Noninvasive Hemodynamic Monitoring by Transthoracic Impedance Cardiography During Different Ventricular Activation Sequences in CRT Patients
Abstract
Introduction
Patients and Methods
Patient Population
Impedance Cardiography
Haemodynamic Assessment
Statistical Analysis
Results
Patient Characteristics
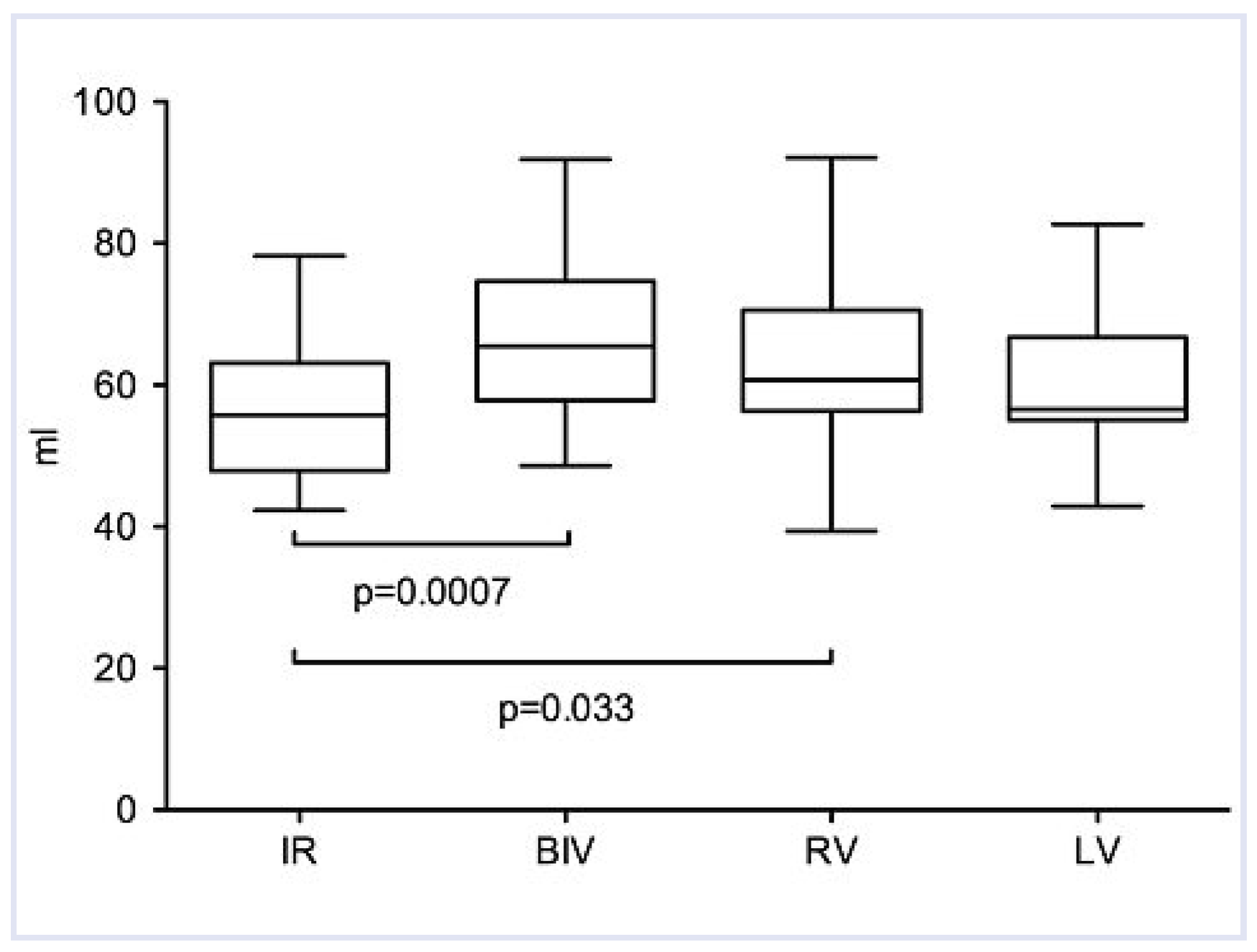
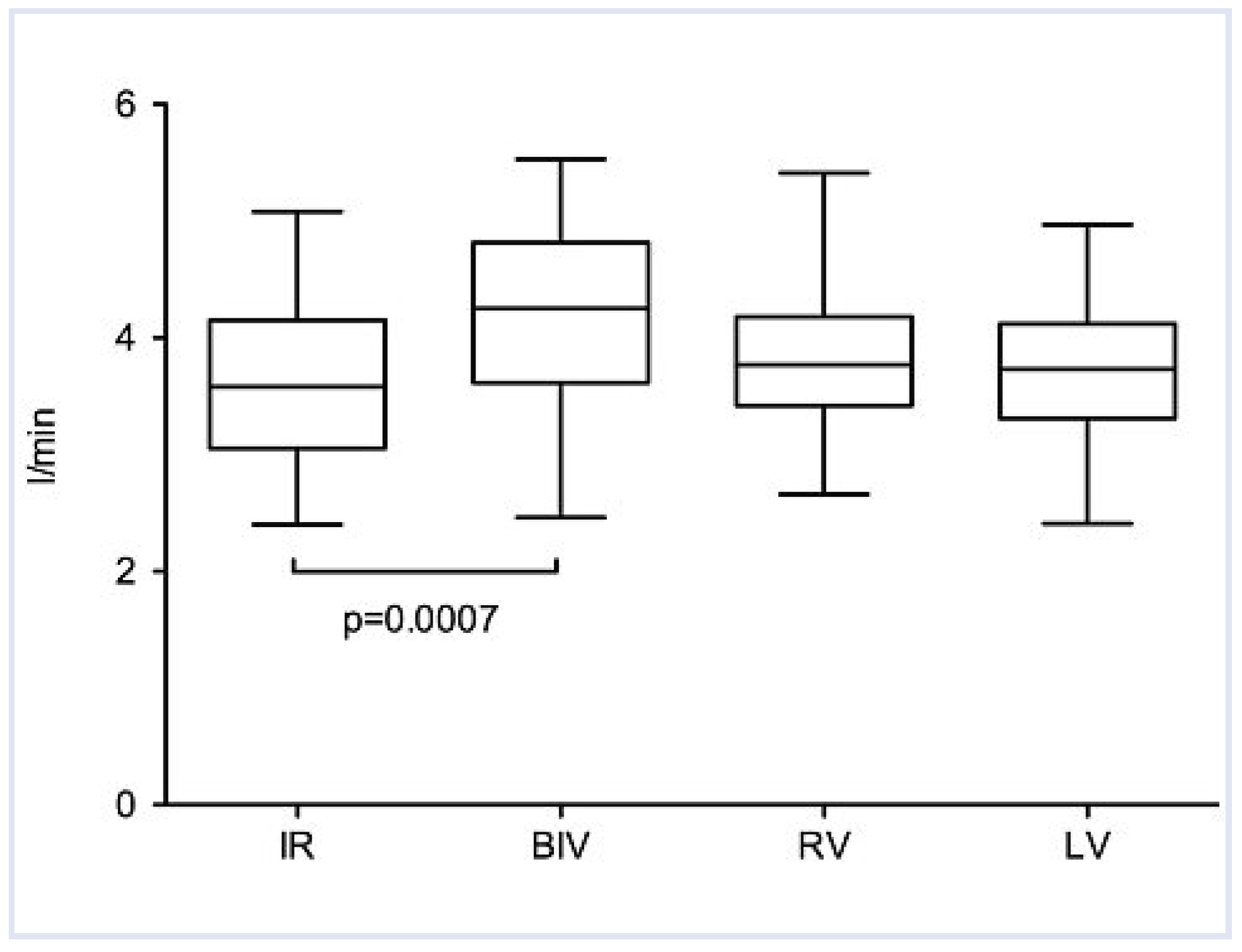
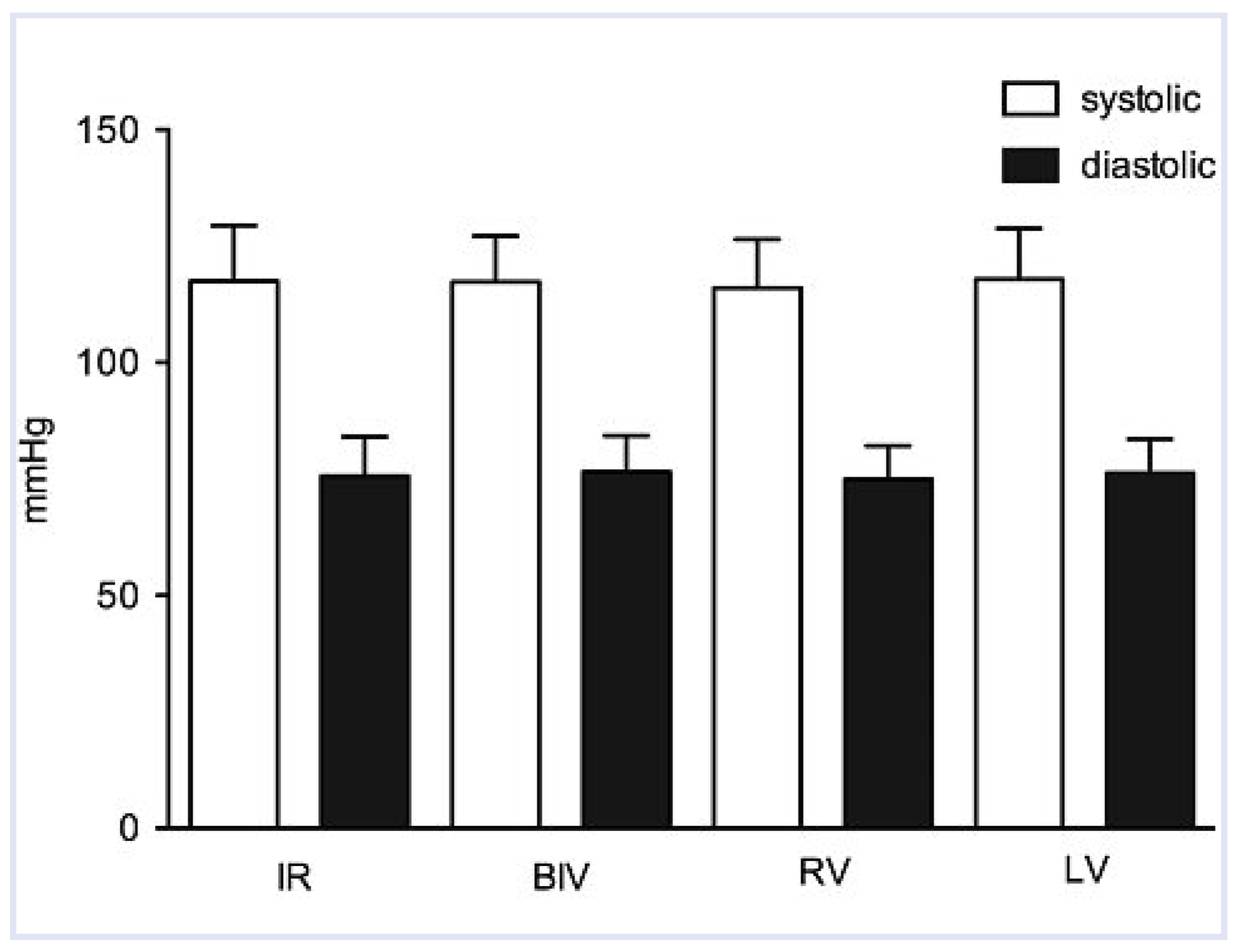
Haemodynamic Measurements and QRS Width During Different Pacing Modes Compared to Intrinsic Rhythm
Discussion
Value of ICG for Noninvasive Haemodynamic Monitoring
Comparison with Previous Studies Comparing Haemodynamics During BIV Pacing
Limitations
Conclusions
Funding
Conflicts of Interest
Abbreviations
| AV | atrioventricular |
| BIV | biventricular pacing |
| cBP | continous blood pressure |
