Abstract
Novel oral anticoagulants such as dabigatran which specifically target thrombin, and rivaroxaban and apixaban which are activated factor X inhibitors are in the process of being approved for use in Switzerland to lower the risk of stroke in patients with atrial fibrillation. Although there is currently no evidence relevant to the clinical benefit for monitoring anticoagulant intensity of these drugs in routine clinical practice, the need for measuring the anticoagulant effect of the novel anticoagulants may arise in some clinical situations such as: haemorrhage and thrombosis occurring under anticoagulation, an emergency surgery, drug interaction, overdose, impaired renal or liver function compliance monitoring. Furthermore, the effect of novel anticoagulants on routine coagulation tests must be known by the clinician. To date, clinical experience is insufficient to definitively guide the management of emergencies including major bleeding in patients re- ceiving these drugs. Indeed, there is currently no specific antidote available. Fortunately, the half-life of these agents is short, hence treatment interruption is most of the time sufficient to reverse the anticoagulant and clinical effect. In case of life-threatening bleeding, dabigatran can be removed by haemodialysis, and rivaroxaban and apixaban can be antagonised by nonactivated prothrombin complex concentrates. More importantly, preventive attitudes should be placed upfront, before the administration of the novel oral anticoagulant. The prescriber has the responsibility to carefully review possible drug interactions as well as to check renal and liver functions and to provide the patient with an identification card containing personal information, the name of the anticoagulant and treatment indication. In addition, blood cell counts, pro- thrombin and activated partial thromboplastin times measurements need to be performed before the introduction of the anticoagulation, at least in elderly patients.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. affecting about 1% of the general population and up to 10% of people older than 80 years [1,2]. AF has adverse consequences related to a reduction in cardiac output, cardioembolic stroke and systemic throm-boembolism, resulting in an increased risk of mortality. In patients with AF, the estimated risk of stroke is in- creased four- to fivefold [3,4]. For decades, vitamin K antagonists (oral anticoagulants) are highly effective for stroke prevention and recommended for most patients with AF according to risk stratification schema [5]. They can decrease the risk for stroke by about 70% [2,5]. However, multiple food and drug interactions, inter-individual variability and a narrow therapeutic window require regular monitoring with dose adjustment and constitute major clinical challenges [6]. Consequently, only about half of the patients who would benefit from this therapy actually receive the drug [7], and among those receiving the treatment, international normalised ratio (INR) is in the therapeutic range for less than two thirds of the time [8]. Suboptimal anti- coagulation constitutes a clinically significant risk for thromboembolism, especially major bleeding [9].
We are now on the verge of an exciting new era as classes of oral anticoagulant specifically targeting thrombin, activated factor II, called factor IIa direct inhibitor (dabigatran etexilate, Pradaxa®), or activated factor X, factor Xa, called factor Xa direct inhibitors (rivaroxaban, Xarelto®; apixaban, Eliquis®), emerge. With a rapid onset of action, the need for an overlap with another anticoagulant such as (low-molecular-weight) heparins vanishes. Their low propensity for food and drug interaction produces a predictable anticoagulant effect after a fixed dose administration and coagulation monitoring is unnecessary. However, the knowledge on the pharmacology of these products mainly comes from animal experiments and studies in young Caucasian subjects. Prescribing these compounds to a wider patient population may lead to unknown side-effect. There is today no antidote and standardised monitoring methods are still lacking. Rivaroxaban (Xarelto®) and apixaban (Eliquis®) are approved in Switzerland for thromboembolic prophylaxis after major orthopedic surgery, such as hip and knee arthroplasty [10]. These drugs and dabigatran etexilate (Pradaxa®) are in the process of being approved for use in Switzerland to lower the risk for stroke in patients with AF. This cur- rent review will provide a short overview of the pharmacology of these novel anticoagulants and will then focus on their impact on coagulation tests, the quantification of their anticoagulant effect and the management of bleeding in patients receiving these drugs.
Pharmacology overview
Clinicians who prescribe the novel anticoagulants need to have a basic understanding of the pharmacology of these agents. Indeed, elimination process and drug interactions are important information for the prescriber in order to prevent major side effects. In addition, knowing time to peak concentration and half-life is necessary for monitoring and reversal processes.
Dabigatran etexilate (Pradaxa®)
Dabigatran is a selective, competitive, reversible, direct thrombin (factor IIa) inhibitor that is not absorbed from the intestine because it is a strongly polar and hydrophobic molecule. It is given as an absorbable prodrug, dabigatran etexilate [6]. It has a low oral bioavailability (5 to 7%) [11]. In order to optimise the intestinal absorption capsules contain tartric acid [12]. This inactive prodrug is rapidly and completely converted to the active compound by plasma esterases. Peak plasma concentration is reached 1 to 2 hours after intake and terminal half-life is 12 to 17 hours with repeated dosing, whereas the terminal half-life following a single dose is ~9 hours in healthy volunteers [6,11]. It takes 2–3 days to reach steady-state levels [6]. About one third of the circulating drug is bound to plasma protein. The drug is predominantly cleared by the kidney (about 80% of the drug is excreted unchanged in the urine). Absorption of dabigatran etexilate is influenced by gastric pH. Therefore, food, proton pump inhibitors, postoperative state and drugs modifying the activity of the efflux transporter P-glycoprotein (dabigatran is a substrate for P-glycoprotein), such as amiodarone, quinidine, ketokonazole, clarithromycin, verapamil (inhibitors), rifampicin and St John’s wort (inducers) interfere with dabigatran etexilate absorption (table 1) [13]. In clinical studies, dabigatran was not administered to patients with a creatinine clearance <30 ml/ min and the same limitation should be applied in clinical practice. A study involving patients with moderate hepatic impairment (Child-Pugh B) showed similar pharmalogical profile; however, it should not be used in monitorpatients with more severe liver dysfunction [6,11,14]. Animal studies suggest fetal toxicity therefore dabigatran should be avoided in pregnant and lactating women [6,11,144].

Table 1.
Drug-drug interactions.
Table 1.
Drug-drug interactions.
