New Anticoagulants for the Prevention and Therapy of Venous Thromboembolism—A Review
Abstract
Introduction
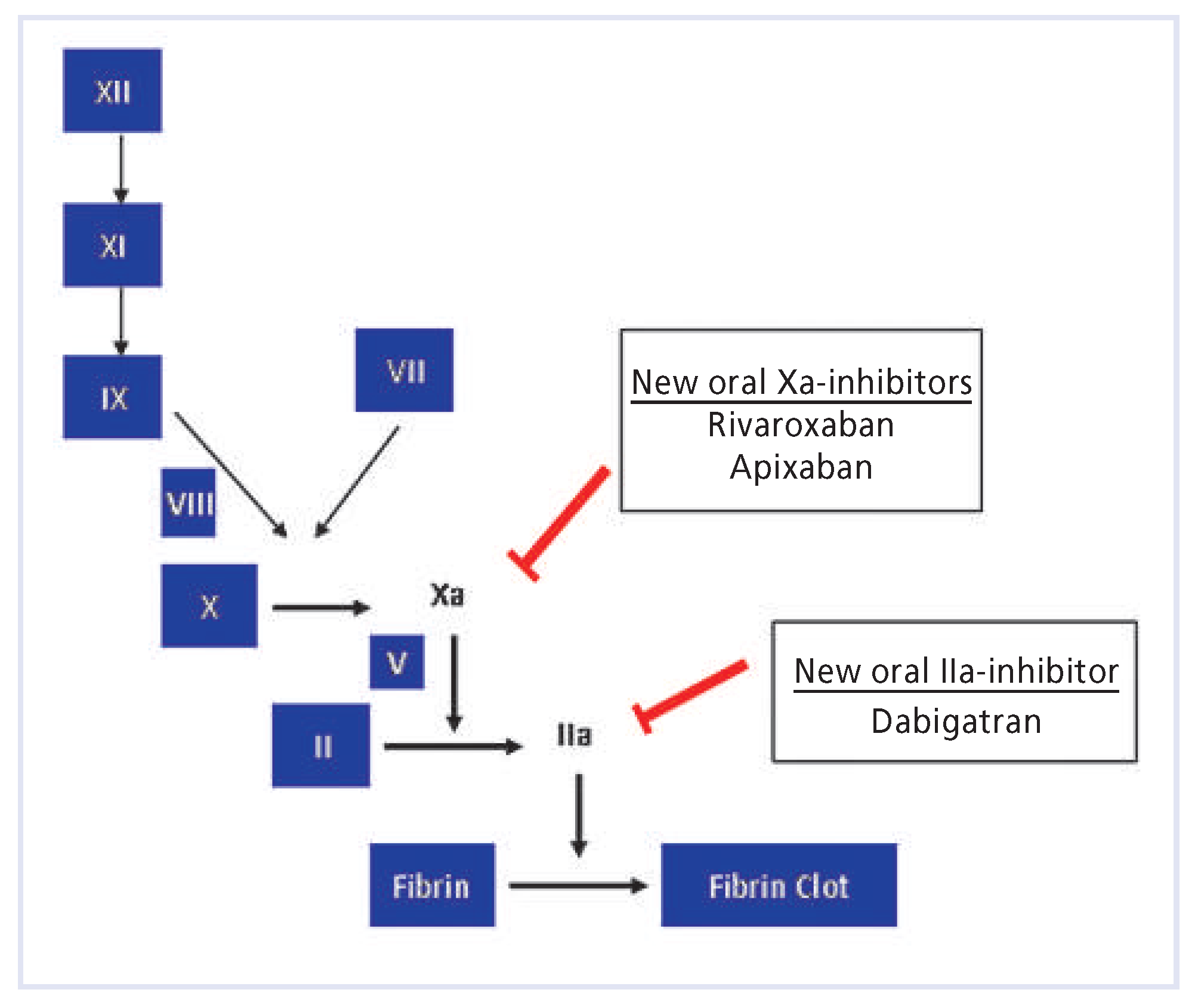
New oral anticoagulants
Dabigatran etexilate
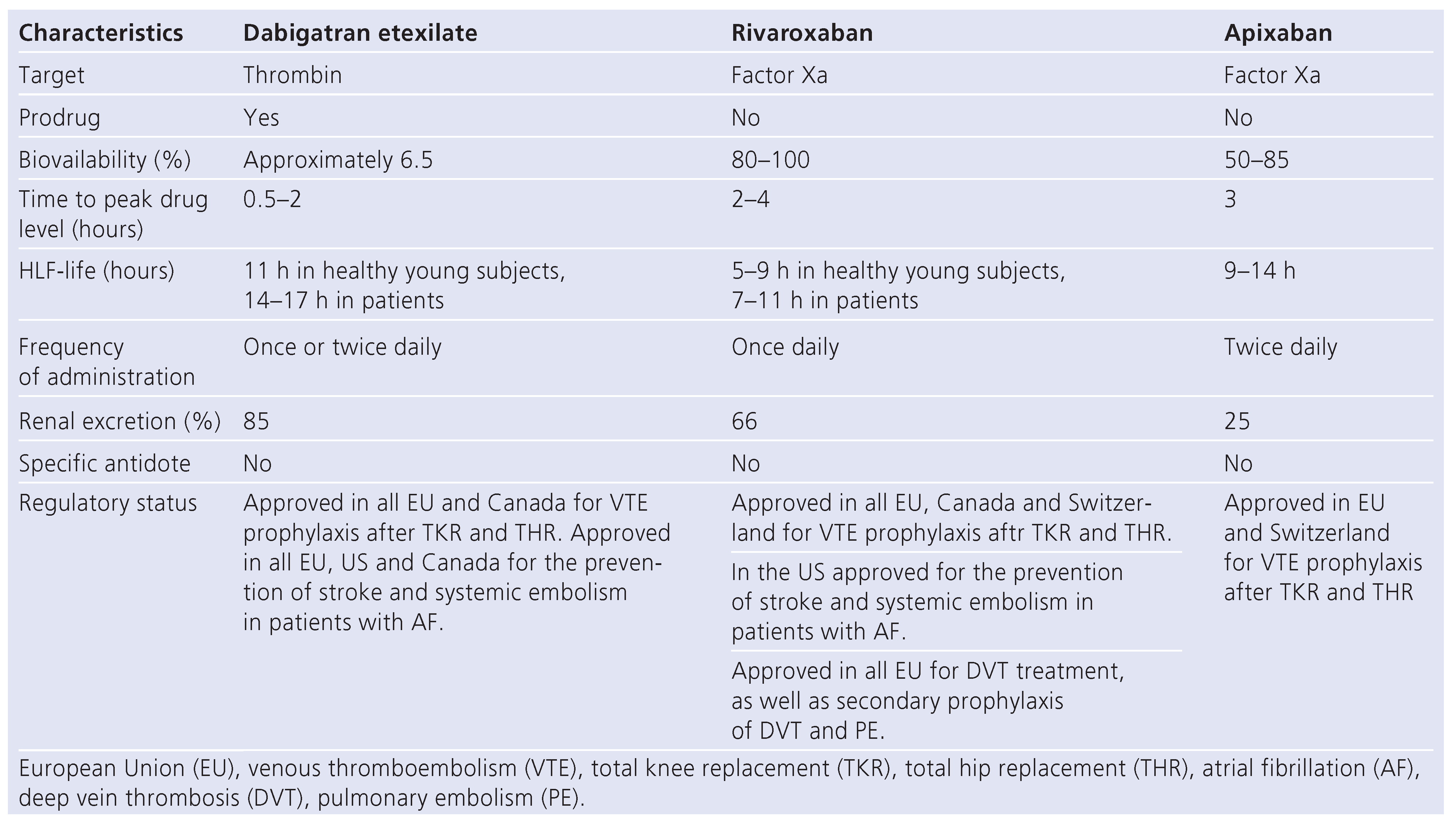
Rivaroxaban
Apixaban
Potential problems with the new oral anticoagulants
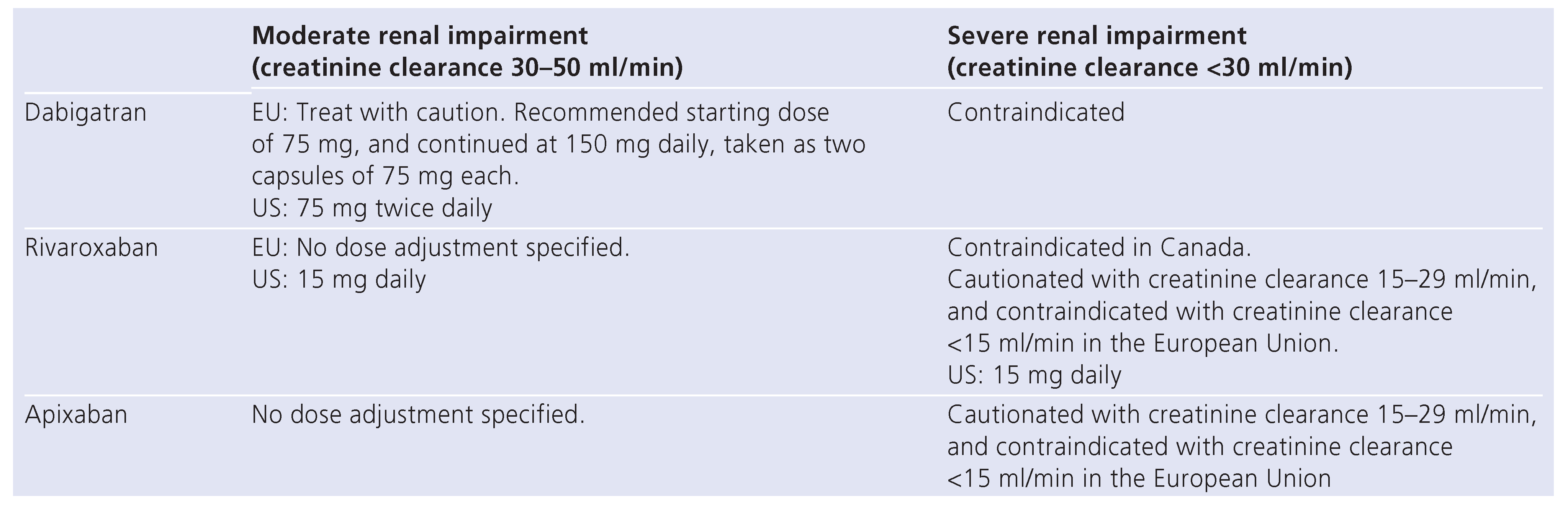
Renal impairment
Liver toxicity
Cardiac toxicity
Drug and food interactions
Conclusion
Funding/potential competing interests
References
- Spencer, F.A.; Emery, C.; Lessard, D.; et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epedimiology of venous thromboembolism. J Gen Intern Med. 2006, 21, 722–727. [Google Scholar] [CrossRef]
- Hutten, B.A.; Prin, M.H. Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst Rev. 2006, 1, CD001367. [Google Scholar]
- Baker, W.L.; Cios, D.A.; Sander, S.D.; Coleman, C.I. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009, 15, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J. Warfarin versus new agents: Interpreting the Data. Hematology. 