Do We Need MR Conditional Pacemakers?
Abstract
Introduction
Potential benefits of magnetic resonance imaging in paced patients
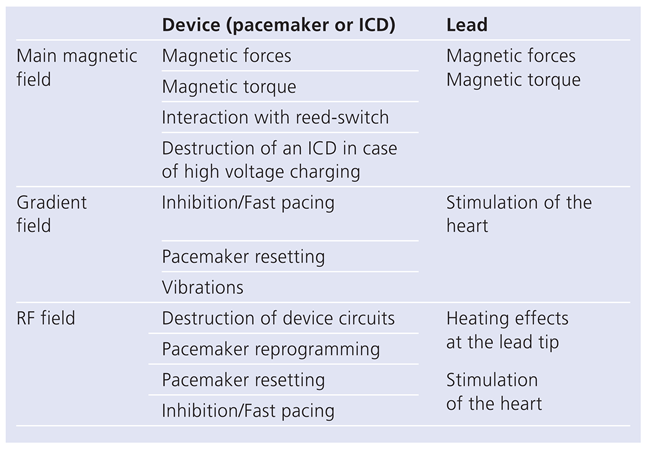
Electromagnetic fields in magnetic resonance imaging
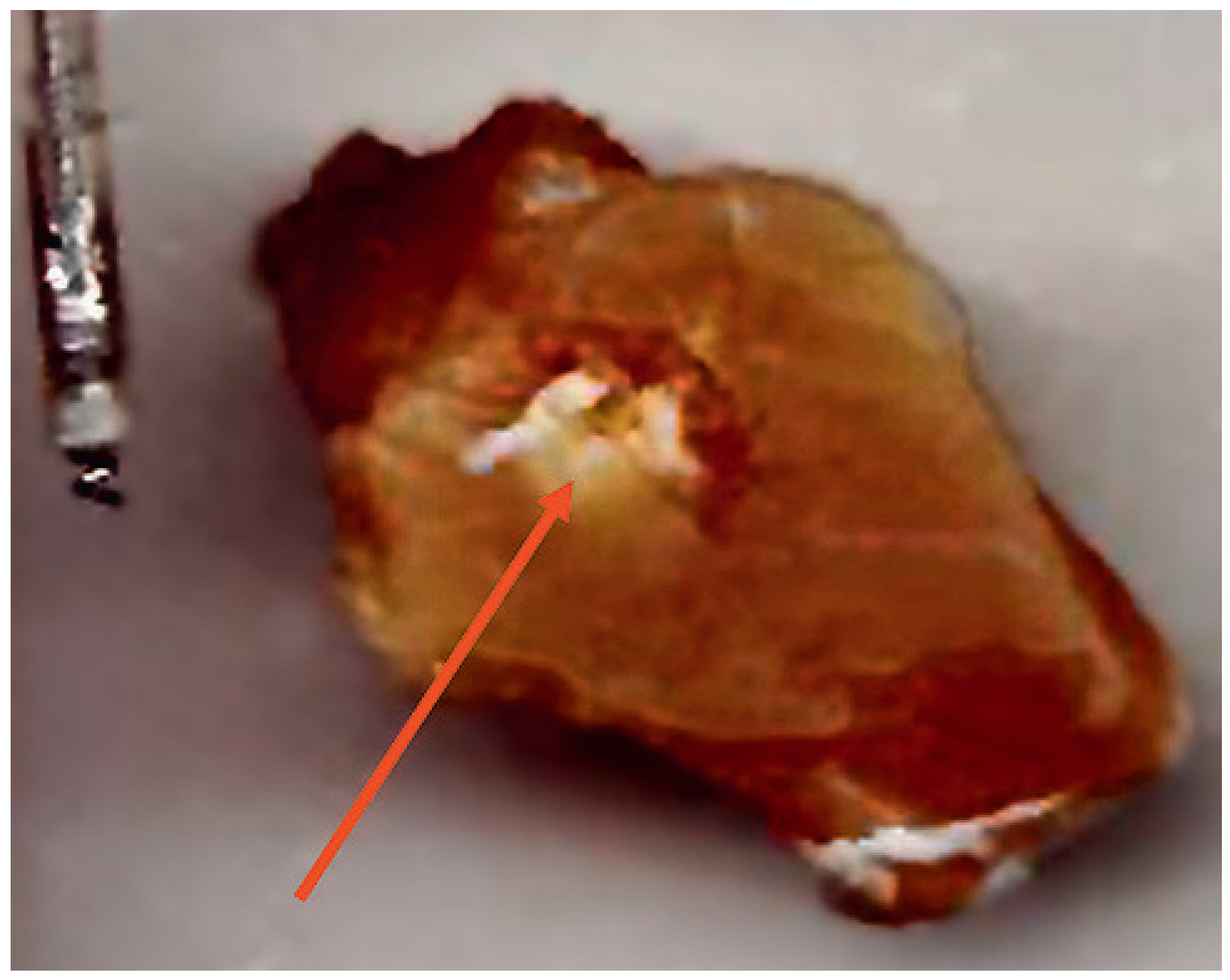
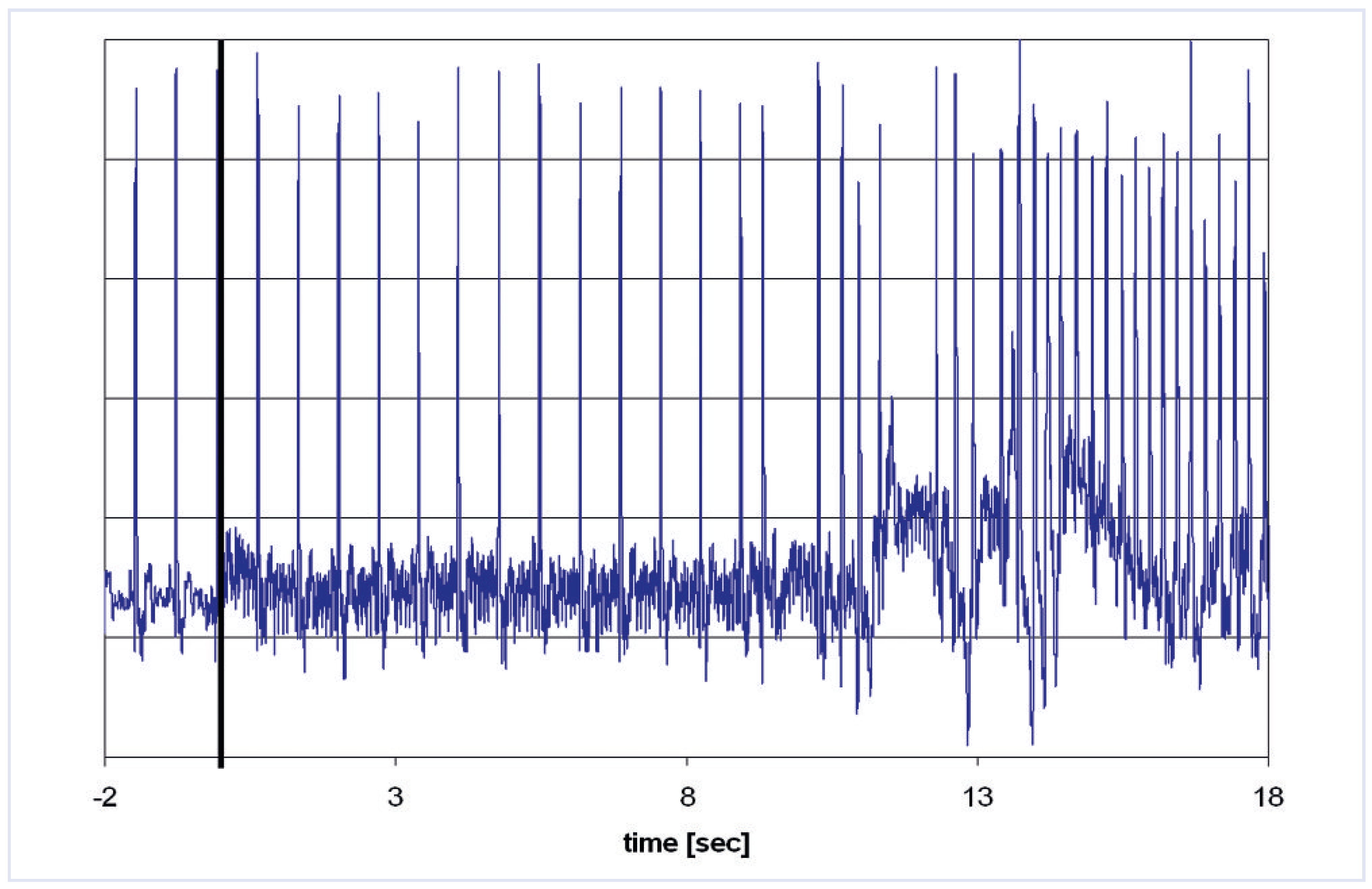
Effects of magnetic resonance imaging on cardiac pacemakers
Animal and human studies
MRI in patients with conventional pacemakers
- -
- Consensus by the radiologist and cardiologist on the need for MR imaging without an imaging alternative. The higher risk of pacemaker-dependent patients must be taken into account.
- -
- Pacemaker patients should only be scanned at experienced centres with expertise in MR imaging and electrophysiology.
- -
