Current Outcome of Acute Coronary Syndromes: Data from the Zurich-Acute Coronary Syndrome (Z-ACS) Registry
Abstract
Introduction
Methods
Results
Discussion
Author Contributions
Acknowledgments
Funding/potential competing interests
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third Universal Definition of Myocardial Infarction. Journal of the American College of Cardiology 2012. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001, 104, 365–372. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. Nat Rev Cardiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Jr Collins, A.; Ferrari, R.; et al. Our Time: A Call to Save Preventable Death From Cardiovascular Disease (Heart Disease and Stroke). Circulation. 2012. [Google Scholar] [CrossRef]
- Steg, P.G.; Goldberg, R.J.; Gore, J.M.; et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol 2002, 90, 358–363. [Google Scholar] [CrossRef]
- Steg, P.G.; James, S.K.; Atar, D.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J. 2012. [Google Scholar]
- Fox, K.A.; Goodman, S.G.; Klein, W.; et al. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2002, 23, 1177–1189. [Google Scholar] [CrossRef]
- Caro, J.J.; Migliaccio-Walle, K. Generalizing the results of clinical trials to actual practice: the example of clopidogrel therapy for the prevention of vascular events. CAPRA (CAPRIE Actual Practice Rates Analysis) Study Group. Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events. Am J Med 1999, 107, 568–572. [Google Scholar]
- Steg, P.G.; Lopez-Sendon, J.; de Sa, E.L.; et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med 2007, 167, 68–73. [Google Scholar] [CrossRef]