2010, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.A.; Baglin, T.P. Targeting thrombin: ratinal drug design from natural mechanism. Trends Pharmacol Sci. 2003, 24, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Leslie, B.; Hudoba, M. Thrombin binds to soluble fibrin degradation products where it is protected from inhibition by heparin-anti- thrombin but susceptible to inactivation by antithrombin-independent inhibitors. Circulation. 1998, 97, 544–552. [Google Scholar] [CrossRef]
- Hankey, G.J.; Eikelboom, J.W. Dabigatran Etexilate. A new oral thrombin inhibitor. Circulation. 2011, 123, 1436–1450. [Google Scholar] [CrossRef]
- Stangier, J.; Rathgen, K.; Stähle, H.; Gannser, D.; Roth, W. The pharmacokinetics, pharmacodynamics, and tolerability of Dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007, 64, 292–303. [Google Scholar] [CrossRef]
- Stangier, J.; Rathgen, K.; Stähle, H.; Mazur, D. Ifluence of renal impairment on the pharmacokinetics and pharmacodynamics of oral Dabiga- tran etexilate: an open label, parallel group, singlecentre study. Clin Pharmacokinet. 2010, 49, 259–268. [Google Scholar] [CrossRef]
- Available online: http://www.tga.gov.au/safety/alerts-medicine-Dabigatran-111005.
- Schulman, S.; Kearon, C.; Kakkar, A.K.; et al. Dabigatran versus Warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009, 361, 2342–52. [Google Scholar] [CrossRef]
- Schulman, S.; Kakkar, A.K.; Schellong, S.M.; et al. A randomized trial of Dabigatran versus warfarin in the treatment of acute venous thrombo-embolism (RE-COVER II). In American Society of Hematology 2011 In Proceedings of the Annual Meeting, December 12, San Diego, CA, USA, Abstract 205. 2011. [Google Scholar]
- Wood, S.; O’Rioran, M. FDA approves Dabigatran for stroke prevention, embolism, in AF patients. Available online: http://www.theheart.org/article/1138703.do. (accessed on October 2010).
- European Medicines Agency (EMEA). European public assessment report: Pradaxa [online]. Available online: http://www.emea.europe.eu (accessed on May 2010).