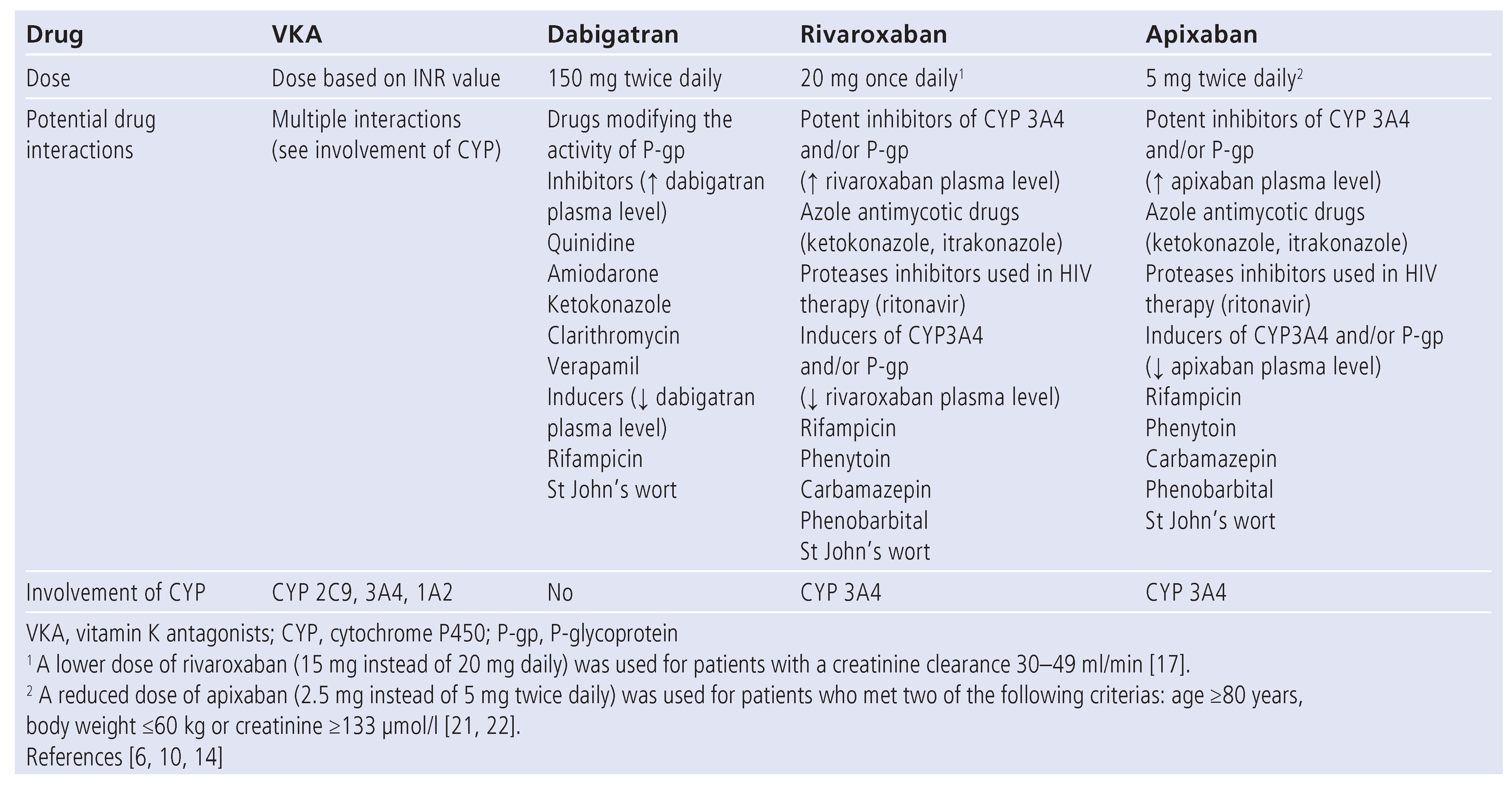
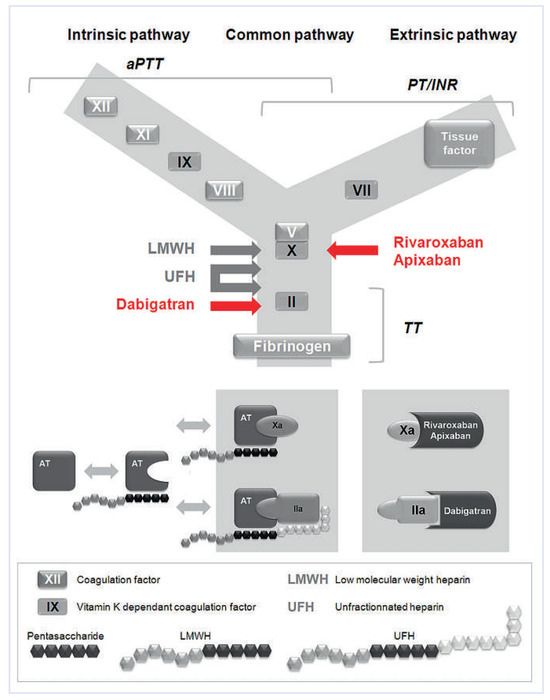
Figure 1.
Anticoagulants and their targets Vitamin K antagonists produce their anticoagulant effect by interfering with the γ-carboxylation of vitamin K-dependant factors II, VII, IX, and X. Unfractionated heparin (UFH) is an indirect anticoagulant that binds to antithrombin (AT), enhancing its ability to inhibit activated factor X (factor Xa), thrombin (factor IIa) and other coagulation factors. Low molecular heparins (LMWH) derive from UFH by chemical or enzymatic depolymerisation and fondaparinux is a synthetic analog of the AT-binding pentasaccharide found in UFH and LMWH. Fondaparinux is too short to enable bridging between AT and thrombin, thus selectively potentiates the anti-factor Xa activity of AT and has no effect on thrombin. Similarely LMWH have only a marginal impact on thrombin. Dabigatran is a direct, selective inhibitor of thrombin, hence independent of AT activity. Rivaroxaban and apixaban are direct, highly selective, factor Xa inhibitors. Clot-based assays comprise the prothrombin time (PT), the activated partial thromboplastin time (aPTT) and the thrombin time (TT). PT is used to assess the extrinsic and the common pathways of coagulation. Clotting is initiated by recalcifying citrated plasma in the presence of thromboplastin (a mixture of tissue factor and phospholipids). In order to promote standardisation of the PT, the World Health Organization (WHO) developed an international reference thromboplastin and recommends that the PT ratio be expressed as the International Normalized Ratio or INR to evaluate the effect of anti-vitamin K anticoagulants. aPTT is used to assess the integrity of the intrinsic coagulation pathway (prekallikrein, high molecular weight kininogen, factors XII, XI, IX, VIII) and final common pathway (factors II, V, X, and fibrinogen). It is performed by recalcifying citrated plasma in the presence of a thromboplastic material that does not have tissue factor activity (hence the term partial thromboplastin) and a negatively charged substance (i.e., celite, kaolin, silica). TT measures the final step of the clotting pathway, the conversion of fibrinogen to fibrin. The test is performed by recalcifying citrated plasma in the presence of dilute bovine or human thrombin.

