The current situation
Ever since their introduction in the middle of the 20th century, vitamin K antagonists (VKAs) have been used in the treatment and prevention of thromboembolic diseases, especially for venous thromboembolism and stroke prevention in atrial fibrillation (AF) [
1]. Under optimal conditions, VKA are very effective (and com- paratively safe) for both these disease entities; how- ever, long-term treatment is often problematic due to several reasons [
2,
3]: Drugs interfering with the me- tabolism and/or high degree of protein binding of VKA, in particular the frequently prescribed non-steroidal anti-inflammatory drugs (NSAIDS) often pose a prob- lem, leading to an unpredictable degree of anticoagula- tion. Furthermore, due to their narrow therapeutic window, meticulous drug dosing and life-long coagula- tion monitoring is necessary to strike the best balance between effective anticoagulation and lowest possible bleeding risk [
4,
5]. Indeed, the risk of major as well as intracranial haemorrhage under VKA is a constant threat in the treatment of these patients. Although frequently overestimated [
5,
6], the inherent risk of this iatrogenic event has led to a substantial underuse of VKA, especially in the setting of stroke prevention in atrial fibrillation.
Novel oral anticoagulants (“NOACs”) – pathophysiology and clinical trial evidence
In order to overcome these limitations, several novel agents have recently been developed with the goal of replacing VKAs [
1]. These substances differ fundamentally from the latter with respect to their mode of action. While vitamin K antagonists such as phenprocoumon (Marcoumar®), acenocoumarol (Sintrom®) or warfarin (Coumadin®) prevent
hepatic de novo synthesis of
several factors by inhibiting vitamin-K dependent y-carboxylation, these novel agents directly and selectively block
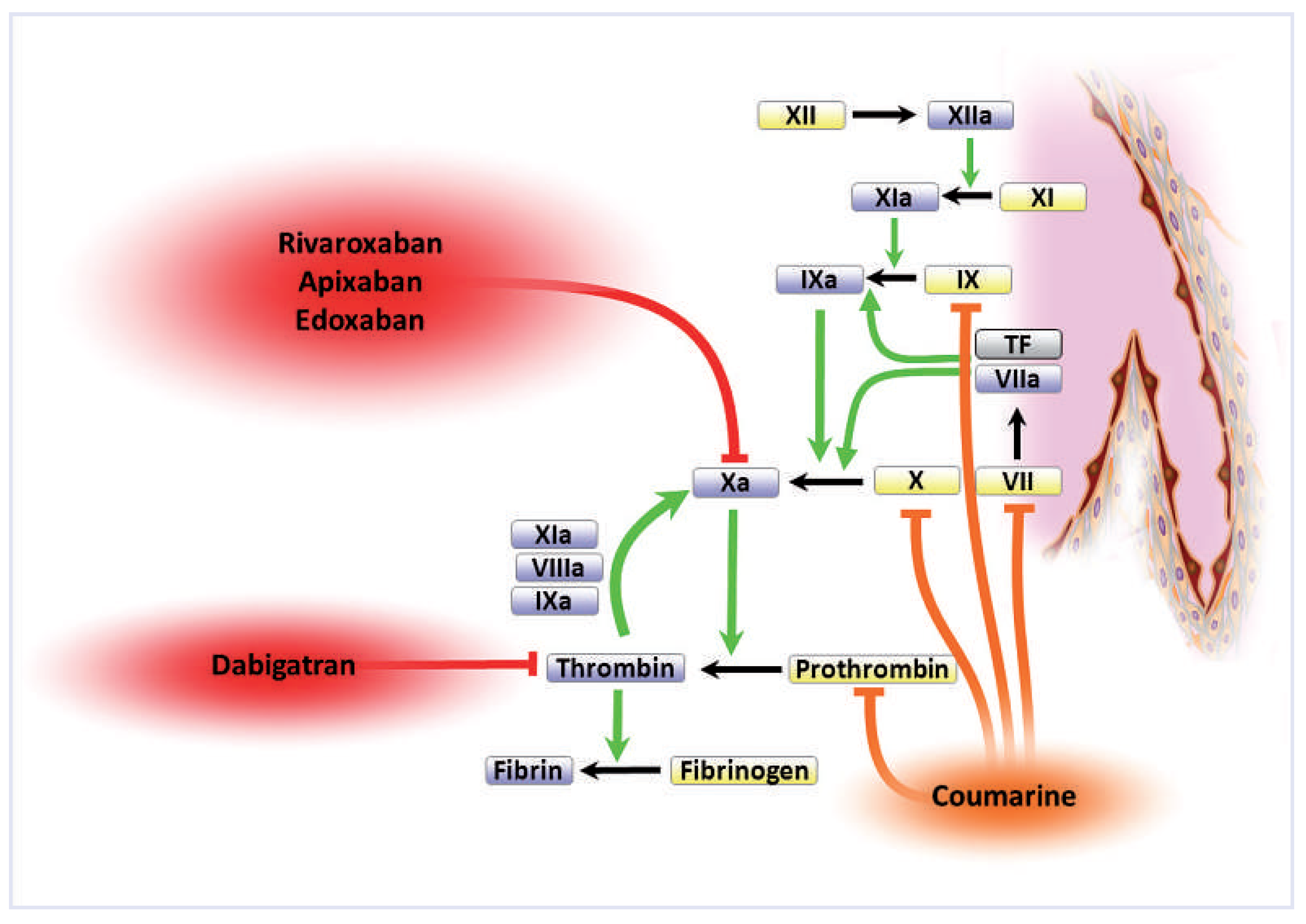
one activated factor of the coagulation cascade (
fig. 1). Two basic principles have turned out to be most successful in this re- gard: direct thrombin (Factor IIa) blockers, which selectively inhibit the activity of thrombin thereby preventing both the conversion of fibrinogen to fibrin and, importantly, the amplifying auto-feedback activa- tion of FXa via its cofactors (
fig. 1, “thrombin burst”); and factor Xa inhibitors, which exert their anticoagu- lant effect by blocking the conversion of prothrombin to thrombin [