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; et al. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N Engl J Med. [Epub ahead of print]. 2011. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; et al. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N Engl J Med. [Epub ahead of print]. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kubitza, D.; Haas, S. Novel factor Xa inhibitors for the prevention and treatment of thromboembolic diseases. Expert opinion investig drugs. 2006, 15, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.I.; Borris, L.C.; Friedman, R.J.; et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008, 358, 2765–2775. [Google Scholar] [CrossRef]
- Kakkar, A.K.; Brenner, B.; Dahl, O.E.; et al. Extended duration Rivaroxa- ban versus short-term enoxaparin for he prevention of venous throm-boembolism after total hip arthroplasty: a double-blind randomized controlled trial. Lancet. 2008, 372, 31–39. [Google Scholar] [CrossRef]
- Lassen, M.R.; Ageno, W.; Borris, L.C.; et al. Rivaroxaban for thrombopro-phylaxis after total knee arthroplasty. N Engl J Med. 2008, 358, 2776–2785. [Google Scholar] [CrossRef]
- Turpie, A.G.; Lassen, M.R.; Davidson, B.L.; et al. Rivaroxaban versus enoxaparin for thrmoboprophylaxis after total knee arthroplasty (RECORD 4): a randomized trial. Lancet. 2009, 373, 1673–1680. [Google Scholar] [CrossRef]
- The EINSTEIN Investigators. Oral Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010, 63, 2499–2510.
- Eriksson, B.I.; Kakkar, A.K.; Turpie, A.G.G.; et al. Oral Rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br. 2009, 91, 636–644. [Google Scholar] [CrossRef]
- Cohen, A.T.; Spiro, T.E.; Büller, H.R.; et al. Extended-duration Rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis. 2011, 31, 407–416. [Google Scholar] [CrossRef]
- Wong, P.C.; Crain, E.J.; Xin, E.; et al. Apixaban, an oral, direct and selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic statdies. J Thromb Haemost. 2007, 6, 820–829. [Google Scholar] [CrossRef]
- Available online: http://www.theheart.org/article/1207331.
- Eriksson, B.I.; Quinlan, D.J.; Weitz, J.I. Comparative pharmacodynamics and pharmakokinetics of oral direct thrombin and factor Xa inhibitors development. Clin Pharmacokinet. 2009, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lassen, M.R; Raskob, G.E.; Gallus, A. Aprixaban or enoxaparin for throm-boprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010, 375, 807–815. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.; Joyner, C.; et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011, 364, 806–817. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.H.; Lopes, R.D.; James, S.; et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011, 365, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z.; Leizorovics, A.; Kakkar, A.K.; et al. Apixaban versus Enoxaparin for Thromboprophylaxis in Medically Ill Patients. N Engl J Med. 2011, 365, 2167–2177. [Google Scholar] [CrossRef]
- Available online: http://www.trialresultscenter.org/study10328-AMPLIFY-%28CV185-056%29.
- Available online: http://www.trialresultscenter.org/study10329-AMPLIFY-EXT-%28CV185-057%29.
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011, 65, 883–891. [Google Scholar] [CrossRef]
- Lassen, M.R.; Raskob, G.E.; Gallus, A.; et al. Apixaban or Enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009, 361, 594–604. [Google Scholar] [CrossRef]
- Eriksson, B.I.; Dahl, O.E.; Rosencher, N.; et al. Oral Dabigatran etecilate vs. Subcutaneous thromboembolism after TKR: the RE-MODEL ran- domized trial. J Thromb Haemost. 2007, 5, 2178–2185. [Google Scholar]
- Eriksson, B.I.; Dahl, O.E.; Rosencher, N.; et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after THR: a randomised, double-blind, non-inferiortiy trial. Lancet. 2007, 370, 949–956. [Google Scholar] [CrossRef]
- Appraise Steering Committee and Investigators, Alexander JH, Becker RC, et al. Apixaban, an oral, direct, selective factor Xa inhibitor, in com- bination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for prevention of acute ischemic and saftey events (APPRAISE) trial. Circulation. 2009, 119, 2877–2885.
- Xarelto®Summary of product characteristics-EU, Bayer Schering Pharma AG, Germany, May 2009. Available online: www.Xarelto.com/htlm/ downloads/Xarelto_Summary_of_product_Characteristics_May2009.pdf (accessed on September 2010).
- Pradax®product monograph, Boehringer Ingelheim, Canada, Juli 2009. Available online: www.boehringeringelheim.ca/en/Home/Human_Health/ Our_products/Product_Monographs/Pradax-pm.pdf (accessed on 2010).
- Xarelto®product monograph, Bayer Inc., Canada, September 2008. Available online: www. Bayer.ca/files/XARELTO-PM-ENG-10SEP2008-119111.pdf (accessed on September 2010).
- Available online: http://www.ema.europa.eu/docs/de_DE/document_library/EPAR_Prod-uct_Information/human/002148/WC500107728.
