Antichemokine Treatments in Acute Ischaemic Cardiovascular Diseases
Abstract
1. Introduction
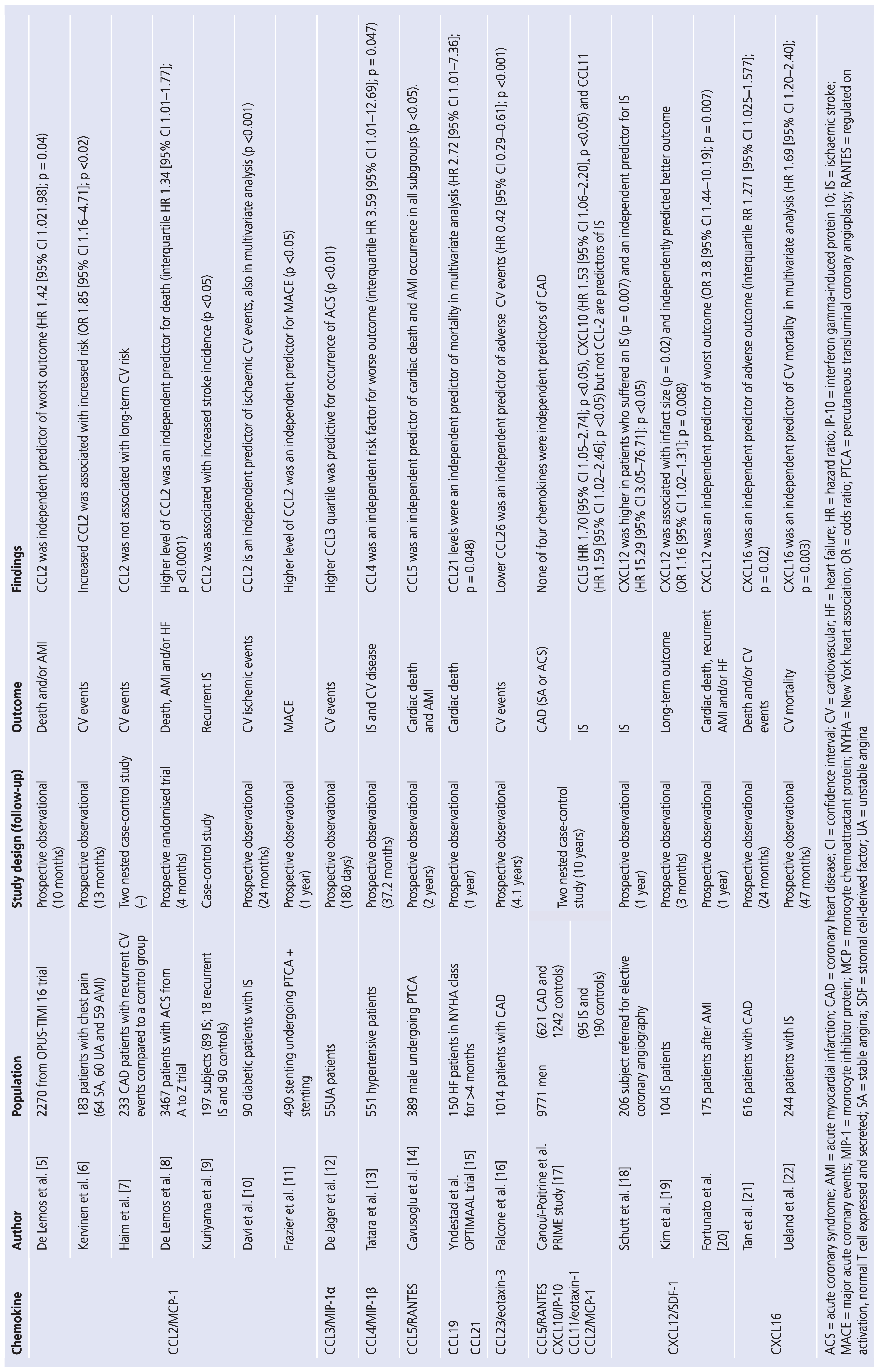
2. Chemokines in Acute Myocardial Infarction and Stroke
3. CXC Chemokines
4. CC Chemokines
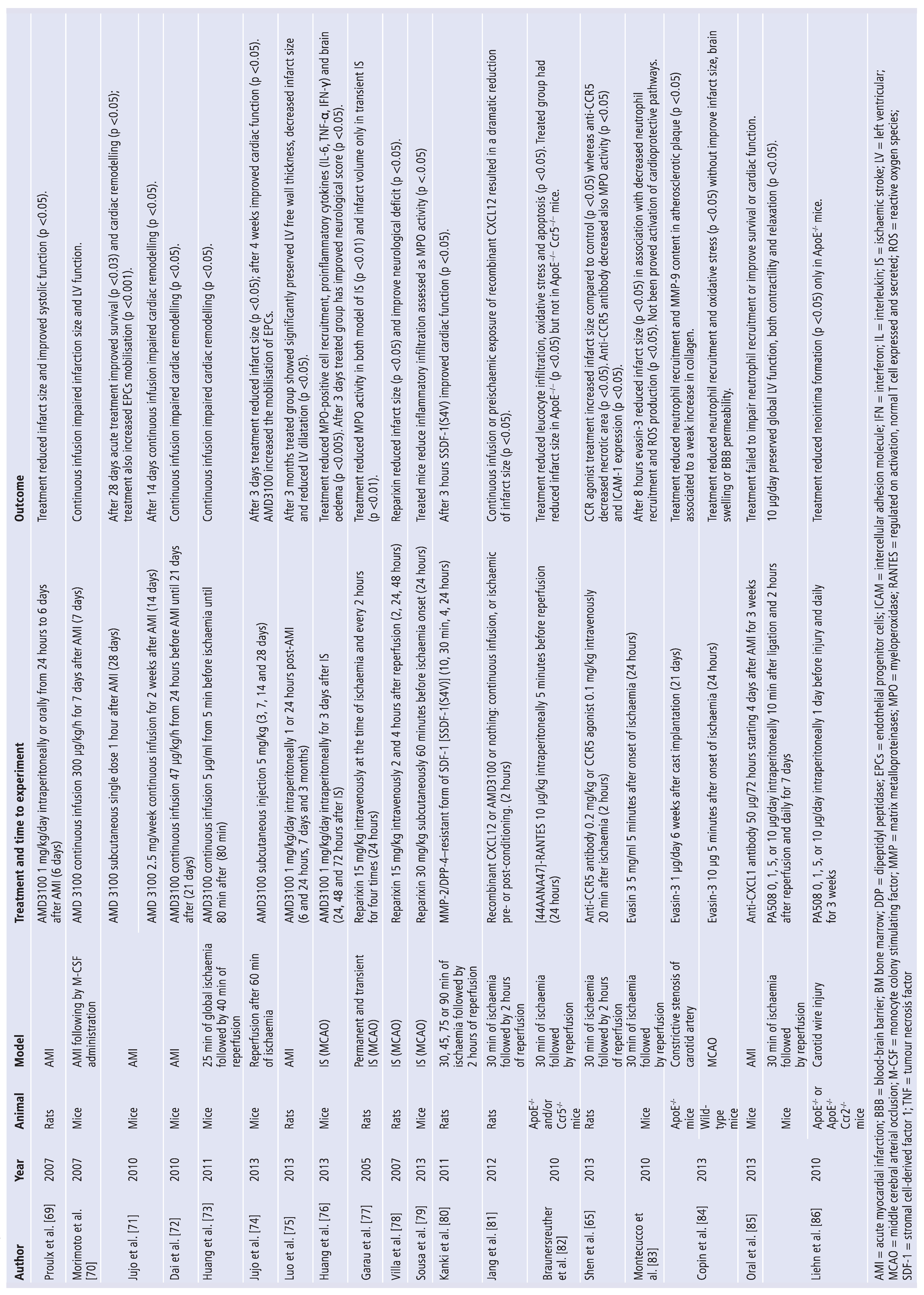
