Routine Robotic and Video—Assisted Mitral Valve Repair in Everyday Surgery
Abstract
Introduction
Methods
Patients and follow-up
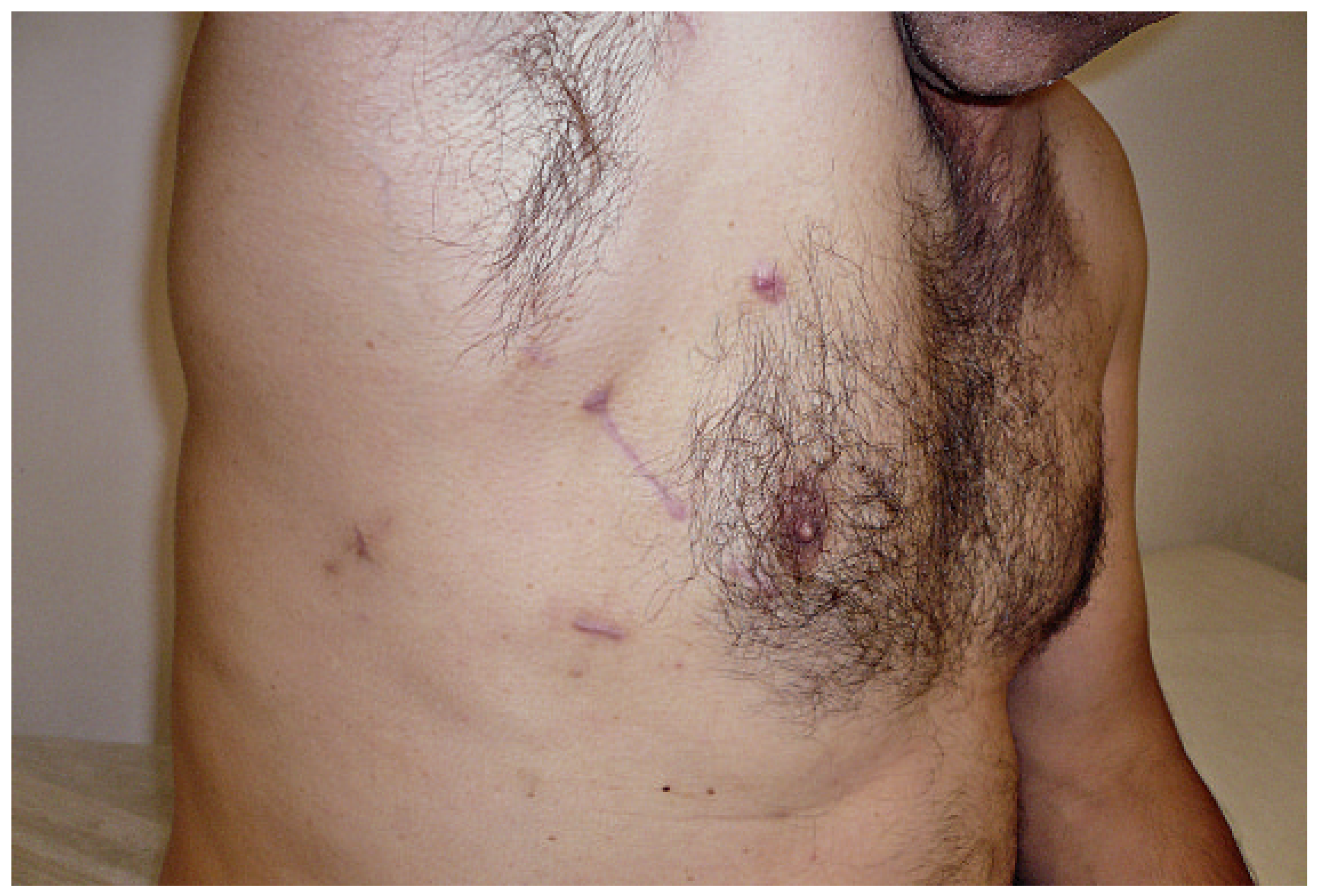
Surgical technique
Results
Discussion
Conclusion
Conflicts of Interest
References
- Modi, P.; Hassan, A.; Chitwood, W.R., Jr. Minimally invasive mitral valve surgery: A systematic review and meta-analysis. Eur J Cardiothorac Surg. 2008, 34, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.J.; Rodriguez, E.; Atluri, P.; Chitwood, W.R., Jr. Minimally invasive, robotic, and off-pump mitral valve surgery. Semin Thorac Cardiovasc Surg 2006, 18, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Panos, A.; Myers, P.O.; Kalangos, A. Thoracoscopic and robotic tricuspid valve annuloplasty with a biodegradable ring: An initial experience. J Heart Valve Dis. 2010, 19, 201–205. [Google Scholar] [PubMed]
- Casselman, F.P.; La Meir, M.; Jeanmart, H.; Mazzarro, E.; Coddens, J.; Van Praet, F.; et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation. 2007, 116 (Suppl. 11), I270–275. [Google Scholar] [CrossRef] [PubMed]
- Nifong, L.W.; Chitwood, W.R.; Pappas, P.S.; Smith, C.R.; Argenziano, M.; Starnes, V.A.; et al. Robotic mitral valve surgery: A United States multicenter trial. J Thorac Cardiovasc Surg. 2005, 129, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Desai, B.; Glower, D.D. Results of 141 consecutive minimally invasive tricuspid valve operations: An 11–year experience. Ann Thorac Surg. 2009, 88, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; Atik, F.A.; Cosgrove, D.M.; Blackstone, E.H.; Rajeswaran, J.; Krishnaswamy, G.; et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J Thorac Cardiovasc Surg. 2010, 139, 926–932 e1-2. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, W.R.; Jr Rodriguez, E.; Chu, M.W.; Hassan, A.; Ferguson, T.B.; Vos, P.W.; et al. Robotic mitral valve repairs in 300 patients: A single-center experience. J Thorac Cardiovasc Surg. 2008, 136, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Miller, J.S.; Langford, D.A. Endoscopic