- Bosch, X.; Delgado, V.; Verbal, F.; et al. Causes of ineligibility in randomized controlled trials and long-term mortality in patients with non-ST-segment elevation acute coronary syndromes. Int J Cardiol 2008, 124, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: a multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J 2001, 141, 190–199. [CrossRef]
- Eagle, K.A.; Goodman, S.G.; Avezum, A.; Budaj, A.; Sullivan, C.M.; Lopez-Sendon, J. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE). Lancet 2002, 359, 373–377. [Google Scholar] [CrossRef]
- Urban, P.; Radovanovic, D.; Erne, P.; et al. Impact of changing definitions for myocardial infarction: a report from the AMIS registry. Am J Med 2008, 121, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Radovanovic, D.; Hunziker, P.R.; et al. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med 2008, 149, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; et al. Third universal definition of myocardial infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Dallongeville, J.; Van Belle, E.; et al. STEMI and NSTEMI: are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur Heart J 2007, 28, 1409–1417. [Google Scholar] [CrossRef]
- Radovanovic, D.; Erne, P. AMIS Plus: Swiss registry of acute coronary syndrome. Heart 2010, 96, 917–921. [Google Scholar] [CrossRef]
- Polonski, L.; Gasior, M.; Gierlotka, M.; et al. A comparison of ST elevation versus non-ST elevation myocardial infarction outcomes in a large registry database: are non-ST myocardial infarctions associated with worse long-term prognoses? Int J Cardiol 2011, 152, 70–77. [Google Scholar] [CrossRef]
- Ino, Y.; Kubo, T.; Tanaka, A.; et al. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. JACC Cardiovasc Interv 2011, 4, 76–82. [Google Scholar] [CrossRef]