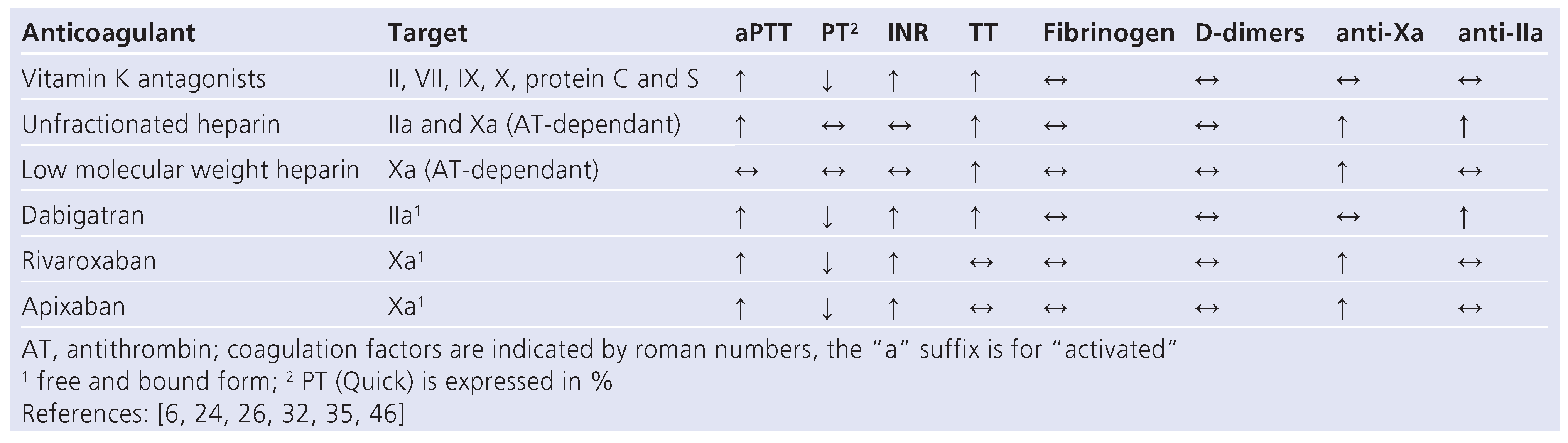
Table 2.
Effects of anticoagulants on coagulation test.
Table 2.
Effects of anticoagulants on coagulation test.
Rivaroxaban (Xarelto®)
Rivaroxaban competitively and specifically binds to the active site of factor Xa and blocks its interaction with prothrombin. It has a high bioavailability (80 to 100%). Peak plasma concentration is reached after 2 to 4 hours and terminal half-life is 5 to 13 hours, increasing with subject’s age [6,15,16]. One third of the drug is excreted unchanged by the kidney, and two thirds are converted to inactive metabolites by hepatic metabolism involving cytochrome P450 family member CYP3A4. In addition, rivaroxaban is a substrate of the transporter protein P-glycoprotein. Hence potent inhibitors of both CYP3A4 and P-glycoprotein such as azole antimycotic drugs (i.e. ketokonazole and itraconazole) and proteases inhibitors used in HIV therapy (such as ritonavir) could lead to clinically relevant drug interaction (table 1). Plasma protein binding is 92 to 95%. In the clinical trial involving stroke prophylaxis in AF patients, rivaroxaban was not used in patients with a creatinine clearance <30 ml/min [17]; the same limitation should be applied in clinical practice [10]. Importantly, in this trial, a lower dose of rivaroxaban (15 mg instead of 20 mg daily) was used for patients with a creatinine clearance 30–49 ml/min [17]. Severe hepatic dysfunction (Child-Pugh C) was an exclusion criteria in clinical studies and should be a contra-indi- cation for rivaroxaban use [10,18]. In milder dysfunction, plasma concentration and anticoagulant effect are increased [19]. In animal models, rivaroxaban passes the placenta and is secreted into breastfeeding milk prohibiting its use for pregnant or lactating women until further notice [10,18].
Apixaban (Eliquis®)
Apixaban is a highly selective, reversible, direct factor Xa inhibitor. It is an active drug with a mean bioavalability of 52.3%. Plasma concentration peaks 3 to 4 hours after drug intake and elimination half-life is 9 to 14 hours [6,16,20]. Plasma protein binding is about 87%. Apixaban is eliminated via multiple pathways including oxidative metabolism, renal (27%) and intestinal (56%) routes [20]. Similar to rivaroxaban, potent CYP3A4 and p-glycoprotein inhibitors such as ketokonazole and ritonavir, or inducers such as phenytoin, carbamazepin, phenobarbital and St John’s wort, should be avoided (table 1). Patients with mild to moderate renal impairment require no dosage adjustment, however apixaban should not be prescribed in patients with severe renal impairment (creatinine clearance <15 ml/min). It is worth noticing that, in clinical stud- ies, a reduced dose of apixaban (2.5 mg instead of 5 mg twice daily) was used for patients who met two of the following criterias: age ≥80 years, body weight ≤60 kg or creatinine ≥133 µmol/l [21,22]. Apixaban should be used with caution by patients with mild to moderate hepatic dysfunction (Child-Pugh A and B) and avoided by those with most severe (Child-Pugh C) stage [10]. Apixaban should not be used for pregnant or lactating women [10].
Effects of novel anticoagulants on coagulation and laboratory monitoring
Unlike vitamin K antagonists and unfractionated heparin, the novel anticoagulants do not require routine anticoagulation monitoring because they have predictable pharmacokinetics and -dynamics, as well as a wide therapeutic window. Indeed, in the various large scale phase 3 trials no routine monitoring of the treatment was per- formed and clinical outcomes in relation to drug levels and to coagulation tests results were not examined [6]. However, the need for monitoring or measuring the anticoagulant effect of these agents might arise in some clinical situations such as haemorrhage or thrombosis occurring under anticoagulation, an emergency surgery, suspicion or known interaction with other drugs, suspected overdose, severely impaired hepatic or renal functions, compliance monitoring and eventually bridging from one anticoagulant to another [6,23]. The coagulation cascade model is a useful tool to integrate the effects of these novel drugs on routine coagulation tests (fig. 1). These effects are detailed in table 2.
Thrombin (factor IIa) represents the last step of the common coagulation pathway responsible for fibrin formation. In addition, it activates platelets leading to platelet aggregation and favours constriction of endothelium-denuded vessels. Dabigatran is a direct competitive thrombin inhibitor, thus independent of antithrombin activity, that interacts specifically with the active site of thrombin. It also inactivates fibrin- or platelet-bound thrombin [6]. The effects of dabigatran on blood coagulation tests are proportional to plasma dabigatran concentration [6,11,14]. Dabigatran pro- longs both the prothrombin time (PT) and the activated partial thromboplastin time (aPTT) in vitro and ex vivo in a concentration-dependant manner [24] (table 2). However, the linear relationship with the plasma con- centration is lost at high concentrations and neither PT nor aPTT are recommended for measuring the precise quantitative effect of dabigatran. aPTT might be useful when no other method is available in determining excessive anticoagulation in presence of bleeding [14].
