Case presentation
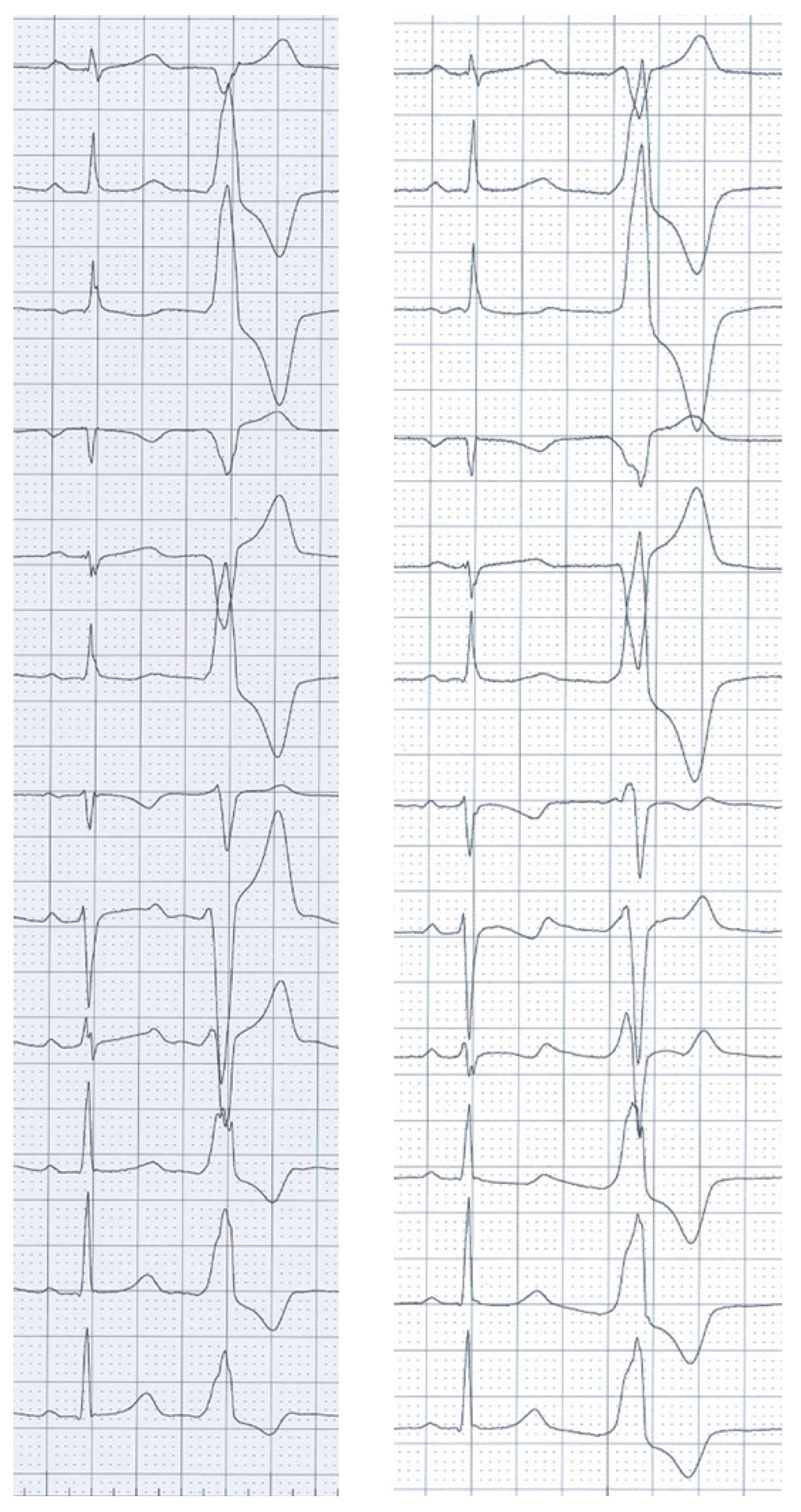
A 25-year-old woman was referred for radiofrequency ablation (RFA) of right ventricular outflow tract (RVOT) tachycardia. The 12-lead ECG in sinus rhythm and echocardiography were unremarkable. Premature ventricular complexes and ventricular tachycardia (VT) with a cycle length of 270 ms showed a left bundle branch block morphology with an inferior axis and R/S transition after V3 (
Figure 1, panel A).