| CRT | cardiac resynchronisation therapy |
| HR | heart rate |
| ICG | impedance cardiography |
| IR | intrinsic rhythm |
| LV | left ventricular pacing |
| LVEF | left ventricular ejection fraction |
| RV | right ventricular pacing |
| SV | stroke volume |
| TPR | total peripheral resistance |
| VV | interventricular |
References
- Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C; et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001, 344, 873–880. [CrossRef]
- Bakker PF, Meijburg HW, de Vries JW, Mower MM, ThomasAC, Hull ML; et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol. 2000, 4, 395–404. [CrossRef]
- Sawhney NS, WaggonerAD, Garhwal S, Chawla MK, Osborn J, Faddis MN. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004, 1, 562–567. [CrossRef] [PubMed]
- Birnie DH, Tang AS. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol. 2006, 21, 20–26. [CrossRef]
- Chung ES, LeonAR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008, 117, 2608–2616. [CrossRef]
- Braun MU, Schnabel A, Rauwolf T, Schulze M, Strasser RH. Impedance cardiography as a noninvasive technique for atrioventricular interval optimization in cardiac resynchronization therapy. J Interv Card Electrophysiol. 2005, 13, 223–229. [CrossRef]
- Heinroth KM, Elster M, Nuding S, Schlegel F, Christoph A, Carter J et al. Impedance cardiography: A useful and reliable tool in optimization of cardiac resynchronization devices. Europace. 2007, 9, 744–750. [CrossRef]
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment ofAcute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart FailureAssociation of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008, 29, 2388–2442.