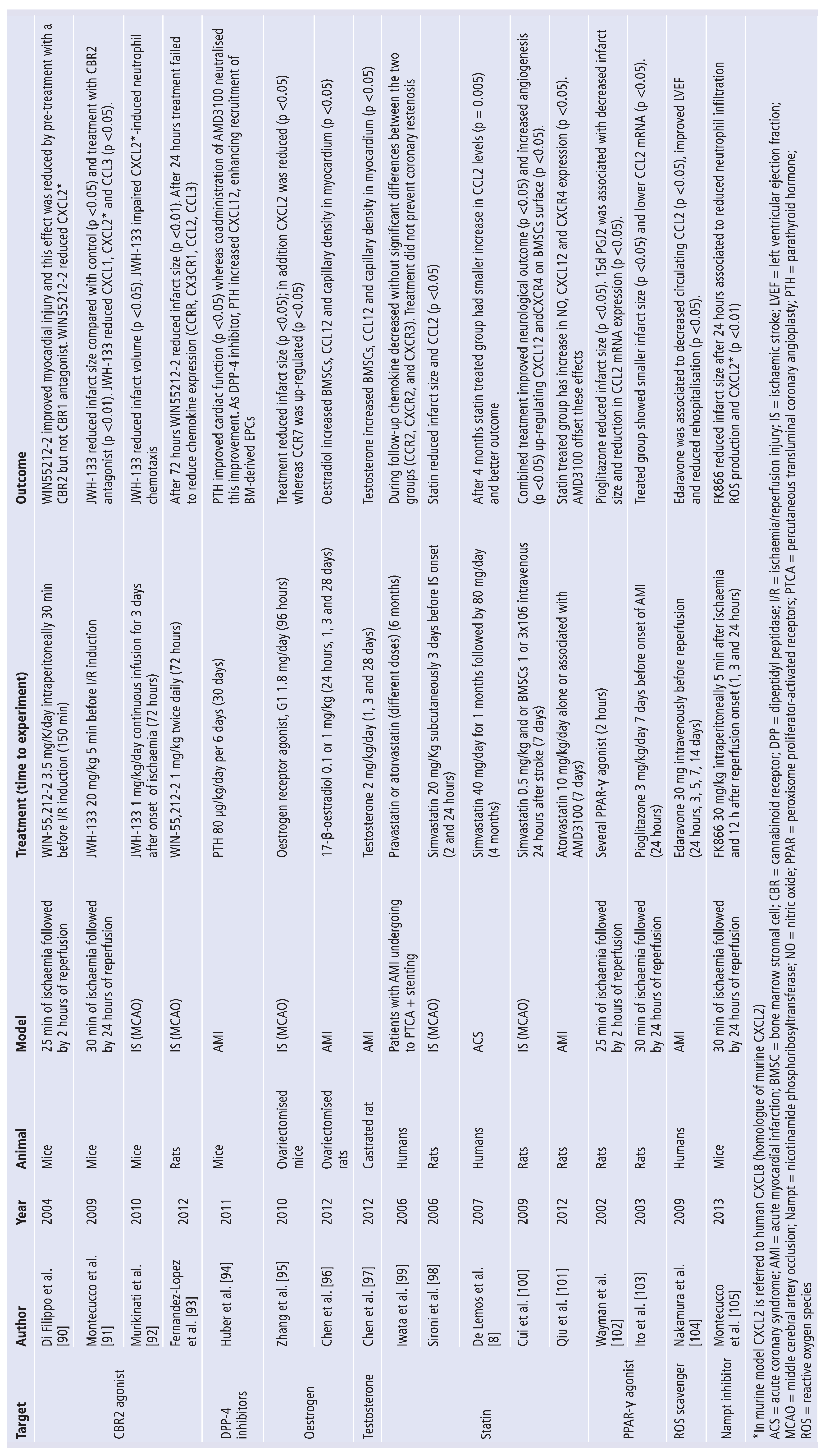
5. Update on Treatments Targeting Chemokines
- modified chemokines;
- synthetic small molecules acting as antagonist or inverse agonist at chemokine receptors;
- neutralising antibodies targeting chemokines or their receptors;
- chemokine-binding proteins.