1].
After decades of research in the preclinical and early phase clinical setting, a host of large scale phase III studies have now surfaced for the treatment and long-term prophylaxis of VTE as well as for stroke prevention in atrial fibrillation. In this special issue of Cardiovacular Medicine, several renown experts weigh in with their interpretation of trial data for the various indications, and discuss – from different points of view – practical aspects and limitations in the use of these novel substances. With the recent approval of rivaroxaban in Switzerland and other drugs close to the introduction into clinical practice, this is an extremely important and timely topic.
First, Wolfgang Korte and Nazanin Sédille-Mosta- faie review the role of novel anticoagulants in the prevention and therapy of venous thromboembolism. Several studies in this field have indicated that the direct thrombin inhibitor dabigatran as well as the factor Xa inhibitor rivaroxaban may be equally effective and safe, if not superior as compared to VKA in this indication. Moreover, data from the EINSTEIN extension study challenge current guidelines with respect to the treatment duration for patients after a venous thromboembolic event.
Jürg Beer and Erik Holy focus in their article on the use of novel anticoagulants for stroke prevention in atrial fibrillation. For this indication, three studies have compared warfarin to either dabigatran (Re-LY), rivaroxaban (ROCKET-AF) and apixaban (ARISTO-TLE), all with compelling evidence of both non-inferiority and, essentially, superiority of the respective novel anticoagulant versus warfarin. Equally, and arguably even more important, all three studies showed at the same time a reduction in intracranial haemorrhage as well as in severe bleedings, indicating an improved safety profile of these novel substances. This is further underlined by a reduction in all-cause mortality, which was observed in ARISTOTLE (p = 0.047), Re-LY (p = 0.051) and ROCKET-AF (p = 0.07, trend).
Figure 1.
Point of action of vitamin K antagonists and novel oral anticoagulants in the coagulation cascade. See text for detail. (Reproduced from [
1]: Steffel J, Braunwald E. Novel oral anticoagulants: focus on stroke prevention and treatment of venous thrombo-embolism. Eur Heart J. 2011;32(16):1968– 76. © Oxford University Press, Oxford, UK. Reprint with kind permission.).