- Tang WH, Tong W. Measuring impedance in congestive heart failure: Current options and clinical applications. Am Heart J. 2009, 157, 402–411. [CrossRef] [PubMed]
- Fortin J, Marte W, Grullenberger R, Hacker A, Habenbacher W, Heller A; et al. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med. 2006, 36, 941–957. [CrossRef] [PubMed]
- Rosenberg P, Yancy CW. Noninvasive assessment of hemodynamics: An emphasis on bioimpedance cardiography. Curr Opin Cardiol. 2000, 15, 151–155. [CrossRef] [PubMed]
- Lababidi Z, Ehmke DA, Durnin RE, Leaverton PE, Lauer RM. The first derivative thoracic impedance cardiogram. Circulation. 1970, 41, 651–658. [CrossRef] [PubMed]
- Drazner MH, Thompson B, Rosenberg PB, Kaiser PA, Boehrer JD, Baldwin BJ; et al. Comparison of impedance cardiography with invasive hemodynamic measurements in patients with heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2002, 89, 993–995. [CrossRef] [PubMed]
- Belardinelli R, Ciampani N, Costantini C, Blandini A, Purcaro A. Comparison of impedance cardiography with thermodilution and direct Fick methods for noninvasive measurement of stroke volume and cardiac output during incremental exercise in patients with ischemic cardiomyopathy. Am J Cardiol. 1996, 77, 1293–1301.
- Schieken RM, Patel MR, Falsetti HL, Lauer RM. Effect of mitral valvular regurgitation on transthoracic impedance cardiogram. Br Heart J. 1981, 45, 166–172. [CrossRef]
- Hubbard WN, Fish DR, McBrien DJ. The use of impedance cardiography in heart failure. Int J Cardiol. 1986, 12, 71–79. [CrossRef]
- Raaijmakers E, Faes TJ, Scholten RJ, Goovaerts HG, Heethaar RM. A meta-analysis of three decades of validating thoracic impedance cardiography. Crit Care Med. 1999, 27, 1203–1213. [CrossRef]
- Fortin J, Habenbacher W, HellerA, HackerA, Grullenberger R, Innerhofer J et al. Non-invasive beat-to-beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Comput Biol Med. 2006, 36, 1185–1203. [CrossRef]
- Wilkoff BL, Cook JR, EpsteinAE, Greene HL, HallstromAP, Hsia H; et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002, 288, 3115–3123. [CrossRef]
- Kindermann M, Hennen B, Jung J, Geisel J, Bohm M, Frohlig G. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: The Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol. 2006, 47, 1927–1937.