In a dose escalation study in healthy subjects, the thrombin time (TT) increases in direct proportion to plasma concentration [11]. However, since the maxi- mum measurement time is regularly exceeded, TT ap- pears to be too sensitive to monitor dabigatran. In addition, TT lacks standardisation across laboratories. The diluted TT might be practically applicable for monitor ing the anticoagulant effect. However, it needs to be standardised. A recent in-vitro study shows that the HEMOCLOT direct thrombin inhibitor assay (Hyphen BioMed, France; http://www.coachrom.com/docs/hyp/ CK002L.pdf) is accurate for the rapid assessment of da- bigatran’s anticoagulant activity [25] (fig. 2). The assay is well established for the measurement of other direct thrombin inhibitors (lepirudin and argatroban). It measures the pharmacodynamic effect of dabigatran, since it is a sensitive assay that involves diluting the test sample with 0.15 M NaCl and adding a constant amount of highly purified human α-thrombin to initiate coagulation. Dilution of the sample avoids the exaggerated sen- sitivity of assay response associated with the conventional TT test. Using dabigatran standards, a calibration curve can be generated and dabigatran plasma concentration can be back-calculated from the coagula- tion time. This standardised method enables the assessment of dabigatran concentrations within the range 100–2000 nM (47–943 ng/ml). Results are independent of coagulation factors. However, unfractionated heparin or other anti-IIa inhibitors may interfere with the test by prolonging the clotting time. The assay should be validated in plasma samples from patients taking dabigatran. Ecarin clotting time (ECT) could be an alternative assay, provided that it is calibrated with dabigatran standards, because ECT offers good linearity with adequate assay sensitivity. The use of this test is recommended by the Italian Federation of Thrombosis Cent- ers (FCSA) [26]. The Ecarin Chromogenic Assay (ECA) (Diagnostica Stago, France) is a promising tool for determining the plasma concentration of dabigatran (fig. 3). This method is an enhancement of the ECT. Ecarin converts prothrombin, in excess in the reagent, into meizothrombin. Meizothrombin is a precursor of α-thrombin in the conversion of prothrombin to α-thrombin. The cleavage of a chromogenic substrate by prothrombin activation products is inhibited by dabigatran. Thus, the generation of para-nitroanilide (pNA) is inversely proportional to the plasma concentration of dabigatran. The ECA dose response results are linear and do not pla- teau at elevated concentrations. Results are independent of coagulation factors and inhibitors within the sam- ple. In addition, it is not influenced by lupus anticoagulant, (low-molecular-weight) heparins, danaparoid, fondaparinux and direct anti-factor Xa anticoagulants. This test might therefore also be useful for bridging and transition to other anticoagulants. The prothrombinase- induced clotting time (PiCT) assay (Pentapharm, Basel, Switzerland; http://www.pentapharm.com/files/PiCT_ Flyer.pdf) is under study for dabigatran quantification [27] (fig. 4).
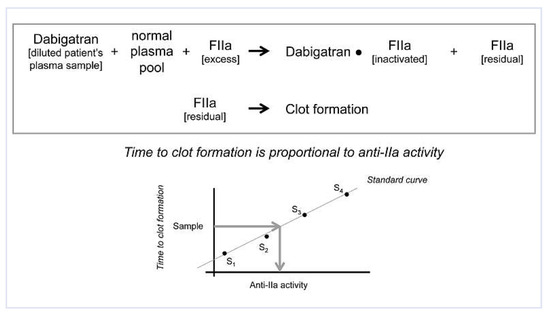
Figure 2.
Quantification of anti-thrombin (anti-IIa) activity – clot based method (HEMO- CLOT). The patient’s plasma sample containing dabigatran is diluted with 0.15 M NaCl and mixed with normal plasma pool. An excess of α-thrombin (FIIa) is then added. Dabigatran inactivates part of the FIIa. The residual FIIa promotes the clot formation. Time to clot formation is proportional to anti-IIa activity. Using dabigatran standards (S1-S4), a calibration curve is generated and dabigatran plasma level back-calculated from the coagulation time (arrows in the diagram).
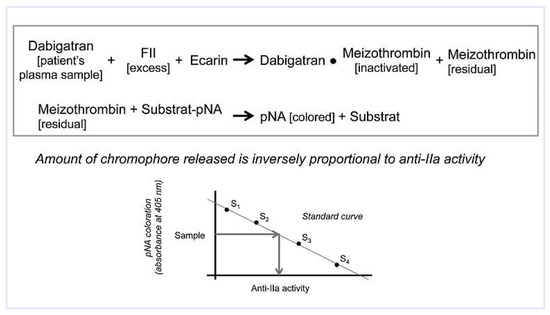
Figure 3.
Quantification of anti-thrombin (anti-IIa) activity – ecarin chromogenic assay (ECA). Ecarin converts prothrombin (FII), in excess in the reagent, into meizothrombin. Residual meizothrombin promotes the cleavage of the chromogenic substrate, generating para-nitroanilide (pNA). The generation of pNA is inversely proportional to the plasma concentration of dabigatran (anti-IIa activity). Using dabigatran standards (S1-S4), a calibration curve is established and dabigatran plasma level back-calculated from the amount of chromophore.
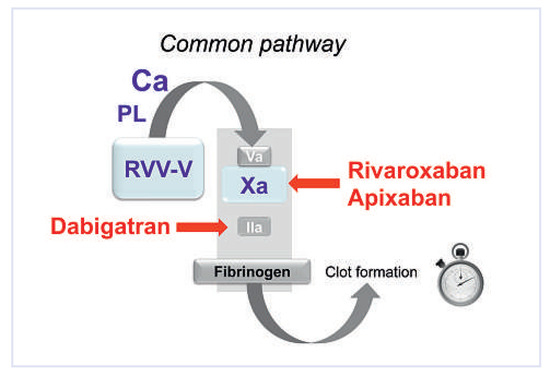
Figure 4.
Quantification of anti-thrombin or anti-activated factor X (anti-Xa) – prothrombinase-induced clotting time (PiCT) assay. RVV-V is a specific factor V activator from Russell’s viper. It activates factor V in activated factor V (Va). In the presence of added phospholipids (PL), a defined amount of activated factor X (Xa) and calcium (Ca) is added and the prothrombinase complex is rapidly formed. Due to the preactivation of factor V, prothrombinase formation depends solely on Xa and IIa activity in the complex. This test has been developed to measure the plasma level of anti-IIa (dabigatran) or anti-Xa (rivaroxabn or apixaban) inhibitors.