6. Selective Chemokines Inhibitors
7. Nonselective Chemokine Inhibitors
8. Limitations of Antichemokine Treatments in Humans
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| BMSCs | bone marrow-derived stem cells |
| CBR | cannabinoid receptor |
| CT | computed tomography |
| CV | cardiovascular |
| DPP | dipeptidyl peptidase |
| ELR | glutamate-leucine-arginine |
| EMEA | European Medicines Evaluation Agency |
| EPCs | endothelial progenitor cells |
| GLP | glucagon-like peptide |
| HIV | human immunodeficiency virus |
| MCP | monocyte chemoattractant protein |
| MIP | macrophage inflammatory protein |
| MMP | matrix metalloproteinase |
| mRNA | messsenger ribonucleic acid ox |
| LDL | oxidised low density lipoprotein |
| PPAR | peroxisome proliferator-activated receptor |
| RANTES | regulated upon activation normal T-cell expressed and secreted |
| ROS | reactive oxygen species |
| SDF | stromal cell-derived factor |
References
- Magnussen, C.G.; Niinikoski, H.; Juonala, M.; Kivimäki, M.; Rönnemaa, T.; Viikari, J.S.A.; Simell, O.; Raitakari, O.T. When and how to start prevention of atherosclerosis? Lessons from the Cardiovascular Risk in the Young Finns Study and the Special Turku Coronary Risk Factor Intervention Project. Pediatr. Nephrol. 2011, 27, 1441–1452. [Google Scholar] [CrossRef]
- Borden, W.B.; Davidson, M.H. Updating the Assessment of Cardiac Risk: Beyond Framingham. Rev. Cardiovasc. Med. 2009, 10, 63–71. [Google Scholar]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, T.; Akasaka, T. Biomarkers Associated with Vulnerable Atheromatous Plaque. Curr. Med. Chem. 2012, 19, 2588–2596. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Morrow, D.A.; Sabatine, M.S.; Murphy, S.A.; Gibson, C.M.; Antman, E.M.; McCabe, C.H.; Cannon, C.P.; Braunwald, E. Association Between Plasma Levels of Monocyte Chemoattractant Protein-1 and Long-Term Clinical Outcomes in Patients With Acute Coronary Syndromes. Circulation 2003, 107, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Kervinen, H.; Mänttäri, M.; Kaartinen, M.; Mäkynen, H.; Palosuo, T.; Pulkki, K.; Kovanen, P.T. Prognostic usefulness of plasma monocyte/macrophage and T-lymphocyte activation markers in patients with acute coronary syndromes. Am. J. Cardiol. 2004, 94, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Haim, M.; Tanne, D.; Boyko, V.; Reshef, T.; Goldbourt, U.; Battler, A.; Mekori, Y.A.; Behar, S. Monocyte chemoattractant protein-1 and recurrent cardiovascular events in patients with stable coronary heart disease. Clin. Cardiol. 2005, 28, 31–35. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Morrow, D.A.; Blazing, M.A.; Jarolim, P.; Wiviott, S.D.; Sabatine, M.S.; Califf, R.M.; Braunwald, E. Serial measurement of monocyte chemo-attractant protein-1 after acute coronary syndromes: Results from the A to Z trial. J. Am. Coll. Cardiol. 2007, 50, 2117–2124. [Google Scholar] [CrossRef]
- Kuriyama, N.; Mizuno, T.; Kita, M.; Nagakane, Y.; Hosomi, A.; Harada, S.; Takeda, K.; Ozasa, K.; Yamada, K.; Tokuda, T.; et al. Predictive Markers of Blood Cytokine and Chemokine in Recurrent Brain Infarction. J. Interf. Cytokine Res. 2009, 29, 729–734. [Google Scholar] [CrossRef]
- Davi, G.; Tuttolomondo, A.; Santilli, F.; Basili, S.; Ferrante, E.; Di Raimondo, D.; Pinto, A.; Licata, G. CD40 ligand and MCP-1 as predictors of car-diovascular events in diabetic patients with stroke. J. Atheroscler. Thromb. 2009, 16, 707–713. [Google Scholar] [CrossRef]
- Frazier, L.; Vaughn, W.K.; Willerson, J.T.; Ballantyne, C.M.; Boerwinkle, E. Inflammatory Protein Levels and Depression Screening After Coronary Stenting Predict Major Adverse Coronary Events. Biol. Res. Nurs. 2009, 11, 163–173. [Google Scholar] [CrossRef]
- de Jager, S.C.; Kraaijeveld, A.O.; Grauss, R.W.; de Jager, W.; Liem, S.-S.; van der Hoeven, B.L.; Prakken, B.J.; Putter, H.; van Berkel, T.J.; Atsma, D.E.; et al. CCL3 (MIP-1α) levels are elevated during acute coronary syndromes and show strong prognostic power for future ischemic events. J. Mol. Cell. Cardiol. 2008, 45, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Tatara, Y.; Ohishi, M.; Yamamoto, K.; Shiota, A.; Hayashi, N.; Iwamoto, Y.; Takeda, M.; Takagi, T.; Katsuya, T.; Ogihara, T.; et al. Macrophage inflammatory protein-1β induced cell adhesion with increased intracellular reactive oxygen species. J. Mol. Cell. Cardiol. 2009, 47, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Eng, C.; Chopra, V.; Clark, L.T.; Pinsky, D.J.; Marmur, J.D. Low Plasma RANTES Levels Are an Independent Predictor of Cardiac Mortality in Patients Referred for Coronary Angiography. Arter. Thromb. Vasc. Biol. 2007, 27, 929–935. [Google Scholar] [CrossRef]
- Yndestad, A.; Finsen, A.V.; Ueland, T.; Husberg, C.; Dahl, C.P.; Øie, E.; Vinge, L.E.; Sjaastad, I.; Sandanger, Ø.; Ranheim, T.; et al. The Homeostatic Chemokine CCL21 Predicts Mortality and May Play a Pathogenic Role in Heart Failure. PLoS ONE 2012, 7, e33038. [Google Scholar] [CrossRef]