- Spencer, F.A.; Santopinto, J.J.; Gore, J.M.; et al. Impact of aspirin on presentation and hospital outcomes in patients with acute coronary syndromes (The Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol 2002, 90, 1056–1061. [Google Scholar] [CrossRef]
- Kastrati, A.; Neumann, F.J.; Schomig, A. Operator volume and outcome of patients undergoing coronary stent placement. J Am Coll Cardiol 1998, 32, 970–976. [Google Scholar] [CrossRef][Green Version]
- Vakili, B.A.; Kaplan, R.; Brown, D.L. Volume-outcome relation for physicians and hospitals performing angioplasty for acute myocardial infarction in New York state. Circulation 2001, 104, 2171–2176. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Gharacholou, S.M.; Lopes, R.D.; Alexander, K.P.; et al. Age and outcomes in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: findings from the APEX-AMI trial. Arch Intern Med 2011, 171, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.P.; Mallory, R.; Heiat, A.; Krumholz, H.M. Reporting the recruitment process in clinical trials: who are these patients and how did they get there? Ann Intern Med 2002, 137, 10–16. [Google Scholar] [CrossRef]
- King, S.B., 3rd; Barnhart, H.X.; Kosinski, A.S.; et al. Angioplasty or surgery for multivessel coronary artery disease: comparison of eligible registry and randomized patients in the EAST trial and influence of treatment selection on outcomes. Emory Angioplasty versus Surgery Trial Investigators. Am J Cardiol 1997, 79, 1453–1459. [Google Scholar] [CrossRef]
- Raber, L.; Kelbaek, H.; Ostoijc, M.; et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA 2012, 308, 777–787. [Google Scholar] [CrossRef]