Factor Xa mediated activation of prothrombin is the first step of the common pathway. Rivaroxaban binds to the active site of factor Xa and blocks the interaction with its substrates. Rivaroxaban prolongs PT in a con- centration-dependent manner, increases the INR and prolongs aPTT [28] (Table 2). The PT seems to be more sensitive than the aPTT which is also known for the other direct factor Xa inhibitors but is not seen with in- direct inhibitors. A possible explanation is that indirect factor Xa inhibitors only inhibit free factor Xa whereas direct inhibitors also bind to factor Xa in the prothrombinase complex, which is a more efficient process to re- duce thrombin generation [6,24]. Rivaroxaban exposure could therefore be determined by using the PT, preferably expressed in INR [29]. However, it is important to note that the thromboplastins used for PT measurement have various sensitivity to factor Xa direct inhibitors and the INR introduced to correct for PT sensitivity when monitoring the vitamin K antagonists does not adequately correct for differences in assay sensitivity to direct factor Xa inhibitors [30]. In a recent study, it has been shown that INR, when calibrated for rivaroxaban, allows PT normalisation. However, it is worth noticing that this is an in-vitro study [31]. TT, assessing the last step of the common pathway, downstream of thrombin, will remain largely unaffected by specific factor Xa inhibitors [28,32]. Rivaroxaban induces underestimation of clotting factor activity measured with PT- and aPTT- clotting-based assay [32,33]. In a recent study, this interference was overcome in vitro by diluting plasma samples prior to the analysis [33]. Rivaroxaban also causes a dose-dependent increase of the diluted Russell viper venom ratio [29]. The PiCT assay is under study for anti-Xa inhibitor quantification [27,34] (). Di- rect quantification of anti-Xa activity can be obtained by the use of colorimetric tests. First generation tests were developed for (low-molecular-weight) heparins monitoring and now largely available but not specific for direct factor Xa inhibitors. New highly sensitive tests are being developed. In absence of confounding anticoagulant, they give results very comparable to the direct concentration measurements and will likely represent the standards in determining rivaroxaban and apixaban anti-Xa activities [29,32,35,36] (fig. 5).
Reversal of novel anticoagulants
There is currently no specific antidote available to antagonise the effects of the novel anticoagulants. In addition, clinical experience is insufficient to definitively guide the management of major bleeding, suspected overdose, urgently needed surgery, urgent invasive di- agnostic and therapeutic procedures in patients receiving the novel anticoagulants [6,26].
Reversal of dabigatran prior to elective surgery
Dabigatran has a relatively short half-life (13 hours) in patients with normal renal function [14]. This means that, for most patients with minor bleeding, interruption of anticoagulation is sufficient to reverse the anticoagulant effect. It is worth noticing the half-life of dabigatran increases in the case of renal dysfunction. This has to be taken into account for the reversal strategy (table 3). Creatinine needs to be checked several days be- fore elective surgery and the clearance calculated. In pa- tients with normal renal function and a standard bleeding risk, dabigatran has to be interrupted 24 hours prior to elective surgery. In patients at high risk of bleeding or in whom a major surgery is planed, dabigatran should be discontinued 2 to 4 days prior to surgery (table 3). TT should be measured 6–12 hours before surgery in patients at high risk of bleeding or if a major surgery is planned, and a normal result should be obtained [14]. If TT is prolonged, assessment of dabigatran’s concentration by specific tests should be performed. In patients with severe renal dysfunction and persistently elevated dabigatran plasma concentration, haemodialysis might be considered [14].
Reversal of dabigatran in emergency
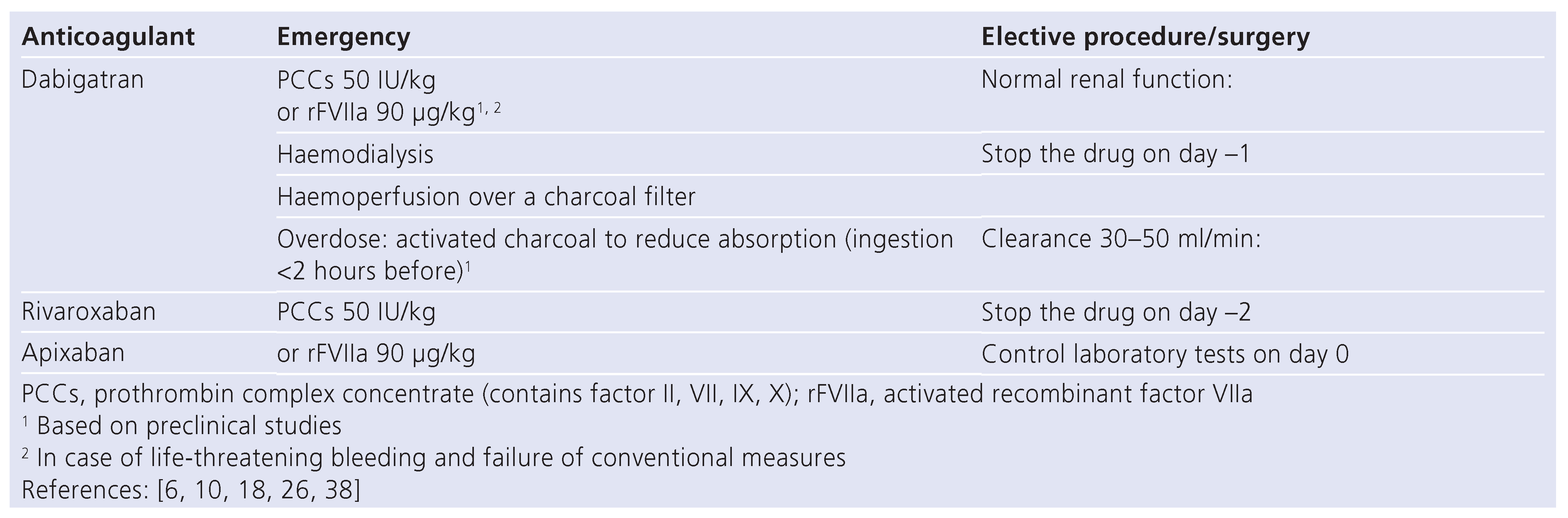
There is no evidence-based strategy when immediate reversal is required [6,26]. In the case of major haemorrhage, general measures [14] comprise the discontinuation of dabigatran, the initiation of an appropriate clinical support comprising mechanical compression and local as well as surgical haemostasis, blood product transfusion, volume substitution, inotropic drugs and the maintenance of an adequate diuresis. Transfusion of platelet concentrates might be considered in case of thrombocytopenia or if antiplatelet drugs have been ad- ministered. The infusion of fresh-frozen plasma is limited for reversing the anticoagulation because of its small content in thrombin and the lack of evidence for its use in this indication [37]. Also, administration of prothrombin complex concentrates (PCCs) did not cause any reversal of the anticoagulant effect of dabigatran in a controlled trial in healthy human subjects [38]. How- ever, based on in-vitro studies and animal models, PCCs or recombinant activated factor VII (rFVIIa) might be infused empirically to bypass the anticoagulant effect of dabigatran in case of life-threatening bleeding (table 4) [6,14]. The decision for administrating these drugs has to be based on the clinical state and not on the laboratory tests. In addition, it is important to be aware that PCCs and rFVIIa administration might be complicated by thrombotic events reinforcing the importance of limiting their prescription to only life-threatening haemorrhages. Dabigatran could be absorbed via haemoperfu- sion over a charcoal filter. In case of major life-threatening bleeding, haemodialysis is a therapeutic option (table 4) [26]. It may take 6–8 hours to clear dabigatran by dialysis. Monitoring of the reversal of the anticoagulant effect of dabigatran can be simply done by measuring TT. A normal TT would exclude a residual anticoagulant effect of dabigatran. Nevertheless, because of the extremely high sensitivity of TT, assessment of dabigatran’s concentration by specific tests would be more appropriate. The availability of these tests is however limited as they are currently only performed by specialised haemostasis laboratories and not always on a 24 hour-based schedule.