- Falcone, C.; Minoretti, P.; D’Angelo, A.; Buzzi, M.P.; Coen, E.; Emanuele, E.; Aldeghi, A.; Olivieri, V.; Geroldi, D. Markers of eosinophilic inflammation and risk pre-diction in patients with coronary artery disease. Eur. J. Clin. Invest. 2006, 36, 211–217. [Google Scholar] [CrossRef]
- Canouï-Poitrine, F.; Luc, G.; Mallat, Z.; Machez, E.; Bingham, A.; Ferrieres, J.; Ruidavets, J.-B.; Montaye, M.; Yarnell, J.; Haas, B.; et al. Systemic chemokine levels, coronary heart disease, and ischemic stroke events: The PRIME study. Neurology 2011, 77, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Schutt, R.C.; Burdick, M.D.; Strieter, R.M.; Mehrad, B.; Keeley, E.C. Plasma CXCL12 Levels as a Predictor of Future Stroke. Stroke 2012, 43, 3382–3386. [Google Scholar] [CrossRef]
- Kim, Y.S.; Baek, W.; Kim, M.K.; Kim, H.Y.; Lee, K.-Y.; Lee, Y.J.; Kim, S.H.; Chang, D.-I.; Koh, S.-H. Association between Serum Stromal Cell-Derived Factor-1α and Long-Term Outcome of Acute Ischemic Stroke. Eur. Neurol. 2012, 67, 363–369. [Google Scholar] [CrossRef]
- Fortunato, O.; Spinetti, G.; Specchia, C.; Cangiano, E.; Valgimigli, M.; Madeddu, P. Migratory activity of circulating progenitor cells and serum SDF-1α predict adverse events in patients with myocardial infarction. Cardiovasc. Res. 2013, 100, 192–200. [Google Scholar] [CrossRef]
- Tan, K.; Lu, S.; Chen, Y.; Song, X.; Wu, X.; Jin, Z.; Yuan, F.; Zhou, Y.; Li, H.; Yang, T.; et al. CXC Chemokine Ligand 16 as a Prognostic Marker in Patients with Intermediate Coronary Artery Lesions: A 2-Year Follow-Up Study. Tohoku J. Exp. Med. 2011, 223, 277–283. [Google Scholar] [CrossRef][Green Version]
- Ueland, T.; Smedbakken, L.; Hallén, J.; Atar, D.; Januzzi, J.; Halvorsen, B.; Jensen, J.; Aukrust, P. Soluble CXCL16 and long-term outcome in acute ischemic stroke. Atherosclerosis 2012, 220, 244–249. [Google Scholar] [CrossRef]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. New Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Chiovato, L. The chemokine system as a therapeutic target in autoimmune thyroid diseases: A focus on the inter-feron-gamma inducible chemokines and their receptor. Curr. Pharm. Des. 2011, 17, 3202–3216. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Chemokines in ischemia and reperfusion. Thromb. Haemost. 2007, 97, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Fish, J.E.; White, M.D.; Yu, S.; Smyth, J.W.; Shaw, R.M.; DiMaio, J.M.; Srivastava, D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation 2008, 117, 2224–2231. [Google Scholar] [CrossRef]
- Morimoto, H.; Hirose, M.; Takahashi, M.; Kawaguchi, M.; Ise, H.; Kolattukudy, P.E.; Yamada, M.; Ikeda, U. MCP-1 induces cardioprotection against ischaemia/reperfusion injury: Role of reactive oxygen species. Cardiovasc. Res. 2008, 78, 554–562. [Google Scholar] [CrossRef]
- Liehn, E.A.; Postea, O.; Curaj, A.; Marx, N. Repair after myocardial infarction, between fantasy and reality: The role of chemokines. J. Am. Coll. Cardiol. 2011, 58, 2357–2362. [Google Scholar] [CrossRef]
- Ruscher, K.; Kuric, E.; Liu, Y.; Walter, H.L.; Issazadeh-Navikas, S.; Englund, E.; Wieloch, T. Inhibition of CXCL12 Signaling Attenuates the Postischemic Immune Response and Improves Functional Recovery after Stroke. J. Cereb. Blood Flow. Metab. 2013, 33, 1225–1234. [Google Scholar] [CrossRef]
- Bogoslovsky, T.; Spatz, M.; Chaudhry, A.; Maric, D.; Luby, M.; Frank, J.; Warach, S. Stromal-Derived Factor-1α Correlates With Circulating Endothelial Progenitor Cells and With Acute Lesion Volume in Stroke Patients. Stroke 2011, 42, 618–625. [Google Scholar] [CrossRef]
- Carbone, F.; Nencioni, A.; Mach, F.; Vuilleumier, N.; Montecucco, F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb. Haemost. 2013, 110, 501–514. [Google Scholar] [CrossRef] [PubMed]
- van Tits, L.; Stienstra, R.; van Lent, P.; Netea, M.; Joosten, L.; Stalenhoef, A. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: A crucial role for Krüppel-like factor 2. Atherosclerosis 2011, 214, 345–349. [Google Scholar] [CrossRef]
- Liu, Y.; Hultén, L.M.; Wiklund, O. Macrophages Isolated From Human Atherosclerotic Plaques Produce IL-8, and Oxysterols May Have a Regulatory Function for IL-8 Production. Arter. Thromb. Vasc. Biol. 1997, 17, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, W.A.; Santiago, R.; Curtiss, L.K.; Terkeltaub, R.A. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J. Clin. Investig. 1998, 101, 353–363. [Google Scholar] [CrossRef]
- Kukielka, G.L.; Smith, C.W.; LaRosa, G.J.; Manning, A.M.; Mendoza, L.H.; Daly, T.J.; Hughes, B.J.; Youker, K.A.; Hawkins, H.K.; Michael, L.H. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J. Clin. Investig. 1995, 95, 89–103. [Google Scholar] [CrossRef]
- Boyle, E.M.; Kovacich, J.C.; Hèbert, C.A.; Canty, T.G.; Chi, E.; Morgan, E.N.; Pohlman, T.H.; Verrier, E.D. Inhibition Of Interleukin-8 Blocks Myocardial Ischemia-Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 1998, 116, 114–121. [Google Scholar] [CrossRef]
