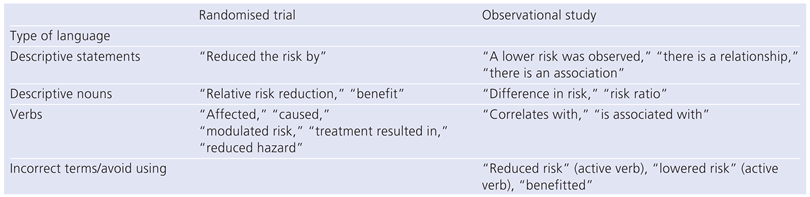
Statement on Matching Language to the Type of Evidence Used in Describing Outcomes Data †
Reference
- Kohli, P.; Cannon, C.P. The importance of matching language to type of evidence: avoiding the pitfalls of reporting outcomes data. Clin Cardiol [Wiley Online Library]. [CrossRef]
 |
© 2013 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
HEART Group Editors. Statement on Matching Language to the Type of Evidence Used in Describing Outcomes Data. Cardiovasc. Med. 2013, 16, 64. https://doi.org/10.4414/cvm.2013.00132
HEART Group Editors. Statement on Matching Language to the Type of Evidence Used in Describing Outcomes Data. Cardiovascular Medicine. 2013; 16(2):64. https://doi.org/10.4414/cvm.2013.00132
Chicago/Turabian StyleHEART Group Editors. 2013. "Statement on Matching Language to the Type of Evidence Used in Describing Outcomes Data" Cardiovascular Medicine 16, no. 2: 64. https://doi.org/10.4414/cvm.2013.00132
APA StyleHEART Group Editors. (2013). Statement on Matching Language to the Type of Evidence Used in Describing Outcomes Data. Cardiovascular Medicine, 16(2), 64. https://doi.org/10.4414/cvm.2013.00132