Table 3.
Dabigatran, renal function and elective procedure.
Table 3.
Dabigatran, renal function and elective procedure.
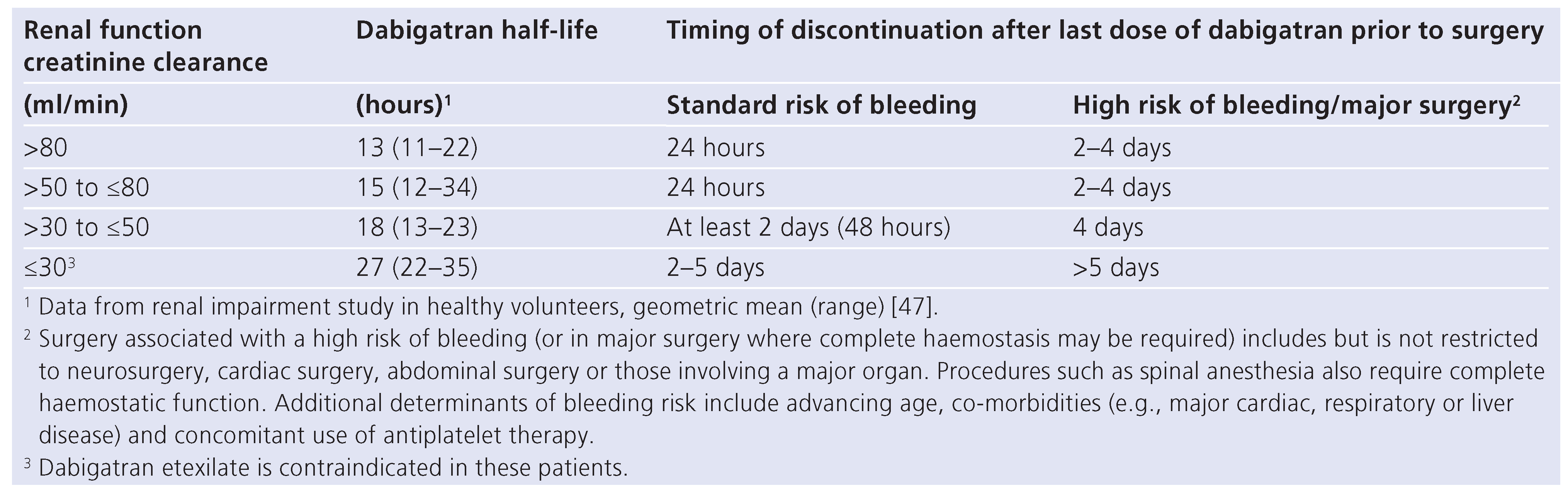
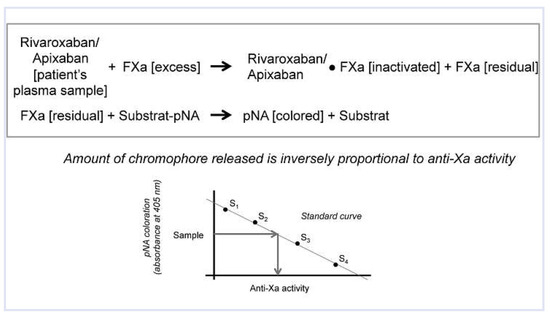
Figure 5.
Quantification of anti-activated factor X (anti-Xa) activity – chromogenic method. These assays measure changes in color (optical density or light absorbance) after cleavage of a synthetic substrate tagged with a chromophore, para-nitroanilide (pNA), by a constant amount of exogenously added excess-activated factor X (FXa). This process is inhibited by anti-Xa anticoagulants (rivaroxaban or apixaban) present in the plasma sample to be tested. The generation of pNA is inversely proportional to the plasma concentration of dabigatran. Using dabigatran standards (S1-S4), a calibration curve is established and dabigatran plasma level back-calculated from the amount of chromophore.

Table 4.
Reversal of novel anticoagulants.
Table 4.
Reversal of novel anticoagulants.
The situation of trauma patients who were under dabigatran for AF and presenting in the emergency room with life-threatening bleeding was recently de- bated. According to ref. [39], the fact that no antidote ex- ists is a crucial problem. The authors expose that, in sharp contrast, VKA can be rapidly reversed, thereby reducing morbidity and mortality. In addition, these au- thors mention that the effect of dabigatran in these pa- tients was not picked up by conventional haemostasis tests such as aPTT, but by thrombelastography. The RELY investigators [40] replied that there is no evidence that the lack of antidote contributed to the death of the reported trauma patients and that the current lack of antidote should not prevent the prescription of dabigatran because this drug is a safe and effective alternative to VKA in patients with AF and associated with lower rates of intracranial bleeding than warfarin (a VKA). Our opinion is that, based on the VKA experience, the administration of an antidote to dabigatran has a good chance of reducing the morbidity and mortal- ity of trauma patients receiving dabigatran. However, we would like to highlight that low-molecular-weight heparins, drugs with a half-life comparable to that of the novel anticoagulants, are only partially antagonised by protamine [41]. Thus, in case of emergency, general measures comparable to those taken for patients under dabigatran are required. If these measures are insufficient, aPCC or rFVIIa might be administered. This cur- rent situation does not prevent the prescription of low- molecular-weight heparins.