Figure 1.
Point of action of vitamin K antagonists and novel oral anticoagulants in the coagulation cascade. See text for detail. (Reproduced from [
1]: Steffel J, Braunwald E. Novel oral anticoagulants: focus on stroke prevention and treatment of venous thrombo-embolism. Eur Heart J. 2011;32(16):1968– 76. © Oxford University Press, Oxford, UK. Reprint with kind permission.).
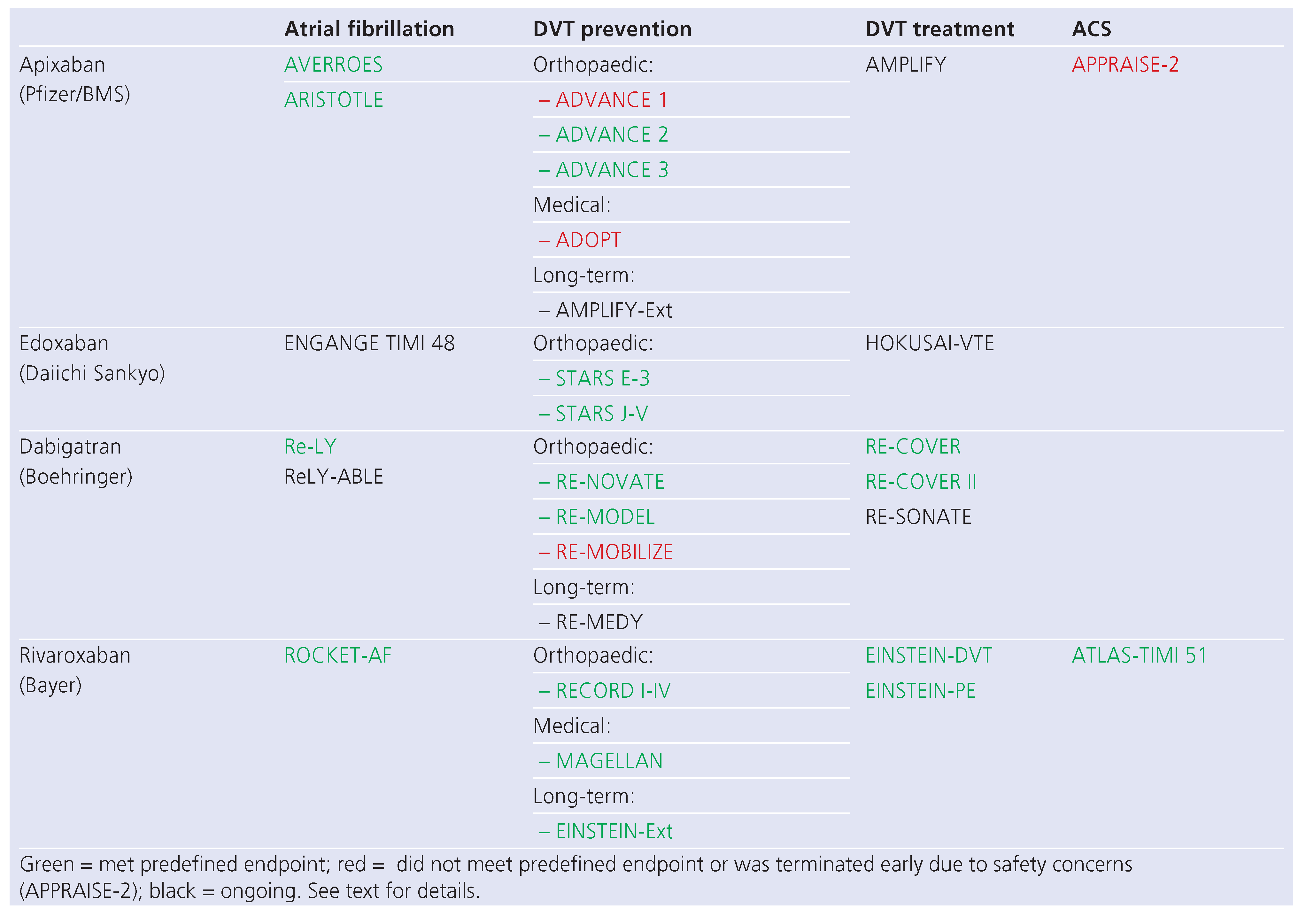
Table 1.