During activation mapping using a 3D electroanatomic mapping system, the earliest bipolar electrogram preceded the onset of the QRS complex by 35 ms in the posteroseptal RVOT. RFA at this location eliminated the VT, but the patient had recurrence 4 weeks later with a slightly different VT morphology showing a slightly larger R-wave in V1 and V2 (
Figure 1, panel B).
During the second procedure, the earliest endocardial activation time (−33 ms) was found in the same region in the posteroseptal RVOT. Pace mapping at this site showed a QRS morphology similar to the VT (pace map match 9/12), but the R-wave in V1/V2 was not reproducible. The paced QRS morphology matched better with the morphology of the VT ablated during the first procedure. However, because of the early endocardial activation time, ablation was performed at that site, which eliminated the VT.
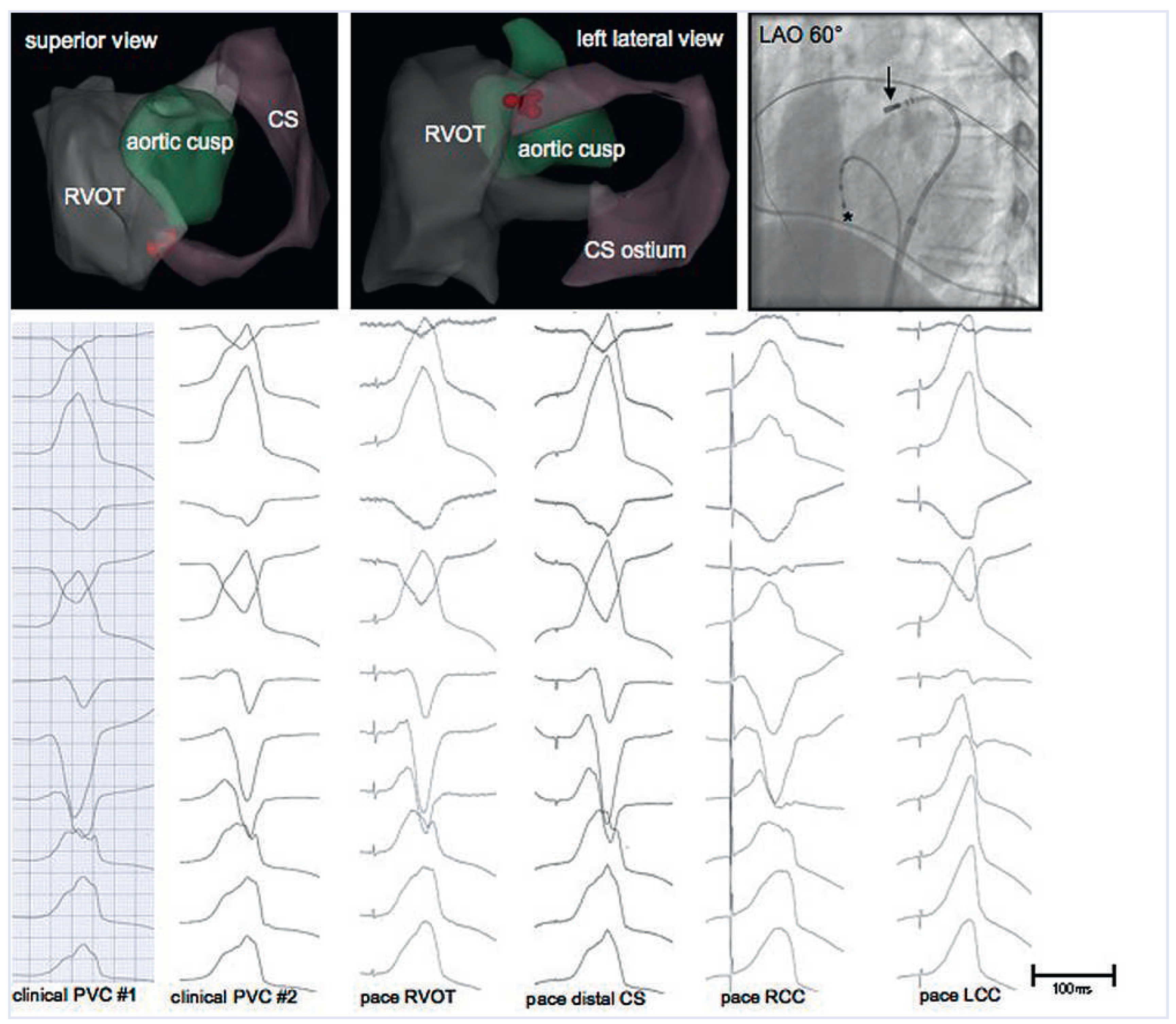
Since the R-wave in V1/V2 of the clinical VT documented during the second procedure suggested a more posterior breakout of the tachycardia, the structures adjacent to the RVOT were studied by means of pace mapping. A 3D electroanatomical map of the RVOT, the coronary cusps and the coronary sinus (CS) shown in
Figure 2 demonstrates the proximity of these three anatomical structures. The coronary cusp pace maps displayed different QRS morphologies compared with the clinical VT, whereas the pace map from the distal CS was nearly identical (pace map match 11/12). Although activation mapping could not be performed because the clinical VT was absent after RFA in the RVOT, and pace mapping is less accurate, additional RFA was performed in the distal CS on the basis of the pace map. RF energy was increased slowly to 20 Watts for 60 s using an irrigated-tip catheter. The patient remained free from arrhythmia during a follow-up of 6 months.
Discussion
The majority of outflow tract arrhythmias originate from the anterior and superior septal aspect of the RVOT, and these arrhythmias are usually caused by triggered activity secondary to cyclic adenosine monophosphate-mediated delayed afterdepolarisations [
1]. However, other origins, including the left ventricular outflow tract, the aortic cusps and the CS, especially when R/S transition occurs at V3 or earlier, have been described [
2,
3]. Studies intended to establish clearly ECG criteria defining the origin from the 12-lead ECG are very helpful in clinical practice, but these criteria still have imperfect diagnostic accuracy, probably because of anatomical variability and differences in QRS axis in sinus rhythm.