- Hochman, J.S. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation 2003, 107, 2998–3002. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Windecker, S.; Roffi, M.; Kaiser, C.A.; Stauffer, J.C.; Pedrazzini, G.; et al. Interventional Cardiology in Switzerland during the year 2007, Cardiovascular Medicine. Cardiovascular Medicine 2010, 13, 18–24. [Google Scholar]
- Dorler, J.; Edlinger, M.; Alber, H.F.; et al. Clopidogrel pre-treatment is associated with reduced in-hospital mortality in primary percutaneous coronary intervention for acute ST-elevation myocardial infarction. Eur Heart J 2011, 32, 2954–2961. [Google Scholar] [CrossRef]
- Stauffer, J.C.; Goy, J.J.; Duvoisin, N.; Radovanovic, D.; Rickli, H.; Erne, P. Dramatic effect of early clopidogrel administration in reducing mortality and MACE rates in ACS patients. Data from the Swiss registry AMIS-Plus. Swiss Med Wkly 2012, 142, w13573. [Google Scholar] [CrossRef] [PubMed]
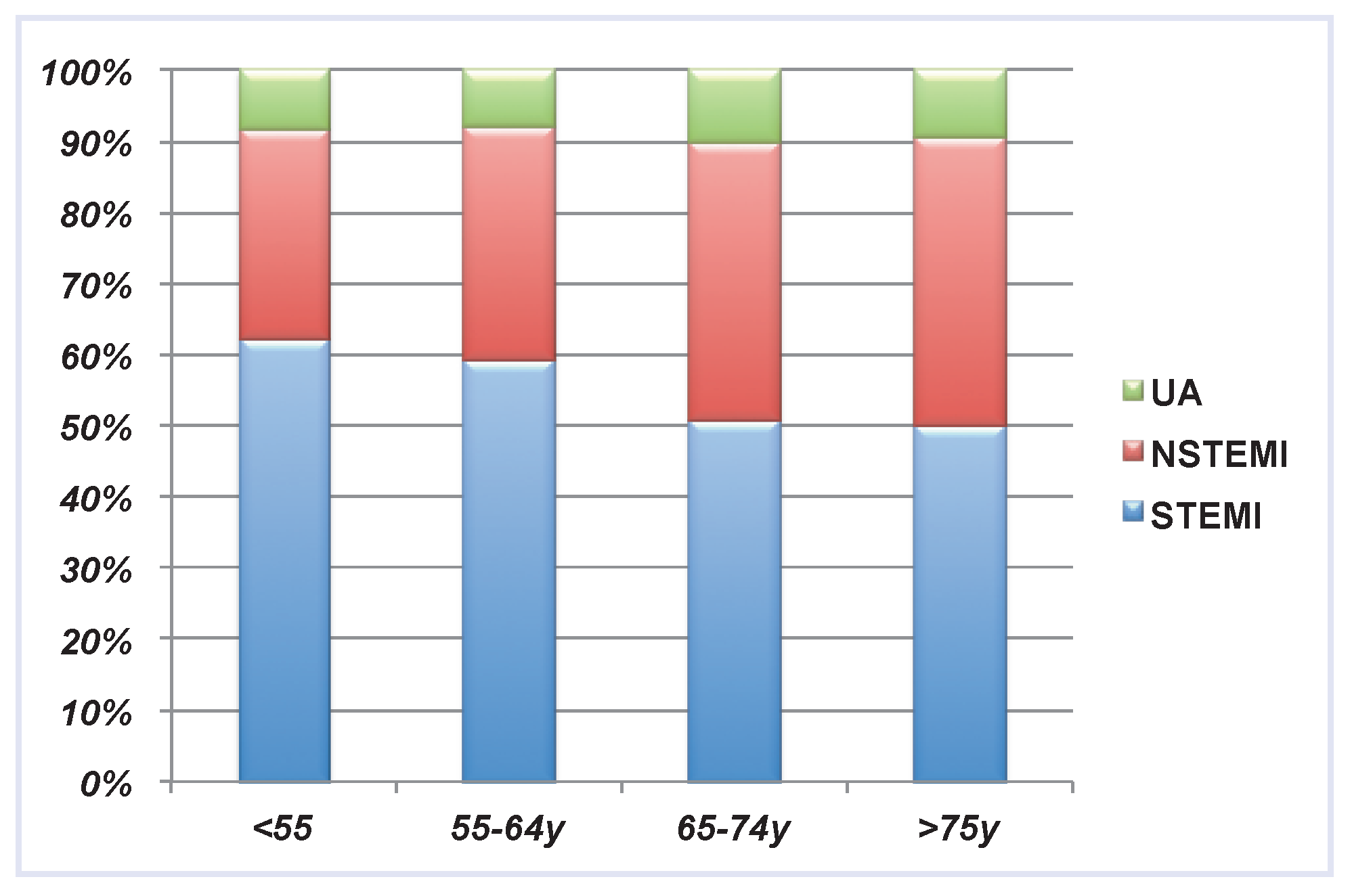
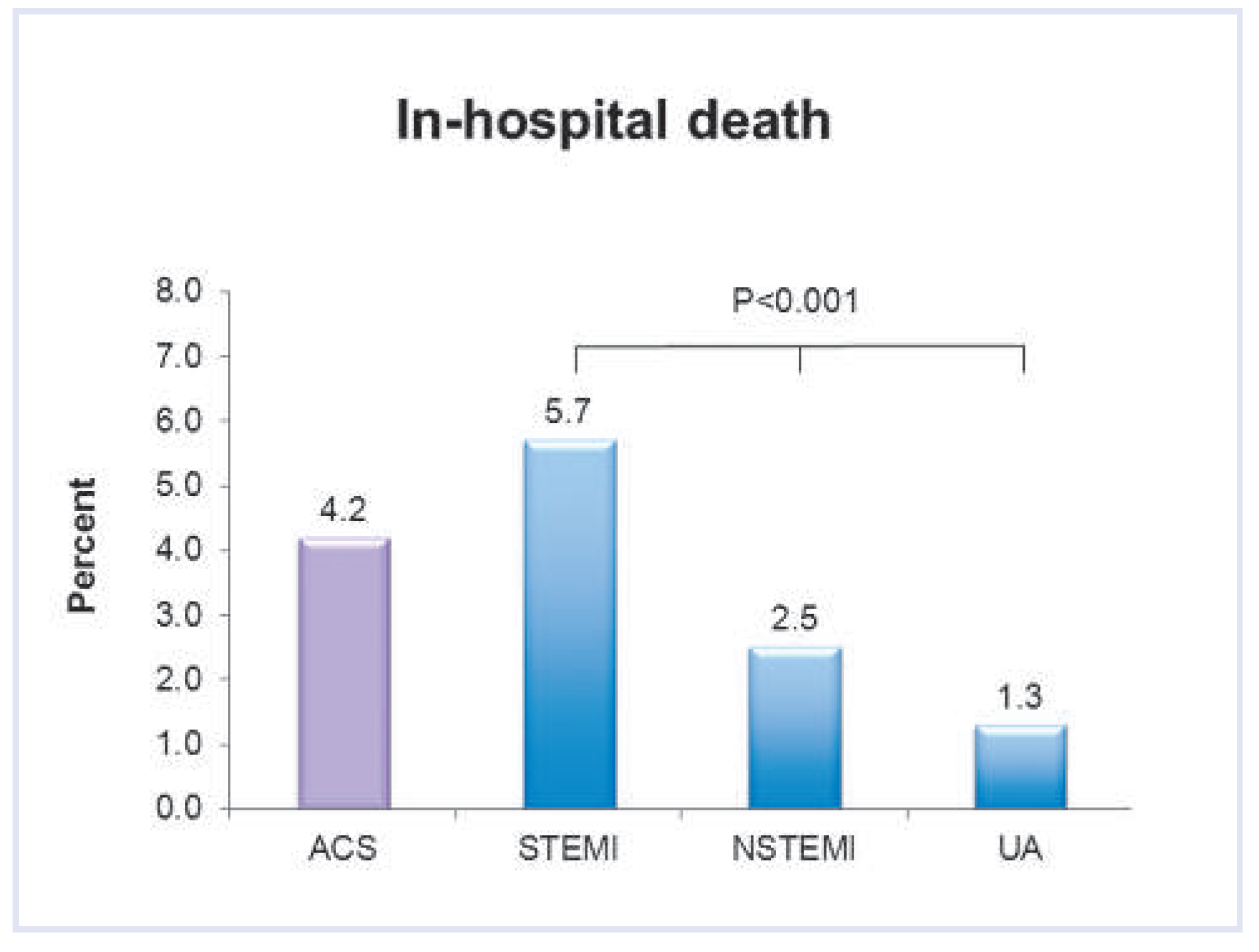
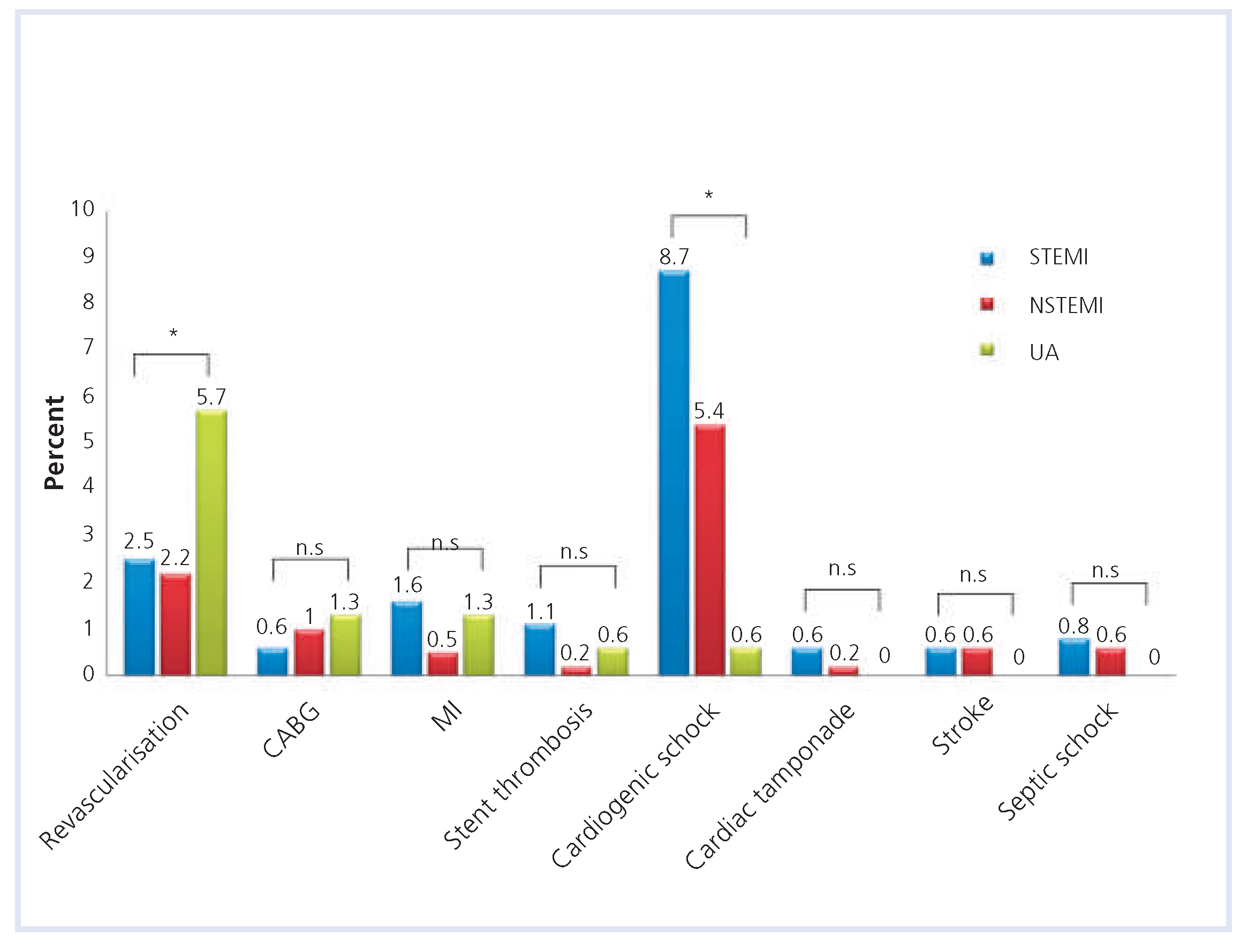
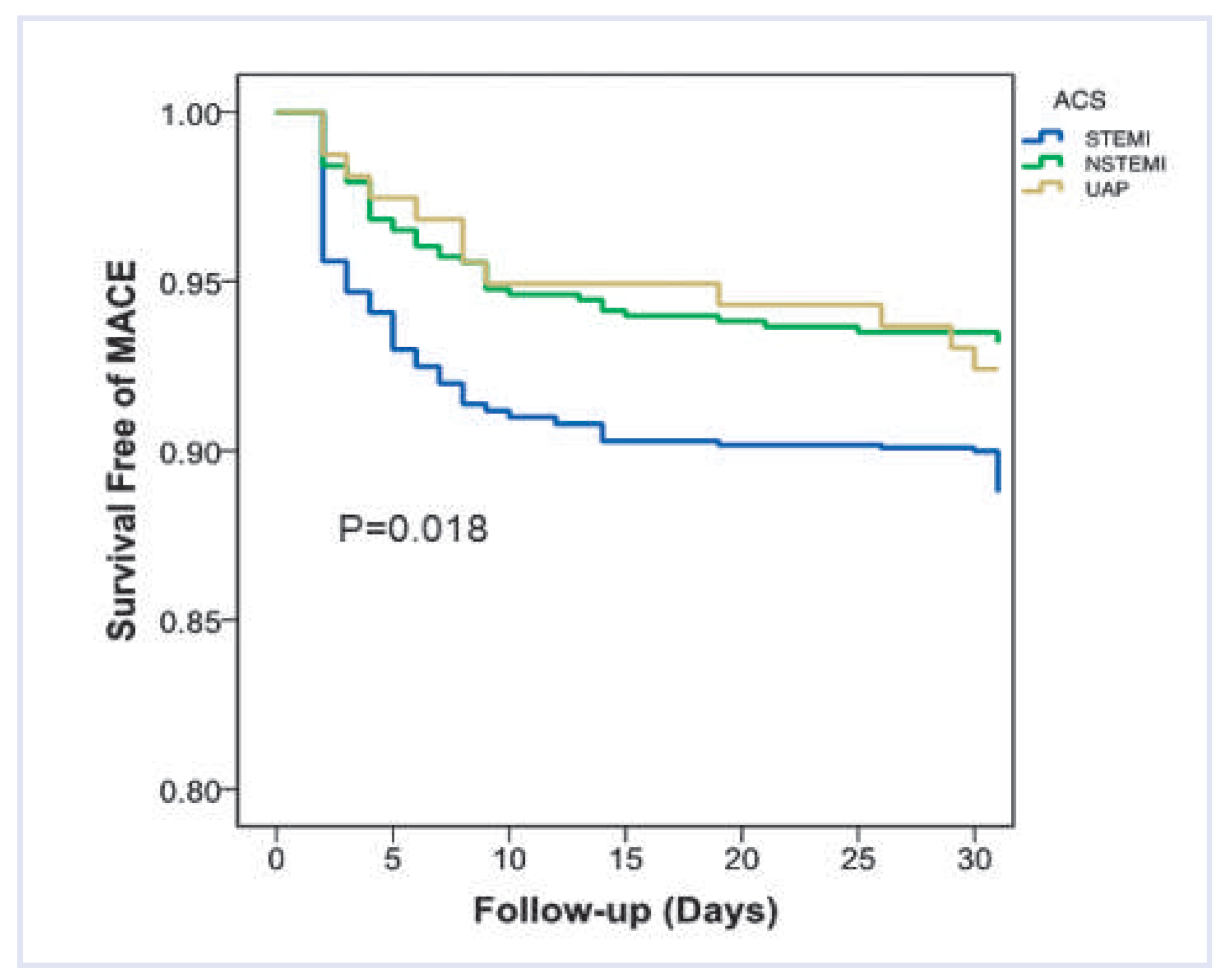
| STEMI | NSTEMI | UA | Total | p-value | |
|---|---|---|---|---|---|
| 998 (55.8%) | 631 (35.3%) | 158 (8.8%) | 1787 | ||
| Male | 766 (76.8%) | 474 (75.1%) | 118 (74.7%) | 1358 (76.0%) | 0.694 |
| Age (yr; mean ± SD) | 62.4 ± 12.5 | 65.3 ± 12.2 | 64.3 ± 12.3 | 63.6 ± 12.5 | <0.001 |
| Cardiovascular risk factors | |||||
| HTN | 506 (50.7%) | 367 (58.2%) | 89 (56.3%) | 962 (53.8%) | 0.011 |
| DM | 145 (14.5%) | 139 (22.0%) | 32 (20.3%) | 316 (17.7%) | <0.001 |
| Hyperlipidaemia | 333 (33.4%) | 266 (42.2%) | 75 (47.5%) | 674 (37.7%) | <0.001 |
| Current smoker | 465 (46.6%) | 230 (36.5%) | 59 (37.3%) | 754 (42.2%) | <0.001 |
| Obesity | 204 (20.4%) | 126 (20%) | 35 (22.2%) | 365 (20.4%) | 0.831 |