Overview over the most important phase III randomised controlled trials involving the substances discussed in the text. (Reproduced from [
1]: Steffel J, Braunwald E. Novel oral anticoagulants: focus on stroke prevention and treatment of venous thrombo-embolism. Eur Heart J. 2011;32(16):1968–76. © Oxford University Press, Oxford, UK. Reprint with kind permission.).
Table 1.
Overview over the most important phase III randomised controlled trials involving the substances discussed in the text. (Reproduced from [
1]: Steffel J, Braunwald E. Novel oral anticoagulants: focus on stroke prevention and treatment of venous thrombo-embolism. Eur Heart J. 2011;32(16):1968–76. © Oxford University Press, Oxford, UK. Reprint with kind permission.).
Roberto Corti and Oliver Gämperli review in their article the potential role of novel anticoagulants in acute coronary syndromes. Although traditionally considered the primary domain of antiplatelet agents (due to the high shear forces encountered in the arterial circulation), data from the ATLAS-TIMI 51 study demonstrating a reduction in ischaemic events as well as all-cause mortality with low dose rivaroxaban equally indicate a potential role for inhibition of plasmatic anticoagulation in this setting. However, this needs to be carefully orchestrated with respect to dosing as well as concomitant antiplatelet therapy. Indeed, data from the APPRAISE-2 trial demonstrated no additional benefit with “normal” dose apixaban in addition to dual antiplatelet therapy, but instead an increase in bleed- ing events. Furthermore, the role of novel antiplatelet agents such as prasugrel and ticagrelor require further study. Can they be combined with low-dose rivaroxaban in this setting? Could tailored therapy with a potent antiplatelet regime (ASS + prasugrel or ticagrelor) for the first 1–3 months after an event, followed by ASS (± clopidogrel) + rivaroxaban for up to 1 year be an option? Unfortunately, there are currently no data for these potentially tempting options, indicating the urgent necessity for further studies.
Practical aspects
With dabigatran, rivaroxaban and apixaban, three novel oral anticoagulants have proven their efficacy and safety against VKAs in the treatment and preven- tion of venous thromboembolism and particularly for stroke prevention in atrial fibrillation. Despite the – understandable – enthusiasm associated with these novels substances, some uncertainties remain which deserve special attention. In their review, Anne Angelillo-Scherrer and Mathilde Gavillet discuss two of the most frequently brought forward concerns: quantification of the anticoagulatory effect and management of emergencies. While the lack of necessity for routine INR monitoring certainly constitutes one of the greatest advantages of these novel agents, rapid assessment of the anticoagulant status in case of emergencies, surgery or trauma would clearly be desirable. This can now readily be achieved with specialised assays both for direct thrombin inhibitors as well as for factor Xa inhibitors. It is important to remember, however, that these applications are only possible for the “quantification of anticoagulation”; a routine “monitoring”, as it is performed for VKAs, is neither possible (due to fluctuating drug levels depending on drug intake) nor backed by any study-derived target values. Although changing from monitoring-based VKA treatment to application of the novel anticoagulants at a fixed dose without routine monitoring will certainly require some getting used to, this paradigm is in a way comparable to the application of aspirin or ADP receptor antagonists such as clopidogrel, prasugrel or ticagrelor: Indeed, the latter are equally given at a fixed dose without “monitoring” of their effect, which, based on large trial evidence (CURE, CREDO, TRITON, PLATO, etc.), has been successfully performed for the last years to decades.