- Montecucco, F.; Lenglet, S.; Gayet-Ageron, A.; Bertolotto, M.; Pelli, G.; Palombo, D.; Pane, B.; Spinella, G.; Steffens, S.; Raffaghello, L.; et al. Systemic and Intraplaque Mediators of Inflammation Are Increased in Patients Symptomatic for Ischemic Stroke. Stroke 2010, 41, 1394–1404. [Google Scholar] [CrossRef]
- Grau, A.J.; Reis, A.; Buggle, F.; Al-Khalaf, A.; Werle, E.; Valois, N.; Bertram, M.; Becher, H.; Grond-Ginsbach, C. Monocyte function and plasma levels of interleukin-8 in acute ischemic stroke. J. Neurol. Sci. 2001, 192, 41–47. [Google Scholar] [CrossRef]
- Tarkowski, E.; Rosengren, L.; Blomstrand, C.; Wikkelsö, C.; Jensen, C.; Ekholm, S.; Tarkowski, A. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin. Exp. Immunol. 1997, 110, 492–499. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Smith, J.B.; Freeman, G.L. Ischemia-Reperfusion of Rat Myocardium Activates Nuclear Factor-κB and Induces Neutrophil Infiltration Via Lipopolysaccharide-Induced CXC Chemokine. Circulation 2001, 103, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Goodison, S.; Urquidi, V.; Giacoia, E.G.; Rosser, C.J. Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Mod. Pathol. 2013, 93, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.; Schuster, M.; Bonaros, N.; Lietz, K.; Xiang, G.; Martens, T.; Kurlansky, P.; Sondermeijer, H.; Witkowski, P.; Boyle, A. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J. Mol. Cell. Cardiol. 2006, 40, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Moldobaeva, A.; Baek, A.; Eldridge, L.; Wagner, E.M. Differential activity of pro-angiogenic CXC chemokines. Microvasc. Res. 2010, 80, 18–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Losy, J.; Zaremba, J.; Skrobański, P. CXCL1 (GRO-alpha) chemokine in acute ischaemic stroke patients. Folia Neuropathol. 2005, 43, 97–102. [Google Scholar][Green Version]
- Struyf, S.; Salogni, L.; Burdick, M.D.; Vandercappellen, J.; Gouwy, M.; Noppen, S.; Proost, P.; Opdenakker, G.; Parmentier, M.; Gerard, C.; et al. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood 2011, 117, 480–488. [Google Scholar] [CrossRef]
- Yates-Binder, C.C.; Rodgers, M.; Jaynes, J.; Wells, A.; Bodnar, R.J.; Turner, T.; Proost, P. An IP-10 (CXCL10)-Derived Peptide Inhibits Angiogenesis. PLoS ONE 2012, 7, e40812. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Dobaczewski, M.; Gonzalez-Quesada, C.; Xia, Y.; Leucker, T.; Zymek, P.; Veeranna, V.; Tager, A.M.; Luster, A.D.; Frangogiannis, N.G. Induction of the CXC Chemokine Interferon-γ-Inducible Protein 10 Regulates the Reparative Response Following Myocardial Infarction. Circ. Res. 2009, 105, 973–983. [Google Scholar] [CrossRef]
- Sokolov, V.O.; Krasnikova, T.L.; Prokofieva, L.V.; Kukhtina, N.B.; Arefieva, T.I. Expression of markers of regulatory CD4+CD25+foxp3+ cells in atherosclerotic plaques of human coronary arteries. Bull. Exp. Biol. Med. 2009, 147, 726–729. [Google Scholar] [CrossRef]
- Takahashi, M. Role of the SDF-1/CXCR4 System in Myocardial Infarction. Circ. J. 2010, 74, 418–423. [Google Scholar] [CrossRef]
- Schönemeier, B.; Schulz, S.; Hoellt, V.; Stumm, R. Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J. Neuroimmunol. 2008, 198, 39–45. [Google Scholar] [CrossRef]
- Penn, M.S.; Pastore, J.; Miller, T.; Aras, R. SDF-1 in myocardial repair. Gene Ther. 2012, 19, 583–587. [Google Scholar] [CrossRef]
- Bakondi, B.; Shimada, I.S.; Peterson, B.M.; Spees, J.L. SDF-1α Secreted by Human CD133-Derived Multipotent Stromal Cells Promotes Neural Progenitor Cell Survival Through CXCR7. Stem Cells Dev. 2011, 20, 1021–1029. [Google Scholar] [CrossRef]
- Peng, H.; Wu, Y.; Duan, Z.; Ciborowski, P.; Zheng, J.C. Proteolytic processing of SDF-1α by matrix metalloproteinase-2 impairs CXCR4 signaling and reduces neural progenitor cell migration. Protein Cell 2012, 3, 875–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.L.; Zhang, P.F.; Ding, S.F.; Wang, Y.; Zhang, M.; Zhao, Y.X.; Ni, M.; Zhang, Y.; Jandeleit-Dahm, K. Local Gene Silencing of Monocyte Chemoattractant Protein-1 Prevents Vulnerable Plaque Disruption in Apolipoprotein E-Knockout Mice. PLoS ONE 2012, 7, e33497. [Google Scholar] [CrossRef] [PubMed]
- Martinovic, I.; Abegunewardene, N.; Seul, M.; Vosseler, M.; Horstick, G.; Buerke, M.; Darius, H.; Lindemann, S. Elevated Monocyte Chemoattractant Protein-1 Serum Levels in Patients at Risk for Coronary Artery Disease. Circ. J. 2005, 69, 1484–1489. [Google Scholar] [CrossRef]
- Kumar, A.G.; Ballantyne, C.M.; Michael, L.H.; Kukielka, G.L.; Youker, K.A.; Lindsey, M.L.; Hawkins, H.K.; Birdsall, H.H.; MacKay, C.R.; LaRosa, G.J.; et al. Induction of Monocyte Chemoattractant Protein-1 in the Small Veins of the Ischemic and Reperfused Canine Myocardium. Circulation 1997, 95, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Hayasaki, T.; Kaikita, K.; Okuma, T.; Yamamoto, E.; Kuziel, W.A.; Ogawa, H.; Takeya, M. CC Chemokine Receptor-2 Deficiency Attenuates Oxidative Stress and Infarct Size Caused by Myocardial Ischemia-Reperfusion in Mice. Circ. J. 2006, 70, 342–351. [Google Scholar] [CrossRef]