| FH | 242 (24.2%) | 152 (24.1%) | 52 (32.9%) | 446 (25.0%) | 0.053 |
| Medication on admission | |||||
| Aspirin | 265 (26.6%) | 277 (43.9%) | 94 (59.5%) | 636 (35.6%) | <0.001 |
| Clopidogrel | 89 (8.9%) | 141 (22.3%) | 39 (24.7%) | 269 (15.1%) | <0.001 |
| Statin | 195 (19.5%) | 236 (37.4%) | 81 (51.3%) | 512 (28.7%) | <0.001 |
| Beta-blocker | 200 (20.0%) | 222 (35.2%) | 69 (43.7%) | 491 (27.5%) | <0.001 |
| ACE inhibitor | 117 (11.7%) | 149 (23.6%) | 36 (22.8%) | 302 (16.9%) | <0.001 |
| Diuretic | 120 (12.0%) | 159 (25.2%) | 44 (27.8%) | 323 (18.1%) | <0.001 |
| ARBS | 103 (10.3%) | 81 (12.8%) | 30 (19.0%) | 214 (12.0%) | 0.005 |
| CCB | 79 (7.9%) | 65 (10.3%) | 18 (11.4%) | 162 (9.1%) | 0.149 |
| Warfarin | 17 (1.7%) | 30 (4.8%) | 6 (3.8%) | 53 (3.0%) | 0.002 |
| STEMI = ST-segment elevation myocardial infarction; NSTEMI = non-ST segment myocardial infarction; UA = unstable angina; HTN = hypertension; DM = diabetes mellitus; FH = known family history; ARBS = angiotensin-receptor blocking agents; CCB = calcium-channel blocker. | |||||
| STEMI | NSTEMI | UA | Total | p-value | |
|---|---|---|---|---|---|
| 998 (55.8%) | 631 (35.3%) | 158 (8.8%) | 1787 | ||
| Acute medication | |||||
| Vasopressors | 110 (11.0%) | 31 (4.9%) | 2 (1.3%) | 143 (8.0%) | <0.001 |
| GP-IIb/IIIa | 295 (29.6%) | 89 (14.1%) | 13 (8.2%) | 397 (22.2%) | <0.001 |
| Emergency procedures | |||||
| Intubation | 88 (8.8%) | 36 (5.7%) | 3 (1.9%) | 127 (7.1%) | 0.002 |
| Resuscitation | 117 (11.7%) | 34 (5.4%) | 5 (3.2%) | 156 (8.7%) | <0.001 |
| IABP | 126 (12.6%) | 51 (8.1%) | 4 (2.5%) | 181 (10.1%) | <0.001 |
| Unstable | 139 (13.9%) | 43 (6.8%) | 2 (1.3%) | 184 (10.3%) | <0.001 |