In emergency situations, the lack of specific antidotes with which an immediate reversal of anticoagulation can be achieved is a potential disadvantage of the novel anticoagulants. Nevertheless, despite the lack of a specific, rapid-acting antidote, results of the available large-scale clinical trials have demonstrated that major and/or intra-cranial bleedings do not occur more frequently in patients treated with these substances as compared to VKAs. This may in part be due to the significantly quicker return of normal coagulation status after cessation of the drug based on the much shorter half-life. In patients with normal kidney and liver function, this time span is comparable to the time it takes for vitamin K application in VKA-treated patients. The particular strategies for urgent and emergent reversal of the novel anticoagulants is re- viewed in detail by Dr Angellilo.
NOACs – a “one size fits all” solution for everyone?
Based on the convincing trial evidence, the obvious question arises whether every patient with a venous thromboembolism, and every patient with atrial fibrillation, should receive a novel anticoagulant. The massive enthusiasm brought about by the possibility of abandoning VKA treatment with the use of novel oral anticoagulants was subsequently dampened by reports of severe and fatal bleeding events. While this was to be expected due to the application of a potent, “active” therapy, it also demonstrates that with every kind of therapy, new or established, an undifferentiated use in a large heterogeneous population of patients is neither justifiable nor advisable.
Some potential pitfalls in the use of these substances have surfaced since their introduction in the US (2010) and in Europe (2011). A decrease in renal function for example may be particularly problematic for dabigatran, which is cleared 80% via the kidneys. As such, its use in patients with severe renal dysfunction is contraindicated (in Europe), and regular monitoring of kidney function is advised before starting treatment and regularly thereafter, especially in situations during which a decrease in renal function is anticipated (intercurrent diseases, new co-medication, etc.). It has to be kept in mind, however, that patients with renal insufficiency pose a problem with any anticoagulant treatment, since both impaired renal function per se as well as frequently present co-morbidities in these patients increase their risk both for embolic as well as for bleeding events.
Furthermore, patient compliance will be a critical issue with the use of novel anticoagulants. With the lack of routine monitoring, the possibility of “controlling” patient compliance equally disappeared. As a result, patients with unstable INR due to poor compliance are likely not good candidates to be switched to any of the new oral anticoagulants, since they are likely to do equally poor if they are unable to improve regular medication intake. Especially with respect to anticoagulation therapy (by whichever means it is performed), it is therefore indispensable to include patients in the decision process regarding the available therapeutic options, and to make them assume responsibility for compliance with their therapy.
Other aspects in other subgroups of patients are equally not finally solved. What is the best way to treat a patient with atrial fibrillation with an acute coronary syndrome (probably, VKA, ASS and clopidogrel, according to the 2010 ESC guidelines, at least for the acute phase <1 year)? Which patients should be switched from VKA? Should also those – rare – patients be switched who have fared well over >4 years on VKA therapy? Based on study results, these patients may equally derive a benefit, especially regarding a reduction in intracranial haemorrhage, but why change a winning team? These and other aspects, well delineated in the reviews of the current issue, certainly deserve further attention.
Summary – the dawn of a new era
The impressive study results of novel anticoagulants in AF and VTE prevention will certainly change the land- scape of anticoagulant therapy. When compared to VKAs, these substances possess a host of desirable features, including lack of routine monitoring, ease of use, and less drug and food interactions, all of which are particularly attractive for the long-term use in stroke prevention and VTE. While, as with every therapy, careful patient selection is indispensable for maximising the individual benefit and reducing the potential risks, it is likely that VKAs will be replaced by the novel anticoagulants for the majority of patients. Currently, underutilisation of anticoagulant therapy in patients who clearly qualify for it is one of the most prevalent problems, particularly in atrial fibrillation, leading to a significant negative impact on morbidity and mortality. Hopes are therefore high that the favourable risk-benefit ratio of the novel anticoagulants – with comparable or greater efficacy and a reduced rate of intracranial or major bleedings – will lead to a substantial increase in the number of patients on anticoagulation, who are currently not or not adequately anticoagulated (e.g., with aspirin) due to the inconvenience and drawbacks of VKA therapy [
7].