- Angelli, G.; Eriksson, B.I.; Cohen, A.T.; et al. Safety assessment of new antithrombotic agents: lessons from teh EXTEND study on ximelagtran. Thromb Res. 2009, 123, 488–497. [Google Scholar]
- Hermans, C.; Claeys, D. Review of the rebound phenomenon in new anticoagulant treatments. Curr Med Res Opin. 2006, 22, 471–81. [Google Scholar] [CrossRef] [PubMed]
- Exanta® (ximelagatran) tablets. FDA Advisory Committe Briefing Document. Cardiovascular and Renal Drugs Advisory Committee. 2004. Available online: www.fda.gov/ohrms/dockets/ac/04/briefing/2004-406B1_01_AstraZen- eca-Backgrounder.pdf (accessed on day month year).
- Ring, A.; Clemens, A.; Rathgen, K.; et al. Dabigatran does not prolong the QTC interval with supratherapeutic exposure: a thorough QTC study. J Am Coll Cardiol. 2011, 57, 56. [Google Scholar] [CrossRef]
- Kubizta, D.; Mueck, W.; Becka, M. Randomized, double-blind, crossover study to investigate the effect of Rivaroxaban on QT-interval prolongation. Drug Saf. 2008, 31, 67–77. [Google Scholar]
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Meeting of the Cardiovascular and Renal Drugs Advisory Committee March 19,2009. Available online: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Cardiovas- cularandRenalDrugsAdvisoryCommittee/UCM 143660.pdf. (accessed on 29 September 2010).
- Lassen, M.R.; Davidson, B.L.; Gallus, A.; et al. The efficacy and safety of Apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost. 2007, 5, 2368–2375. [Google Scholar] [CrossRef]
- Lassen, M.R.; Gallus, A.; Raskob, G.E.; Pineo, G.; Chen, D.; Ramirez, L.M. AD-VANCE-3 Investigators. Apixaban versus enoxaparin for thrombopro-phylaxis after hip replacement. N Engl J Med. 2010, 363, 2487–2498. [Google Scholar]
- Aszalos, A. Drug-drug interactions affected by the transporter protein, P-glycoprotein (ABCB1, MDR1) II. clinical aspects. Drug Discov Today. 2007, 12, 838–843. [Google Scholar] [CrossRef]
- Ezekowitz, M.D.; Connolly, S.; Parekh, A.; et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulation therapy, warfarin, compared with Dabigatran. Am Heart J. 2009, 157, 805–810. [Google Scholar] [CrossRef]
- Pradax®product monograph, Boehringer Ingelheim, Canada, Juli 2009. Available online: www.boehringeringelheim.ca/en/Home/Human_Health/ Our_products/Product_Monographs/Pradax-pm.pdf (accessed on 2010).
- Xarelto®product monograph, Bayer Inc., Canada, September 2008. Available online: www. Bayer.ca/files/XARELTO-PM-ENG-10SEP2008-119111.pdf (accessed on September 2010).
- He, K.; He, B.; Grace, J.E.; et al. Preclinical pharmacokinetic and metabolism of Apixaban, a potent and selective factor Xa inhibitor. Blood. 2006, 108, Abstract 910. [Google Scholar] [CrossRef]
- Huo, M.H. New oral anticoagulants in venous thromboembolism prophylaxis in orthopaedic patients: are they really better? Thromb Haemost. 2011, 106, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.W.; Ansell, J. New oral anticoagulants: will the potential ad- vantages be achieved? Thrombo Haemost. 2010, 103, 34–39. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sédille-Mostafaie, N.; Korte, W. New Anticoagulants for the Prevention and Therapy of Venous Thromboembolism—A Review. Cardiovasc. Med. 2012, 15, 147. https://doi.org/10.4414/cvm.2012.01665
Sédille-Mostafaie N, Korte W. New Anticoagulants for the Prevention and Therapy of Venous Thromboembolism—A Review. Cardiovascular Medicine. 2012; 15(5):147. https://doi.org/10.4414/cvm.2012.01665
Chicago/Turabian StyleSédille-Mostafaie, Nazanin, and Wolfgang Korte. 2012. "New Anticoagulants for the Prevention and Therapy of Venous Thromboembolism—A Review" Cardiovascular Medicine 15, no. 5: 147. https://doi.org/10.4414/cvm.2012.01665
APA StyleSédille-Mostafaie, N., & Korte, W. (2012). New Anticoagulants for the Prevention and Therapy of Venous Thromboembolism—A Review. Cardiovascular Medicine, 15(5), 147. https://doi.org/10.4414/cvm.2012.01665