robotic mitral valve surgery. J Thorac Cardiovasc Surg. 2007, 133, 1119–1120, author reply 20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Fontana, G.P.; De Robertis, M.A.; Mirocha, J.; Czer, L.S.; Kass, R.M.; et al. Is robotic mitral valve repair a reproducible approach? J Thorac Cardiovasc Surg. 2010, 139, 628–633. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, M.; Suenaga, E.; Koga, S.; Matsuyama, S.; Kawasaki, H.; Maki, F. Early tracheal extubation after on-pump coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2009, 15, 239–242. [Google Scholar] [PubMed][Green Version]
- Greelish, J.P.; Cohn, L.H.; Leacche, M.; Mitchell, M.; Karavas, A.; Fox, J.; Byrne, J.G.; Aranki, S.F.; Couper, G.S. Minimally invasive mitral valve repair suggests earlier operations for mitral valve disease. J Thorac Cardiovasc Surg. 2003, 126, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, T.; Jarrett, C.M.; Gillinov, A.M.; Williams, S.J.; Devilliers, P.A.; Stewart, W.J.; Svensson, L.G.; Sabik, J.F.; Blackstone, E.H. Robotic repair of posterior mitral valve prolapse versus conventional approaches: Potential realized. J Thorac Cardiovasc Surg. 2011, 141, 72–80. [Google Scholar] [PubMed]
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Panos, A.; Myers, P.O. Routine Robotic and Video—Assisted Mitral Valve Repair in Everyday Surgery. Cardiovasc. Med. 2011, 14, 92. https://doi.org/10.4414/cvm.2011.01573
Panos A, Myers PO. Routine Robotic and Video—Assisted Mitral Valve Repair in Everyday Surgery. Cardiovascular Medicine. 2011; 14(3):92. https://doi.org/10.4414/cvm.2011.01573
Chicago/Turabian StylePanos, Aristotelis, and Patrick O. Myers. 2011. "Routine Robotic and Video—Assisted Mitral Valve Repair in Everyday Surgery" Cardiovascular Medicine 14, no. 3: 92. https://doi.org/10.4414/cvm.2011.01573
APA StylePanos, A., & Myers, P. O. (2011). Routine Robotic and Video—Assisted Mitral Valve Repair in Everyday Surgery. Cardiovascular Medicine, 14(3), 92. https://doi.org/10.4414/cvm.2011.01573