| Vital signs on admission (mean ± SD) | |||||
| HR (beats per min.) | 74.4 ± 15.6 | 73 ± 15.3 | 68.9 ± 12.9 | 73 ± 15.4 | 0.001 |
| SBP (mm Hg) | 124.4 ± 27.0 | 131.1 ± 27.2 | 136.6 ± 26.0 | 127.7 ± 27.3 | <0.001 |
| DBP (mm Hg) | 71.6 ± 15.3 | 69.9 ± 15.2 | 69.9 ± 12.8 | 70.9 ± 15.1 | 0.076 |
| Haemodynamic parameters (mean ±SD) | |||||
| LVEDP (mm Hg) | 21 ± 8.8 | 19.2 ± 8.0 | 17.6 ± 8.0 | 20 ± 8.5 | <0.001 |
| EF (%) | 51.2 ± 11.6 | 55.2 ± 12.0 | 59.3 ± 8.7 | 53.4 ± 11.8 | <0.001 |
| Location of the lesion | |||||
| LM | 9 (0.9%) | 15 (2.4%) | 8 (5.1%) | 32 (1.8%) | <0.001 |
| LAD | 467 (46.8%) | 262 (41.5%) | 72 (45.6%) | 801 (44.8%) | 0.112 |
| LCX | 126 (12.6%) | 177 (28.1%) | 47 (29.7%) | 350 (19.6%) | <0.001 |
| RCA | 383 (38.4%) | 151 (23.9%) | 25 (15.8%) | 559 (31.3%) | <0.001 |
| Graft | 13 (1.3%) | 26 (4.1%) | 6 (3.8%) | 45 (2.5%) | 0.001 |
| Coronary angiography findings | |||||
| Single-vessel disease | 512 (51.3%) | 238 (37.7%) | 69 (43.7%) | 819 (45.8%) | <0.001 |
| Multivessel disease | 486 (48.7%) | 391 (62.0%) | 89 (56.3%) | 966 (54.1%) | <0.001 |
| STEMI = ST-segment elevation myocardial infarction; NSTEMI = non-ST segment myocardial infarction; UA= unstable angina; IABP = intra-aortic balloon pump; HR = heart rate; SBP = systolic blood pressure; DP = diastolic blood pressure; LVEDP = left ventricular end-diastolic pressure; EF = ejection fraction; LM = left main artery; LAD = left anterior descending artery; LCX = left circumflex artery; RCA = right coronary artery. | |||||
| STEMI | NSTEMI | UA | Total | p-value | |
|---|---|---|---|---|---|
| 998 (55.8%) | 631 (35.3%) | 158 (8.8%) | 1787 | ||
| Cholesterol | 4.9 (± 0.05) | 4.7 (± 0.07) | 4.6 (± 0.14) | 4.8 (± 0.04) | 0.017 |