Reversal of novel direct factor Xa inhibitors
For the direct factor Xa inhibitors, in view of their short half-life, cessation of medication may be sufficient to re- verse the anticoagulant effect in the case of mild bleeding (table 4) [6,26]. However, if immediate reversal of anticoagulation is required, there is no solid evidence for any reversing agent for the anticoagulant effect of any of these orally available factor Xa inhibitors so far [6,26]. Recently it was shown that the administration of PCCs resulted in a correction of the prolonged PT and restored depressed thrombin generation after rivaroxaban treatment in a controlled trial in healthy human subjects [38]. Importantly, in a rabbit model, rFVIIa and PCCs both partially improved in-vitro coagulation tests but did not reduce rivaroxaban-induced bleeding [42]. However, it is worth noticing that the dose of rivaroxaban used in the model would be considered an overdose in the clinical setting [43]. The inefficacy of the treatment may therefore reflect a relative underdosing of rFVIIa and PCCs [43]. In view of the relatively wide availability of PCCs, this remains an interesting option for reversal, if PCCs clinical effect can be demonstrated in patients on oral factor Xa inhibitors who present with bleeding complications. For the time being, we propose to empirically antagonise direct factor Xa inhibitors by nonactivated PCCs (50 UI/kg by one shot administration) (Table 4) [6,26]. rFVIIa could represent an alternative [6,18]. Monitoring of the reversal of the anticoagulant effect of factor Xa inhibitors is most simply done by measuring the PT. However, antifactor Xa assays are more reliable [35,36].
Clues to prevention of bleeding complications
A well organised follow-up after the introduction of one of the novel anticoagulants is essential. Prevention of complications starts before the prescription. First, the indication to the treatment has to be confirmed and a careful review of potential drug interactions has to be conducted. Second, renal and liver functions need to be checked. In subjects over 75 years old, blood cell count, PT and aPTT measurements have to be performed prior to the administration of the novel anticoagulants [26]. The HAS-BLED score may constitute an useful tool [44]. Monitoring after 2–3 months from the introduction of the anticoagulation in order to have a steady-state laboratory value (clotting times and, when available, specific anti-IIa/anti-Xa assessment) that may be useful in the future if adverse events occur [26]. A follow-up visit at least every 6 months to check for adverse events, renal function, dyspepsia for dabigatran has to be organised [26]. Evaluation of renal function has to be performed every year in case of mild renal failure and every 6 months in case of moderate renal failure [26]. The patient should receive an identification card containing personal information, the type of anticoagulation pre- scribed and treatment indication. Finally, the patient her-/himself should be aware of the potential side-effects and drug interactions.
Conclusion and persectives
Although there is currently no evidence relevant to the clinical benefit for monitoring anticoagulant intensity of the novel anticoagulants in routine clinical practice, the need for measuring the anticoagulant effect of the novel anticoagulants may arise in some clinical situations such as haemorrhage and thrombosis occurring under anticoagulation, an emergency surgery, drug interaction, overdose, impaired renal or liver function, compliance monitoring.
The novel anticoagulants influence clotting times and their anticoagulant effect can be quantified by specific assays. Although these assays are not yet widely used, they are performed by most routine laboratories specialised in haemostasis.
Conventional oral anticoagulation can be reversed by specific interventions when the clinical situation re- quires immediate correction of haemostasis. For the novel anticoagulants, no specific antidotes or reversing agents are currently available. Although some reversal treatment can be proposed in emergencies, they need further evaluation.
Perspectives comprise the administration of novel antidotes such as antibodies directed against the anticoagulant (dabigatran, rivaroxaban or apixaban) or recombinant antidote proteins. Portola Pharmaceuticals developed a novel recombinant protein acting as a universal factor Xa inhibitor antidote (r-Antidote, PRT064445) that was shown to reverse rivaroxaban mediated anticoagulation in animal models [45].
Funding/potential competing interests
No financial support and no other potential conflict of interest relevant to this article was reported.
References
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007, 146(12), 857–867. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Wolf, P.A.; Kelly-Hayes, M.; Beiser, A.S.; Kase, C.S.; Benjamin, E.J.; et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996, 27(10), 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991, 22(8), 983–988. [Google Scholar] [CrossRef]
- Singer, D.E.; Albers, G.W.; Dalen, J.E.; Fang, M.C.; Go, A.S.; Halperin, J.L.; et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133(6 Suppl), 546S–592S. [Google Scholar] [CrossRef]
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G. Oral Anticoagulant Therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141(2 Suppl), e44S–88S. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Borowsky, L.H.; Phillips, K.A.; Selby, J.V.; Singer, D.E. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999, 131(12), 927–934. [Google Scholar] [CrossRef]
- Baker, W.L.; Cios, D.A.; Sander, S.D.; Coleman, C.I. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009, 15(3), 244–252. [Google Scholar] [CrossRef]
- Oake, N.; Fergusson, D.A.; Forster, A.J.; van Walraven, C. Frequency of adverse events in patients with poor anticoagulation: a meta-analysis. CMAJ 2007, 176(11), 1589–1594. [Google Scholar] [CrossRef]
- Arzneimittel–Kompendium der Schweiz / Compendium Suisse des Médicaments; Documed AG, 2012.
- Stangier, J.; Rathgen, K.; Stahle, H.; Gansser, D.; Roth, W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007, 64(3), 292–303. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009, 361(12), 1139–1151. [Google Scholar] [CrossRef]
- Perzborn, E.; Strassburger, J.; Wilmen, A.; Pohlmann, J.; Roehrig, S.; Schlemmer, K.H.; et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939 – an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005, 3(3), 514–521. [Google Scholar] [CrossRef]
- van Ryn, J.; Stangier, J.; Haertter, S.; Liesenfeld, K.H.; Wienen, W.; Feuring, M.; et al. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010, 103(6), 1116–1127. [Google Scholar]
- Kubitza, D.; Becka, M.; Voith, B.; Zuehlsdorf, M.; Wensing, G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Therap. 2005, 78(4), 412–421. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I. New oral anticoagulants in development. Thromb Haemost. 2010, 103(1), 62–70. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011, 365(10), 883–891. [Google Scholar] [CrossRef] [PubMed]
- Pharma Bayer Schering: Xarelto. Summary of Product Characteristics. 2009. Available online: www.xarelto.com/html/downloads/Xarelto_Summary_of_Product_ Characteristics_May2009.pdf (accessed on day month year).
