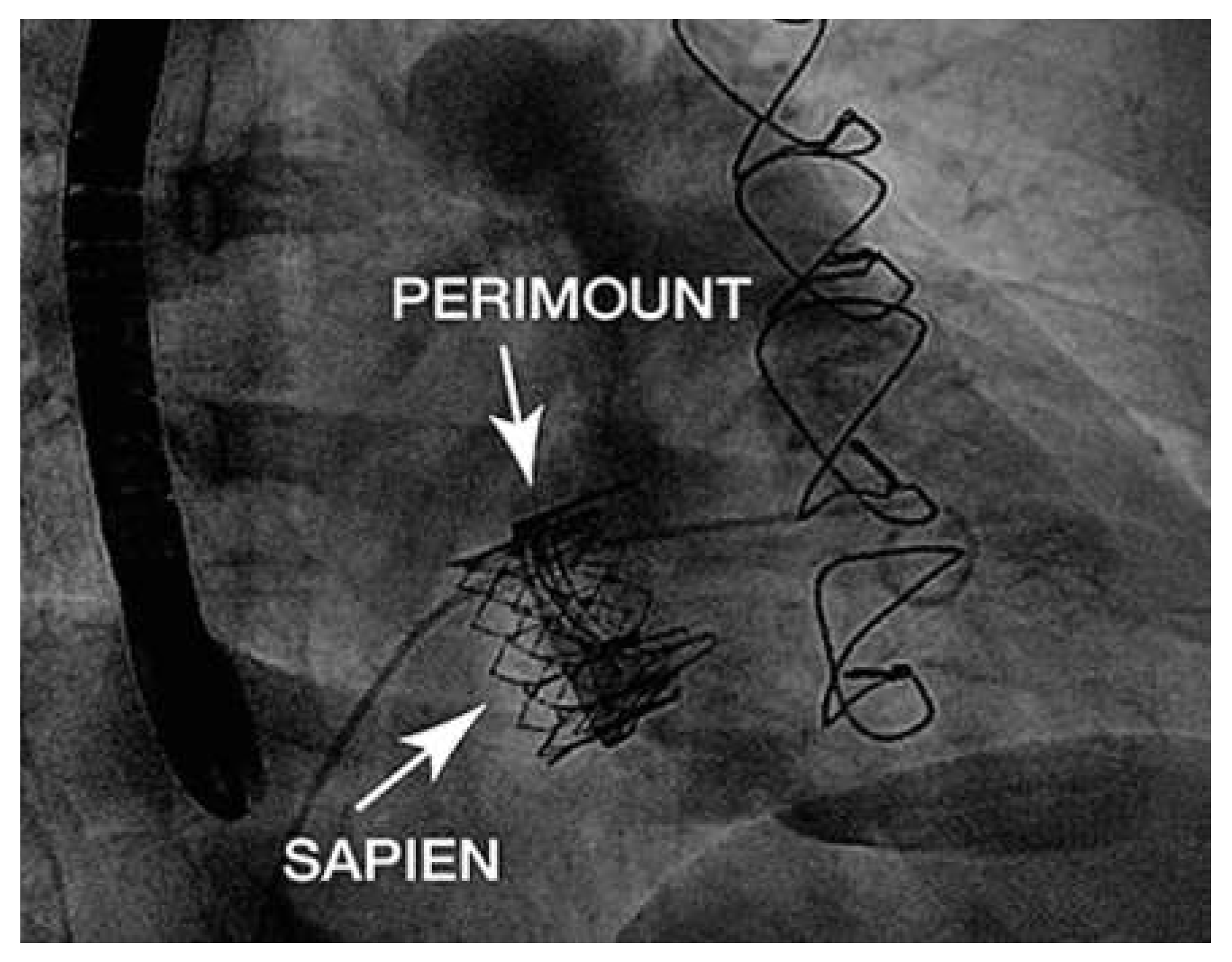
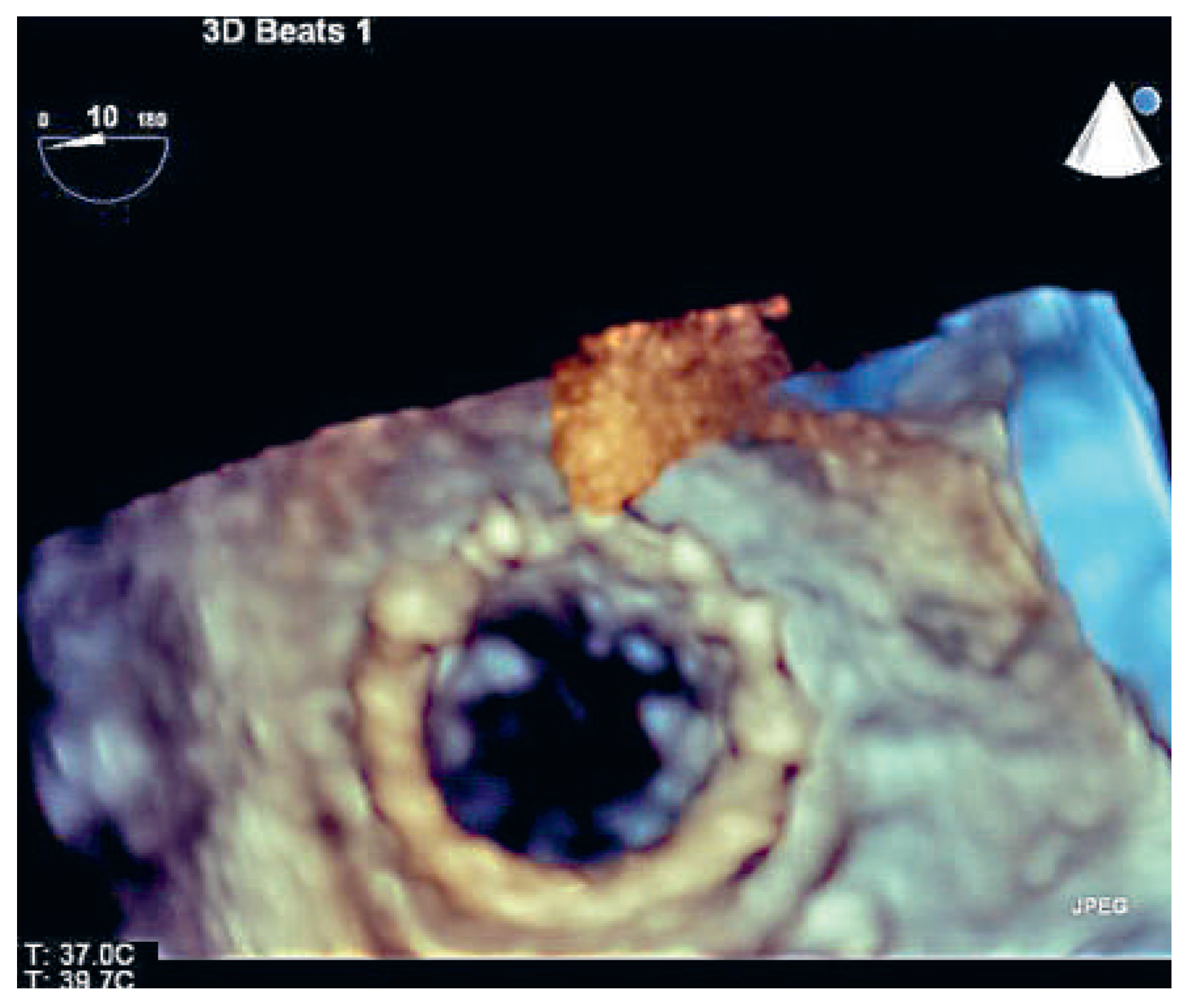
Case report
Discussion
Conclusions
Disclosures
References
- Attenhofer Jost, C.H.; Connolly, H.M.; Dearani, J.A.; Edwards, W.D.; Danielson, G.K. Ebstein’s anomaly. Circulation. 2007, 115, 277–85. [Google Scholar] [CrossRef] [PubMed]
- Brickner, M.E.; Hillis, L.D.; Lange, R.A. Congenital heart disease in adults. Second of two parts. N Engl J Med. 2000, 342, 334–42. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Bonhoeffer, P.; De Groot, N.M.; de Haan, F.; Deanfield, J.E.; Galie, N.; Westby, J. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010, 31, 2915–57. [Google Scholar] [CrossRef] [PubMed]
- Van Arsdell, G. Can we modify late functional outcome in Ebstein anomaly by altering surgical strategy? J Am Coll Cardiol. 2008, 52, 467–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warnes, C.A. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol. 2000, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.N.; Harrison, D.A.; Connelly, M.S.; Webb, G.D.; Siu, S.C. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000, 86, 1111–6. [Google Scholar] [CrossRef] [PubMed]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Kachaner, J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000, 356, 1403–5. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.A.; Boudjemline, Y.; Cheatham, J.P.; Eicken, A.; Ewert, P.; McElhinney, D.B.; Zahn, E. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011, 58, 117–22. [Google Scholar] [CrossRef] [PubMed]