| HDL | 1.1 (± 0.02) | 1.1 (± 0.02) | 1.1 (± 0.05) | 1.1 (± 0.01) | 0.838 |
| LDL | 3.3 (± 0.06) | 3.0 (± 0.07) | 2.8 (± 0.15) | 3.1 (± 0.04) | 0.002 |
| TG | 1.4 (± 0.04) | 1.5 (± 0.06) | 1.5 (± 0.10) | 1.4 (± 0.03) | 0.014 |
| CRP on admission | 15.7 (± 1.25) | 18.3 (± 1.66) | 9.5 (± 2.04) | 16 (± 0.93) | <0.001 |
| CRP maximum | 60.8 (± 3.17) | 58.7 (± 3.94) | 33.1 (± 5.27) | 57.7 (± 2.31) | <0.001 |
| WBC on admission | 12.2 (± 0.15) | 10.0 (± 0.16) | 8.6 (± 0.25) | 11.1 (± 0.11) | <0.001 |
| WBC maximum | 13.7 (± 0.17) | 11.5 (± 0.20) | 9.9 (± 0.35) | 12.6 (± 0.13) | <0.001 |
| CK on admission | 926.5 (± 53.70) | 445.6 (± 27.68) | 167.0 (± 24.87) | 693.7 (± 32.72) | <0.001 |
| CK maximum | 2386.5 (± 86.96) | 870.7 (± 51.43) | 317.2 (± 45.20) | 1680.8 (± 56.32) | <0.001 |
| CK-MB on admission | 114.0 (± 5.53) | 62.2 (± 3.84) | 28.0 (± 2.88) | 88.6 (± 3.50) | <0.001 |
| CK-MB maximum | 235.7 (± 8.57) | 100.1 (± 5.31) | 45.1 (± 5.26) | 172.1 (± 5.53) | <0.001 |
| Myoglobin on admission | 745.7 0±(44.02) | 257.6 (17.96) | 163.6 (± 51.30) | 532.2 (± 27.32) | <0.001 |
| Myoglobin maximum | 1284.1 (± 71.71) | 522.2 (± 56.98) | 323.2 (± 89.04) | 945.4 (± 47.37) | <0.001 |
| Troponin T on admission | 2.0 (± 0.16) | 0.9 (± 0.09) | 0.2 (± 0.09) | 1.5 (± 0.10) | <0.001 |
| Troponin T maximum | 6.1 (± 0.27) | 2.2 (± 0.15) | 4.1 (± 3.49) | 4.5 (± 0.35) | <0.001 |
| NT-proBNP on admission | 1758.7 (± 183.06) | 2535.5 (± 253.78) | 1895.9 (± 571.62) | 2027.9 (± 144.68) | <0.001 |
| NT-proBNP maximum | 3370.5 (± 245.68) | 3928.3 (± 376.26) | 2210.7 (± 580.05) | 3456.9 (± 196.65) | <0.001 |