- If abandoned endocardial and/or epicardial leads are present, reconsider the need for MRI; minimise SAR and avoid the thoracic region.
- -
- A person with expertise in MRI physics and safety should be involved with the scan to ensure optimal planning of the MRI procedure to minimise risk, and consideration should be given to using scanning parameters that are believed to minimise study risk (e.g., lowest RF power levels, weakest/slowest necessary gradient magnetic fields etc.).
- -
- The pacemaker must be programmed to OFF; i.e., sensing (monitoring)-only mode [OAO, OVO, ODO] or to subthreshold outputs in patients with a reliable intrinsic rhythm. Lead polarity should if possible be programmed to bipolar. Additional diagnostic functions such as magnet response, rate response, ventricular rate regulation and capture management features will need to be disabled. In pacemaker-dependent patients an asynchronous mode should be programmed (VOO or DOO).
- -
- The patient should be monitored by ECG and pulse oximetry during the entire exam.
- -
- An advanced cardiac life support (ACLS)-certified physician should be present at the MRI console during the entire examination to monitor the patient and perform basic and ACLS if needed. A crash cart with an external defibrillator must be present at the MR scanner.
- -
- After completion of the MRI study, the device should be re-interrogated and the parameters reprogrammed to the original settings. Sensing and pacing thresholds should be measured and repeated 1 week and 3 months after the exam.