- Kaikita, K.; Hayasaki, T.; Okuma, T.; Kuziel, W.A.; Ogawa, H.; Takeya, M. Targeted Deletion of CC Chemokine Receptor 2 Attenuates Left Ventricular Remodeling after Experimental Myocardial Infarction. Am. J. Pathol. 2004, 165, 439–447. [Google Scholar] [CrossRef]
- Dewald, O.; Zymek, P.; Winkelmann, K.; Koerting, A.; Ren, G.; Abou-Khamis, T.; Michael, L.H.; Rollins, B.J.; Entman, M.L.; Frangogiannis, N.G. CCL2/Monocyte Chemoattractant Protein-1 Regulates Inflammatory Responses Critical to Healing Myocardial Infarcts. Circ. Res. 2005, 96, 881–889. [Google Scholar] [CrossRef]
- Haudek, S.B.; Xia, Y.; Huebener, P.; Lee, J.M.; Carlson, S.; Crawford, J.R.; Pilling, D.; Gomer, R.H.; Trial, J.; Frangogiannis, N.G.; et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 18284–18289. [Google Scholar] [CrossRef]
- Hughes, P.M.; Allegrini, P.R.; Rudin, M.; Perry, V.H.; Mir, A.K.; Wiessner, C. Monocyte chemoattractant protein-1 deficiency is pro-tective in a murine stroke model. J. Cereb. Blood Flow. Metab. 2002, 22, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Absence of the Chemokine Receptor CCR2 Protects Against Cerebral Ischemia/Reperfusion Injury in Mice. Stroke 2007, 38, 1345–1353. [Google Scholar] [CrossRef]
- Yang, B.; Xi, X.; Aronowski, J.; Savitz, S.I. Ischemic Stroke May Activate Bone Marrow Mononuclear Cells to Enhance Recovery After Stroke. Stem Cells Dev. 2012, 21, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-P.; A Sailor, K.; Lang, B.T.; Park, S.-W.; Vemuganti, R.; Dempsey, R.J. Monocyte Chemoattractant Protein-1 Plays a Critical Role in Neuroblast Migration after Focal Cerebral Ischemia. J. Cereb. Blood Flow. Metab. 2007, 27, 1213–1224. [Google Scholar] [CrossRef]
- Shen, B.; Li, J.; Gao, L.; Zhang, J.; Yang, B. Role of CC-chemokine receptor 5 on myocardial ischemia-reperfusion injury in rats. Mol. Cell Biochem. 2013, 378, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Braunersreuther, V.; Lenglet, S.; Delattre, B.M.; Pelli, G.; Buatois, V.; Guilhot, F.; Galan, K.; Vuilleumier, N.; Ferlin, W.; et al. CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur. Hear. J. 2011, 33, 1964–1974. [Google Scholar] [CrossRef]
- Terao, S.; Yilmaz, G.; Stokes, K.Y.; Russell, J.; Ishikawa, M.; Kawase, T.; Granger, D.N. Blood Cell-Derived RANTES Mediates Cerebral Microvascular Dysfunction, Inflammation, and Tissue Injury After Focal Ischemia–Reperfusion. Stroke 2008, 39, 2560–2570. [Google Scholar] [CrossRef]
- Tokami, H.; Ago, T.; Sugimori, H.; Kuroda, J.; Awano, H.; Suzuki, K.; Kiyohara, Y.; Kamouchi, M.; Kitazono, T. RANTES has a potential to play a neuroprotective role in an autocrine/paracrine manner after ischemic stroke. Brain Res. 2013, 1517, 122–132. [Google Scholar] [CrossRef]
- Proulx, C.; El-Helou, V.; Gosselin, H.; Clement, R.; Gillis, M.-A.; Villeneuve, L.; Calderone, A. Antagonism of stromal cell-derived factor-1α reduces infarct size and improves ventricular function after myocardial infarction. Pflug. Arch. Eur. J. Physiol. 2007, 455, 241–250. [Google Scholar] [CrossRef]
- Morimoto, H.; Takahashi, M.; Shiba, Y.; Izawa, A.; Ise, H.; Hongo, M.; Hatake, K.; Motoyoshi, K.; Ikeda, U. Bone Marrow-Derived CXCR4+ Cells Mobilized by Macrophage Colony-Stimulating Factor Participate in the Reduction of Infarct Area and Improvement of Cardiac Remodeling after Myocardial Infarction in Mice. Am. J. Pathol. 2007, 171, 755–766. [Google Scholar] [CrossRef]
- Jujo, K.; Hamada, H.; Iwakura, A.; Thorne, T.; Sekiguchi, H.; Clarke, T.; Ito, A.; Misener, S.; Tanaka, T.; Klyachko, E.; et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc. Natl. Acad. Sci. USA 2010, 107, 11008–11013. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Yuan, F.; Mu, J.; Li, C.; Chen, N.; Guo, S.; Kingery, J.; Prabhu, S.D.; Bolli, R.; Rokosh, G. Chronic AMD3100 antagonism of SDF-1α–CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J. Mol. Cell. Cardiol. 2010, 49, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gu, H.; Zhang, W.; Manukyan, M.C.; Shou, W.; Wang, M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am. J. Physiol. Circ. Physiol. 2011, 301, H1496–H1505. [Google Scholar] [CrossRef]
- Jujo, K.; Ii, M.; Sekiguchi, H.; Klyachko, E.; Misener, S.; Tanaka, T.; Tongers, J.; Roncalli, J.; Renault, M.-A.; Thorne, T.; et al. CXC-Chemokine Receptor 4 Antagonist AMD3100 Promotes Cardiac Functional Recovery After Ischemia/Reperfusion Injury via Endothelial Nitric Oxide Synthase–Dependent Mechanism. Circulation 2013, 127, 63–73. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, X.; Zhou, X.; Ji, W.; Zhang, L.; Luo, T.; Liu, H.; Huang, T.; Jiang, T.; Li, Y. Short-term intermittent administration of CXCR4 antagonist AMD3100 facilitates myocardial repair in experimental myocardial infarction. Acta Biochim. Biophys. Sin. 2013, 45, 561–569. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Li, Y.; Tang, Y.; Tang, G.; Yang, G.-Y.; Wang, Y. CXCR4 Antagonist AMD3100 Protects Blood–Brain Barrier Integrity and Reduces Inflammatory Response After Focal Ischemia in Mice. Stroke 2013, 44, 190–197. [Google Scholar] [CrossRef]