| STEMI = ST-segment elevation myocardial infarction; NSTEMI = non-ST segment myocardial infarction; UA = unstable angina; HDL = high-density lipoprotein; LDL = low-density lipoprotein; TG = triglycerides; CRP = c-reactive protein; WBC = white blood count; CK = creatinine-kinase; BNP = brain-natriuretic peptide. | |||||
© 2013 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Ghadri, J.R.; Jaguszewski, M.; Sacron, A.; Srikantharupan, S.; Pfister, P.; Siddique, A.; Kaufmann, P.A.; Wyss, C.A.; Gaemperli, O.; Landmesser, U.; et al. Current Outcome of Acute Coronary Syndromes: Data from the Zurich-Acute Coronary Syndrome (Z-ACS) Registry. Cardiovasc. Med. 2013, 16, 115. https://doi.org/10.4414/cvm.2013.00156
Ghadri JR, Jaguszewski M, Sacron A, Srikantharupan S, Pfister P, Siddique A, Kaufmann PA, Wyss CA, Gaemperli O, Landmesser U, et al. Current Outcome of Acute Coronary Syndromes: Data from the Zurich-Acute Coronary Syndrome (Z-ACS) Registry. Cardiovascular Medicine. 2013; 16(4):115. https://doi.org/10.4414/cvm.2013.00156
Chicago/Turabian StyleGhadri, Jelena R., Milosz Jaguszewski, Annahita Sacron, Shajanth Srikantharupan, Pascal Pfister, Asim Siddique, Philipp A. Kaufmann, Christophe A. Wyss, Oliver Gaemperli, Ulf Landmesser, and et al. 2013. "Current Outcome of Acute Coronary Syndromes: Data from the Zurich-Acute Coronary Syndrome (Z-ACS) Registry" Cardiovascular Medicine 16, no. 4: 115. https://doi.org/10.4414/cvm.2013.00156
APA StyleGhadri, J. R., Jaguszewski, M., Sacron, A., Srikantharupan, S., Pfister, P., Siddique, A., Kaufmann, P. A., Wyss, C. A., Gaemperli, O., Landmesser, U., Altwegg, L., Maier, W., Corti, R., Lüscher, T. F., & Templin, C. (2013). Current Outcome of Acute Coronary Syndromes: Data from the Zurich-Acute Coronary Syndrome (Z-ACS) Registry. Cardiovascular Medicine, 16(4), 115. https://doi.org/10.4414/cvm.2013.00156