- Hoendermis, E.S.; Douglas, Y.L.; van den Heuvel, A.F. Percutaneous Edwards SAPIEN valve implantation in the tricuspid position: case report and review of literature. EuroIntervention. 2012, 8, 628–33. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, C.; Hoschtitzky, J.A.; Hasan, R.; Clarke, B.; Mahadevan, V.S. Percutaneous tricuspid valve-in-valve implantation in Ebstein’s anomaly: One-year follow-up of valve function. Int J Cardiol. 2014, 174, e77–8. [Google Scholar] [CrossRef] [PubMed]

© 2015 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Schwitz, F.; Wenaweser, P.; Kadner, A.; Wustmann, K.; Windecker, S.; Schwerzmann, M. A Valve-in-Valve Approach for Ebstein’s Anomaly. Cardiovasc. Med. 2015, 18, 139. https://doi.org/10.4414/cvm.2015.00331
Schwitz F, Wenaweser P, Kadner A, Wustmann K, Windecker S, Schwerzmann M. A Valve-in-Valve Approach for Ebstein’s Anomaly. Cardiovascular Medicine. 2015; 18(4):139. https://doi.org/10.4414/cvm.2015.00331
Chicago/Turabian StyleSchwitz, Fabienne, Peter Wenaweser, Alexander Kadner, Kerstin Wustmann, Stephan Windecker, and Markus Schwerzmann. 2015. "A Valve-in-Valve Approach for Ebstein’s Anomaly" Cardiovascular Medicine 18, no. 4: 139. https://doi.org/10.4414/cvm.2015.00331
APA StyleSchwitz, F., Wenaweser, P., Kadner, A., Wustmann, K., Windecker, S., & Schwerzmann, M. (2015). A Valve-in-Valve Approach for Ebstein’s Anomaly. Cardiovascular Medicine, 18(4), 139. https://doi.org/10.4414/cvm.2015.00331