- Garau, A.; Bertini, R.; Colotta, F.; Casilli, F.; Bigini, P.; Cagnotto, A.; Mennini, T.; Ghezzi, P.; Villa, P. Neuroprotection with the CXCL8 inhibitor repertaxin in tran-sient brain ischemia. Cytokine 2005, 30, 125–131. [Google Scholar] [CrossRef]
- Villa, P.; Triulzi, S.; Cavalieri, B.; Di Bitondo, R.; Bertini, R.; Barbera, S.; Bigini, P.; Mennini, T.; Gelosa, P.; Tremoli, E.; et al. The Interleukin-8 (IL-8/CXCL8) Receptor Inhibitor Reparixin Improves Neurological Deficits and Reduces Long-term Inflammation in Permanent and Transient Cerebral Ischemia in Rats. Mol. Med. 2007, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.d.C.; Coelho, F.M.; Rodrigues, D.H.; Campos, A.C.; Barcelos, L.d.S.; Teixeira, M.M.; Rachid, M.A.; Teixeira, A.L. Blockade of CXCR1/2 chemokine receptors protects against brain damage in ischemic stroke in mice. Clinics 2013, 68, 391–394. [Google Scholar] [CrossRef]
- Kanki, S.; Segers, V.F.; Wu, W.; Kakkar, R.; Gannon, J.; Sys, S.U.; Sandrasagra, A.; Lee, R.T. Stromal Cell-Derived Factor-1 Retention and Cardioprotection for Ischemic Myocardium. Circ. Hear. Fail. 2011, 4, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, J.; Ban, C.; Ahn, K.; Cheong, J.; Kim, H.; Kim, J.; Park, Y.; Kim, J.; Chun, K.; et al. Stromal Cell Derived Factor-1 (SDF-1) Targeting Reperfusion Reduces Myocardial Infarction in Isolated Rat Hearts. Cardiovasc. Ther. 2011, 30, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Braunersreuther, V.; Pellieux, C.; Pelli, G.; Burger, F.; Steffens, S.; Montessuit, C.; Weber, C.; Proudfoot, A.; Mach, F.; Arnaud, C. Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice. J. Mol. Cell. Cardiol. 2010, 48, 789–798. [Google Scholar] [CrossRef]
- Montecucco, F.; Lenglet, S.; Braunersreuther, V.; Pelli, G.; Pellieux, C.; Montessuit, C.; Lerch, R.; Deruaz, M.; Proudfoot, A.E.; Mach, F. Single Administration of the CXC Chemokine-Binding Protein Evasin-3 During Ischemia Prevents Myocardial Reperfusion Injury in Mice. Arter. Thromb. Vasc. Biol. 2010, 30, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Copin, J.-C.; da Silva, R.F.; A Fraga-Silva, R.; Capettini, L.; Quintao, S.; Lenglet, S.B.; Pelli, G.; Galan, K.; Burger, F.; Braunersreuther, V.; et al. Treatment with Evasin-3 Reduces Atherosclerotic Vulnerability for Ischemic Stroke, but Not Brain Injury in Mice. J. Cereb. Blood Flow. Metab. 2012, 33, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Kanzler, I.; Tuchscheerer, N.; Curaj, A.; Simsekyilmaz, S.; Sönmez, T.T.; Radu, E.; Postea, O.; Weber, C.; Schuh, A.; et al. CXC chemokine KC fails to induce neutrophil infiltration and neoangiogenesis in a mouse model of myocardial infarction. J. Mol. Cell. Cardiol. 2013, 60, 1–7. [Google Scholar] [CrossRef]
- Liehn, E.A.; Piccinini, A.-M.; Koenen, R.R.; Soehnlein, O.; Adage, T.; Fatu, R.; Curaj, A.; Popescu, A.; Zernecke, A.; Kungl, A.J.; et al. A New Monocyte Chemotactic Protein-1/Chemokine CC Motif Ligand-2 Competitor Limiting Neointima Formation and Myocardial Ischemia/Reperfusion Injury in Mice. JACC 2010, 56, 1847–1857. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Steffens, S.; Arnaud, C.; Pelli, G.; Burger, F.; Proudfoot, A.; Mach, F. A Novel RANTES Antagonist Prevents Progression of Established Atherosclerotic Lesions in Mice. Arter. Thromb. Vasc. Biol. 2008, 28, 1090–1096. [Google Scholar] [CrossRef]
- Jantunen, E. Novel strategies for blood stem cell mobilization: Special focus on plerixafor. Expert. Opin. Biol. Ther. 2011, 11, 1241–1248. [Google Scholar] [CrossRef]
- DérUaz, M.; Frauenschuh, A.; Alessandri, A.L.; Dias, J.M.; Coelho, F.M.; Russo, R.C.; Ferreira, B.R.; Graham, G.J.; Shaw, J.P.; Wells, T.N.; et al. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J. Exp. Med. 2008, 205, 2019–2031. [Google Scholar] [CrossRef]
- Di Filippo, C.; Rossi, F.; Rossi, S.; D’aMico, M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: Involvement of cytokine/chemokines and PMN. J. Leukoc. Biol. 2003, 75, 453–459. [Google Scholar] [CrossRef]
- Montecucco, F.; Lenglet, S.; Braunersreuther, V.; Burger, F.; Pelli, G.; Bertolotto, M.; Mach, F.; Steffens, S. CB(2) cannabinoid receptor activation is car-dioprotective in a mouse model of ischemia/reperfusion. J. Mol. Cell Cardiol. 2009, 46, 612–620. [Google Scholar] [CrossRef]
- Murikinati, S.; Jüttler, E.; Keinert, T.; Ridder, D.A.; Muhammad, S.; Waibler, Z.; Ledent, C.; Zimmer, A.; Kalinke, U.; Schwaninger, M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2009, 24, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Faustino, J.; Derugin, N.; Wendland, M.; Lizasoain, I.; Moro, M.; Vexler, Z. Reduced infarct size and accumulation of microglia in rats treated with WIN 55,212-2 after neonatal stroke. Neuroscience 2012, 207, 307–315. [Google Scholar] [CrossRef]