- Halibi, A.; Kubitza, D.; Zuehlsdorf, M.; Becka, M.; Mueck, W.; Maatouk, H. Effect of heoatic impairment on the pharmacokinetics, pharmacodynamics and tolerability of rivaroxaban – an oral direct Factor Xa inhibitor. J Thromb Haemost. 2007, 5 (Suppl 2), Abstract P-M-635. [Google Scholar]
- Zhang, D.; He, K.; Raghavan, N.; Wang, L.; Mitroka, J.; Maxwell, B.D.; et al. Comparative metabolism of 14C-labeled apixaban in mice, rats, rabbits, dogs, and humans. Drug Metab Dispos. 2009, 37(8), 1738–1748. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.; Joyner, C.; Diener, H.C.; Hart, R.; Golitsyn, S.; et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011, 364(9), 806–817. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011, 365(11), 981–992. [Google Scholar] [CrossRef]
- Mismetti, P.; Laporte, S. New oral antithrombotics: a need for laboratory monitoring. For. J Thromb Haemost. 2010, 8(4), 621–626. [Google Scholar] [CrossRef]
- Samama, M.M.; Guinet, C. Laboratory assessment of new anticoagulants. Clin Chem Lab Med. 2011, 49(5), 761–772. [Google Scholar] [CrossRef]
- Stangier, J.; Feuring, M. Using the HAEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coag Fibrinolysis. 2012, 23(2), 138–143. [Google Scholar] [CrossRef]
- Pengo, V.; Crippa, L.; Falanga, A.; Finazzi, G.; Marongiu, F.; Palareti, G.; et al. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost. 2011, 106(5), 868–876. [Google Scholar] [PubMed]
- Hoppensteadt, D.; Walenga, J.M.; Cunanan, J.; Iqbal, O.; Lewis, B.E.; Kalodiki, E.; et al. selection of a suitable rapid turn around and sensitive test for the laboratory monitoring of newer oral anticoagulants, rivaroxaban, apixaban and dabigatran. Blood 2011, 118(21), 3363. [Google Scholar] [CrossRef]
- Lindhoff-Last, E.; Samama, M.M.; Ortel, T.L.; Weitz, J.I.; Spiro, T.E. Assays for measuring rivaroxaban: their suitability and limitations. Therap Drug Monit. 2010, 32(6), 673–9. [Google Scholar] [CrossRef] [PubMed]
- Samama, M.M.; Martinoli, J.L.; LeFlem, L.; Guinet, C.; Plu-Bureau, G.; Depasse, F.; et al. Assessment of laboratory assays to measure rivaroxaban – an oral, direct factor Xa inhibitor. Thromb Haemost. 2010, 103(4), 815–825. [Google Scholar]
- Smith, S.A. Thromboplastin composition controls the sensitivity of prothrombin time (PT) clotting tests to rivaroxaban, a direct factor Xa inhibitor. Blood 2007, 110, 928. [Google Scholar] [CrossRef]
- Tripodi, A.; Chantarangkul, V.; Guinet, C.; Samama, M.M. The International Normalized Ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: results of an in vitro study. J Thromb Haemost. 2011, 9(1), 226–228. [Google Scholar] [CrossRef]
- Asmis, L.M.; Alberio, L.; Angelillo-Scherrer, A.; Korte, W.; Mendez, A.; Reber, G.; et al. Rivaroxaban: Quantification by anti-FXa assay and influence on coagulation tests A study in 9 Swiss laboratories. Thromb. Res. 2011. [Google Scholar]
- Gerotziafas, G.T.; Baccouche, H.; Sassi, M.; Galea, V.; Chaari, M.; Hatmi, M.; et al. Optimisation of the assays for the measurement of clotting factor ac- tivity in the presence of rivaroxaban. Thromb Res. 2012, 129(1), 101–103. [Google Scholar] [CrossRef]
- Graff, J.; von Hentig, N.; Misselwitz, F.; Kubitza, D.; Becka, M.; Breddin, H.K.; et al. Effects of the oral, direct factor xa inhibitor rivaroxaban on plate- let-induced thrombin generation and prothrombinase activity. J Clin Pharm. 2007, 47(11), 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Barrett, Y.C.; Wang, Z.; Frost, C.; Shenker, A. Clinical laboratory measure- ment of direct factor Xa inhibitors: anti-Xa assay is preferable to pro- thrombin time assay. Thromb Haemost. 2010, 104(6), 1263–1271. [Google Scholar] [PubMed]
- Samama, M.M.; Contant, G.; Spiro, T.E.; Perzborn, E.; Guinet, C.; Gourmelin, Y.; et al. Evaluation of the anti-factor Xa chromogenic assay for the mea- surement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012, 107(2), 379–387. [Google Scholar] [PubMed]
- Crowther, M.A.; Warkentin, T.E. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost. 2009, 7 (Suppl 1), 107–110. [Google Scholar] [CrossRef]
- Eerenberg, E.S.; Kamphuisen, P.W.; Sijpkens, M.K.; Meijers, J.C.; Buller, H.R.; Levi, M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011, 124(14), 1573–1579. [Google Scholar] [CrossRef]
- Cotton, B.A.; McCarthy, J.J.; Holcomb, J.B. Acutely injured patients on dabigatran. N Engl J Med. 2011, 365(21), 2039–2040. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Weitz, J.I. More on acutely injured patients receiving dabigatran. N Engl J Med. 2012, 366(9), 863; author reply 864. [Google Scholar]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141(2 Suppl), e24S–43S. [Google Scholar] [CrossRef]
- Godier, A.; Miclot, A.; Le Bonniec, B.; Durand, M.; Fischer, A.M.; Emmerich, J.; et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology 2012, 116(1), 94–102. [Google Scholar] [CrossRef]
- Spahn, D.R.; Korte, W. New challenges for anesthesiologists in bleeding patients. Anesthesiology 2012, 116, 1–3. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: Euro Heart Survey. Chest 2010, 138(5), 1093–1100. [Google Scholar] [CrossRef]
- Lu, G.; Degzman, F.R.; Karbarz, M.J.; Hollenbach, S.J.; Conley, P.B.; Hutchaleelaha, A.; Sinha, U. Reversal of rivaroxaban mediated anticoagulation in animal models by a recombinant antidote protein (r-Antidote, PRT064445). Eur Heart J. 2011, 32, 640–641. [Google Scholar]
- Garcia, D.; Libby, E.; Crowther, M.A. The new oral anticoagulants. Blood 2010, 115, 15–20. [Google Scholar] [CrossRef]
- Stangier, J.; Rathgen, K.; Stahle, H.; Mazur, D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokin. 2010, 49(4), 259–268. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).