In the case presented, there was recurrence of VT with a slightly different QRS morphology and the same cycle length after an initially successful ablation in the RVOT. Although the presence of a second arrhythmia focus in the outflow tract cannot be ruled out, the most likely explanation for this observation is an alteration of the arrhythmia breakout caused by the ablation lesions in the RVOT during the first procedure. When the pace maps from the RVOT and the distal CS during the second procedure are looked at more closely, it is evident that neither of the two match the clinical VT perfectly, but the pace map from the distal CS is the better match and shows the described R-wave in V1/ V2. It may be that a perfect match was not found because pace mapping was only performed from an endocardial site in the RVOT, an epicardial site in the CS and from the coronary cusps, and the true origin of the tachycardia may be somewhere in this “Bermuda triangle” of the heart.
Mapping the coronary venous system may be technically challenging and the inability to advance the ablation catheter to the great cardiac vein and anterior interventricular branch or to deliver sufficient energy are reasons for unsuccessful procedures. In some cases, a small sized ablation catheter (e.g. 5 F) and CS venography may be helpful. Because of the proximity of the coronary arteries and reports of coronary artery injury, coronary angiography should be performed prior to ablation in order to ensure an adequate safety margin. Using an irrigated-tip catheter, power delivery should start low, especially at sites with high impedance (5–10 Watts) in order to avoid perforation and tamponade. Power may then be increased to 20 Watts (higher if needed) during careful monitoring of impedance [
4,
5].
Conclusion
In our patient, the appearance of a more prominent R-wave in V1/V2 in the recurrent VT is most likely explained by a posterior shift of the arrhythmia breakout following the first RFA. Although the limitations of pace mapping with regard to accuracy are well known, and the authors certainly do not generally want to advocate additional ablation based on pace mapping, the use of pace mapping of the structures adjacent to the RVOT appeared to help to completely eliminate the arrhythmia focus in this case of recurrence of VT after a seemingly successful initial procedure. However, the possibility that repeat ablation in the RVOT alone would have been sufficient to eliminate the arrhythmia cannot be ruled out. Nonetheless, the case presented nicely illustrates the complex anatomy of the outflow tract region and its electrocardiographic implications.