- Huber, B.C.; Brunner, S.; Segeth, A.; Nathan, P.; Fischer, R.; Zaruba, M.M.; Vallaster, M.; Theiss, H.D.; David, R.; Gerbitz, A.; et al. Parathyroid hormone is a DPP-IV inhibitor and increases SDF-1-driven homing of CXCR4+ stem cells into the ischaemic heart. Cardiovasc. Res. 2011, 90, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Subramanian, S.; Dziennis, S.; Jia, J.; Uchida, M.; Akiyoshi, K.; Migliati, E.; Lewis, A.D.; Vandenbark, A.A.; Offner, H.; et al. Estradiol and G1 reduce infarct size and improve im-munosuppression after experimental stroke. J. Immunol. 2010, 184, 4087–4094. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, X.; Zeng, Z.; Liu, W.; Wang, B.; Wang, H. Estrogen-replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing SDF-1 and estrogen receptor expression. Microvasc. Res. 2009, 77, 71–77. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, L.; Han, Y.; Teng, Y.; Sun, J.; Xie, R.; Cao, J. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. Eur. J. Pharmacol. 2012, 684, 116–124. [Google Scholar] [CrossRef]
- Sironi, L.; Banfi, C.; Brioschi, M.; Gelosa, P.; Guerrini, U.; Nobili, E.; Gianella, A.; Paoletti, R.; Tremoli, E.; Cimino, M. Activation of NF-kB and ERK1/2 after permanent focal ischemia is abolished by simvastatin treatment. Neurobiol. Dis. 2006, 22, 445–451. [Google Scholar] [CrossRef]
- Iwata, A.; Miura, S.-I.; Shirai, K.; Kawamura, A.; Tomita, S.; Matsuo, Y.; Zhang, B.; Nishikawa, H.; Kumagai, K.; Matsuo, K.; et al. Lower Level of Low-density Lipoprotein Cholesterol by Statin Prevents Progression of Coronary Restenosis after Successful Stenting in Acute Myocardial Infarction. Intern. Med. 2006, 45, 885–890. [Google Scholar] [CrossRef][Green Version]
- Cui, X.; Chopp, M.; Zacharek, A.; Roberts, C.; Lu, M.; Savant-Bhonsale, S.; Chen, J. Chemokine, vascular and therapeutic effects of com-bination Simvastatin and BMSC treatment of stroke. Neurobiol. Dis. 2009, 36, 35–41. [Google Scholar] [CrossRef]
- Qiu, R.; Cai, A.; Dong, Y.; Zhou, Y.; Yu, D.; Huang, Y.; Zheng, D.; Rao, S.; Feng, Y.; Mai, W. SDF-1α upregulation by atorvastatin in rats with acute myocardial infarction via nitric oxide production confers anti-inflammatory and anti-apoptotic effects. J. Biomed. Sci. 2012, 19, 99. [Google Scholar] [CrossRef]
- Wayman, N.S.; Hattori, Y.; McDonald, M.C.; Mota-Filipe, H.; Cuzzocrea, S.; Pisano, B.; Chatterjee, P.K.; Thiemermann, C. Ligands of the peroxisome prolifera-tor-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. Faseb J. 2002, 16, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Nakano, A.; Kinoshita, M.; Matsumori, A. Pioglitazone, a Peroxisome Proliferator-Activated Receptor-γ Agonist, Attenuates Myocardial Ischemia/Reperfusion Injury in a Rat Model. Mod. Pathol. 2003, 83, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamada, Y.; Shimomura, H.; Nagayoshi, Y.; Tsujita, K.; Yamashita, T.; Fukuda, M.; Ohba, K.; Nako, H.; Ogura, Y.; et al. The effect of edaravone on plasma monocyte chemoattractant protein-1 levels in patients with acute myocardial infarction. J. Cardiol. 2009, 54, 416–424. [Google Scholar] [CrossRef]
- Montecucco, F.; Bauer, I.; Braunersreuther, V.; Bruzzone, S.; Akhmedov, A.; Luscher, T.F.; Speer, T.; Poggi, A.; Mannino, E.; Pelli, G.; et al. Inhibition of nicotinamide phos-phoribosyltransferase reduces neutrophil-mediated injury in myocardial infarction. Antioxid. Redox Signal 2013, 18, 630–641. [Google Scholar] [CrossRef]
- Horuk, R. Chemokine receptor antagonists: Overcoming developmental hurdles. Nat. Rev. Drug Discov. 2008, 8, 23–33. [Google Scholar] [CrossRef]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.N.; Paludan, S.R. Blocking CC Chemokine Receptor (CCR) 1 and CCR5 During Herpes Simplex Virus Type 2 Infection In Vivo Impairs Host Defence and Perturbs the Cytokine Response. Scand. J. Immunol. 2004, 59, 321–333. [Google Scholar] [CrossRef]
- Wallis, R.S. Infectious complications of tumor necrosis factor blockade. Curr. Opin. Infect. Dis. 2009, 22, 403–409. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Kontoyiannis, D.P. Glucocorticoids and invasive fungal infections. Lancet 2003, 362, 1828–1838. [Google Scholar] [CrossRef]
 |
 |
 |
© 2013 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Carbone, F.; Montecucco, F. Antichemokine Treatments in Acute Ischaemic Cardiovascular Diseases. Cardiovasc. Med. 2013, 16, 225. https://doi.org/10.4414/cvm.2013.00182
Carbone F, Montecucco F. Antichemokine Treatments in Acute Ischaemic Cardiovascular Diseases. Cardiovascular Medicine. 2013; 16(9):225. https://doi.org/10.4414/cvm.2013.00182
Chicago/Turabian StyleCarbone, Federico, and Fabrizio Montecucco. 2013. "Antichemokine Treatments in Acute Ischaemic Cardiovascular Diseases" Cardiovascular Medicine 16, no. 9: 225. https://doi.org/10.4414/cvm.2013.00182
APA StyleCarbone, F., & Montecucco, F. (2013). Antichemokine Treatments in Acute Ischaemic Cardiovascular Diseases. Cardiovascular Medicine, 16(9), 225. https://doi.org/10.4414/cvm.2013.00182