- Nahlawi M, Waligora M, Spies SM, Bonow RO, KadishAH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. 2004, 44, 1883–1888. [CrossRef] [PubMed]
- Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, Bax JJ. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol. 2006, 48, 1642–1648. [CrossRef] [PubMed]

| Variable | |
|---|---|
| Age (yrs) | 67 ± 10 |
| Male | 16 (94%) |
| Body Mass Index (kg/m2) | 29 ± 5 |
| Left bundle branch block | 14 (82%) |
| QRS duration (ms) | 173 ± 28 |
| NYHA II/III | 6/11 |
| Left ventricular ejection fraction (%) | 26 ± 6 |
| Ischaemic cardiomyopathy | 12 (71%) |
| IR vs. BIV | IR vs. RV | IR vs. LV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | IR | BIV | Δ (%) | p | RV | Δ (%) | p | LV | Δ (%) | p |
| QRS duration (ms) | 173 ± 28 | 165 ± 24 | –5 | 0.25 | 210 ± 37 | 21 | 0.0006 | 187 ± 30 | 8 | 0.15 |
| Stroke volume (ml) | 58 ± 11 | 67 ± 12 | 16 | 0.0007 | 62 ± 13 | 7 | 0.03 | 60 ± 11 | 3 | 0.13 |
| Cardiac output (l/min) | 3.6 ± 0.7 | 4.2 ± 0.8 | 17 | 0.0007 | 3.8 ± 0.6 | 6 | 0.1 | 3.7 ± 0.6 | 3 | 0.21 |
| TPR (dyn*s/cm5) | 1975 ± 410 | 1694 ± 390 | –14 | 0.0007 | 1783 ± 328 | –10 | 0.009 | 1868 ± 318 | –5 | 0.14 |
| Systolic BP (mm Hg) | 118 ± 12 | 117 ± 10 | –1 | 0.86 | 116 ± 10 | –2 | 0.29 | 118 ± 11 | 0 | 0.68 |
| Diastolic BP (mm Hg) | 76 ± 8 | 77 ± 8 | 1 | 0.78 | 75 ± 5 | –1 | 0.78 | 76 ± 7 | 0 | 0.56 |
| Heart rate (bpm) | 64 ± 10 | 63 ± 10 | –2 | 1.0 | 63 ± 10 | –2 | 1.0 | 63 ± 10 | –2 | 0.52 |
| Patient | Variable | IR | BIV | RV | LV | Responder |
|---|---|---|---|---|---|---|
| 1 | Cardiac output | 3.7 ± 1.1 | 4.2 ± 1.0 | 3.4 ± 0.3 | 4.2 ± 2.5 | no |
| Total peripheral resistance | 1821 ± 350 | 1700 ± 263 | 1949 ± 199 | 1834 ± 464 | ||
| 2 | Cardiac output | 2.5 ± 0.2 | 3.4 ± 0.4 | 3.3 ± 0.6 | 3.1 ± 0.3 | yes |
| Total peripheral resistance | 2889 ± 281 | 2046 ± 219 | 2209 ± 240 | 2380 ± 205 | ||
| 3 | Cardiac output | 4.4 ± 0.5 | 5.1 ± 1.9 | 5.1 ± 2.1 | 4.2 ± 0.6 | yes |
| Total peripheral resistance | 1457 ± 146 | 1464 ± 492 | 1249 ± 293 | 1526 ± 166 | ||
| 4 | Cardiac output | 3.7 ± 0.5 | 4.5 ± 0.5 | 3.8 ± 0.1 | 4.1 ± 1.0 | no |
| Total peripheral resistance | 3303 ± 410 | 2626 ± 177 | 3084 ± 204 | 3131 ± 436 | ||
| 5 | Cardiac output | 3.6 ± 1.0 | 3.6 ± 0.6 | 3.6 ± 0.6 | 3.4 ± 0.6 | yes |
| Total peripheral resistance | 2003 ± 382 | 1874 ± 314 | 1863 ± 292 | 1990 ± 350 | ||
| 6 | Cardiac output | 3.5 ± 0.2 | 3.6 ± 0.6 | 4.0 ± 0.2 | 3.1 ± 0.3 | yes |
| Total peripheral resistance | 1871 ± 108 | 1840 ± 258 | 1480 ± 160 | 2046 ± 207 | ||
| 7 | Cardiac output | 3.3 ± 0.5 | 4.3 ± 0.5 | 3.9 ± 0.5 | 3.7 ± 0.4 | yes |
| Total peripheral resistance | 1946 ± 224 | 1588 ± 130 | 1727 ± 178 | 1804 ± 142 | ||
| 8 | Cardiac output | 2.8 ± 0.4 | 3.8 ± 0.1 | 3.5 ± 0.2 | 3.4 ± 0.1 | yes |
| Total peripheral resistance | n/a | n/a | n/a | n/a | ||
| 9 | Cardiac output | 2.4 ± 0.1 | 2.5 ± 0.2 | 2.7 ± 0.8 | 2.4 ± 0.2 | yes |
| Total peripheral resistance | 2394 ± 275 | 2249 ± 291 | 2393 ± 354 | 2331 ± 245 | ||
| 10 | Cardiac output | 5.1 ± 2.1 | 5.5 ± 0.3 | 5.4 ± 1.1 | 4.4 ± 0.3 | yes |
| Total peripheral resistance | 1809 ± 936 | 1215 ± 82 | 1291 ± 294 | 1509 ± 76 | ||
| 11 | Cardiac output | 3.1 ± 0.4 | 3.3 ± 0.2 | 3.8 ± 0.2 | 3.4 ± 0.2 | yes |
| Total peripheral resistance | 2023 ± 253 | 1998 ± 101 | 1702 ± 87 | 1891 ± 110 | ||
| 12 | Cardiac output | 4.1 ± 0.9 | 3.8 ± 0.3 | 3.4 ± 0.4 | 3.7 ± 0.2 | no |
| Total peripheral resistance | 1502 ± 262 | 1799 ± 150 | 1814 ± 147 | 1724 ± 95 | ||
| 13 | Cardiac output | 3.6 ± 0.1 | 3.9 ± 0.3 | 3.4 ± 0.4 | 3.9 ± 0.1 | yes |
| Total peripheral resistance | 2250 ± 195 | 2279 ± 137 | 2146 ± 140 | 1898 ± 121 | ||
| 14 | Cardiac output | 3.6 ± 0.1 | 4.7 ± 0.4 | 4.0 ± 0.8 | 4.0 ± 0.3 | no |
| Total peripheral resistance | 1755 ± 168 | 1323 ± 102 | 1516 ± 117 | 1654 ± 134 | ||
| 15 | Cardiac output | 3.2 ± 0.3 | 3.2 ± 0.3 | 3.7 ± 0.4 | 3.8 ± 0.6 | yes |
| Total peripheral resistance | 2562 ± 223 | 2562 ± 223 | 2250 ± 283 | 2300 ± 317 | ||
| 16 | Cardiac output | 2.3 ± 0.8 | 4.3 ± 0.2 | 4.4 ± 0.5 | 4.3 ± 0.7 | no |
| Total peripheral resistance | 1746 ± 397 | 1655 ± 90 | 1576 ± 57 | 1641 ± 234 | ||
| 17 | Cardiac output | 4.2 ± 0.4 | 4.9 ± 0.5 | 4.6 ± 0.5 | 4.3 ± 0.5 | yes |
| Total peripheral resistance | 1740 ± 202 | 1479 ± 191 | 1537 ± 168 | 1612 ± 216 |
© 2010 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Altmann, D.; Schaer, B.; Blank, R.; Jeger, R.; Sticherling, C.; Osswald, S. Noninvasive Hemodynamic Monitoring by Transthoracic Impedance Cardiography During Different Ventricular Activation Sequences in CRT Patients. Cardiovasc. Med. 2010, 13, 208. https://doi.org/10.4414/cvm.2010.01506
Altmann D, Schaer B, Blank R, Jeger R, Sticherling C, Osswald S. Noninvasive Hemodynamic Monitoring by Transthoracic Impedance Cardiography During Different Ventricular Activation Sequences in CRT Patients. Cardiovascular Medicine. 2010; 13(6):208. https://doi.org/10.4414/cvm.2010.01506
Chicago/Turabian StyleAltmann, David, Beat Schaer, Robert Blank, Raban Jeger, Christian Sticherling, and Stefan Osswald. 2010. "Noninvasive Hemodynamic Monitoring by Transthoracic Impedance Cardiography During Different Ventricular Activation Sequences in CRT Patients" Cardiovascular Medicine 13, no. 6: 208. https://doi.org/10.4414/cvm.2010.01506
APA StyleAltmann, D., Schaer, B., Blank, R., Jeger, R., Sticherling, C., & Osswald, S. (2010). Noninvasive Hemodynamic Monitoring by Transthoracic Impedance Cardiography During Different Ventricular Activation Sequences in CRT Patients. Cardiovascular Medicine, 13(6), 208. https://doi.org/10.4414/cvm.2010.01506