MR conditional pacemaker
Future directions for magnetic resonance imaging compatible pacing
Conclusions
Conflicts of Interest
References
- Sutton, R.; Kanal, E.; Wilkoff, B.L.; Bello, D.; Luechinger, R.; Jenniskens, I.; Hull, M.; Sommer, T. Safety of magnetic resonance imaging of patients with a new Medtronic EnRhythm MRI SureScan pacing system: clinical study design. Trials. 2008, 9, 68. [Google Scholar] [CrossRef]
- Kalin, R.; Stanton, M.S. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005, 28, 326–328. [Google Scholar] [CrossRef]
- Duru, F.; Luechinger, R.; Scheidegger, M.B.; Luscher, T.F.; Boesiger, P.; Candinas, R. Pacing in magnetic resonance imaging environment: clinical and technical considerations on compatibility. Eur Heart J. 2001, 22, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J.A.; Cahill, P.T.; Pannizzo, F.; Knowles, R.J. Effect of magnetic resonance imaging on DDD pacemakers. Am J Cardiol. 1986, 57, 437–40. [Google Scholar] [CrossRef] [PubMed]
- Fetter, J.; Aram, G.; Holmes, D.R., Jr; Gray, J.E.; Hayes, D.L. The effects of nuclear magnetic resonance imagers on external and implantable pulse generators. Pacing Clin Electrophysiol. 1984, 7, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Pavlicek, W.; Geisinger, M.; Castle, L.; Borkowski, G.P.; Meaney, T.F.; Bream, B.L.; Gallagher, J.H. The effects of nuclear magnetic resonance on patients with cardiac pacemakers. Radiology. 1983, 147, 149–153. [Google Scholar] [CrossRef]
- Bhachu, D.S.; Kanal, E. Implantable pulse generators (pacemakers) and electrodes: safety in the magnetic resonance imaging scanner environment. J Magn Reson Imaging. 2000, 12, 201–204. [Google Scholar] [CrossRef]
- Mollerus, M.; Albin, G.; Lipinski, M.; Lucca, J. Ectopy in patients with permanent pacemakers and implantable cardioverter-defibrillators undergoing an MRI scan. Pacing Clin Electrophysiol. 2009, 32, 772–778. [Google Scholar] [CrossRef]
- Avery, J.K. Loss Prevention case of the month. Not my responsibility! J Tenn Med Assoc. 1988, 81, 523. [Google Scholar]
- Center for Devices and Radiological Health MR Product Reporting Program and Medical Device Report Program. FDA. Available at: http://www.fda.gov/cdrh/ode/primerf6.html. Accessed Nov. 2002.
- Schiebler, M.; Kaut-Watson, C.; Williams, D.L. Both sedated and critically ill require monitoring during MRI (survey). Winter MR. 1994, 41–45. [Google Scholar]
- Irnich, W. Schrittmacher und Kernspintomographie: Frage der Programmierung oder doch kontraindiziert? (Letter). Deutsches Ärzteblatt. 2002, 11, A692. [Google Scholar]
- Irnich, W.; Irnich, B.; Bartsch, C.; Stertmann, W.A.; Gufler, H.; Weiler, G. Do we need pacemakers resistant to magnetic resonance imaging? Europace. 2005, 7, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Alagona, P.; Toole, J.C.; Maniscalco, B.S.; Glover, M.U.; Abernathy, G.T.; Prida, X.E. Nuclear magnetic resonance imaging in a patient with a DDD Pacemaker. Pacing Clin Electrophysiol. 1989, 12 4 Pt 1, 619. [Google Scholar] [CrossRef] [PubMed]
- Inbar, S.; Larson, J.; Burt, T.; Mafee, M.; Ezri, M.D. Case report: nuclear magnetic resonance imaging in a patient with a pacemaker. Am J Med Sci. 1993, 305, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Sommer, T.; Vahlhaus, C.; Lauck, G.; von Smekal, A.; Reinke, M.; Hofer, U.; Block, W.; Traber, F.; Schneider, C.; Gieseke, J.; et al. MR imaging and cardiac pacemakers: in-vitro evaluation and in-vivo studies in 51 patients at 0.5 T. Radiology. 2000, 215, 869–879. [Google Scholar] [CrossRef]
- Schmiedel, A.; Yang, A.; Hackenbroch, M.; Hofer, U.; Schild, H.; Sommer, T. MR imaging of the brain at 1.5Tesla and cardiac pacemakers: In vitro evaluation and safe performance of 71 patient exams (abstract). Paper presented at: MAGMA, 2002; Cannes.
- Garcia-Bolao, I.; Albaladejo, V.; Benito, A.; Alegria, E.; Zubieta, J.L. Magnetic resonance imaging in a patient with a dual-chamber pacemaker. Acta Cardiol. 1998, 53, 33–35. [Google Scholar]
- Gimbel, J.R.; Johnson, D.; Levine, P.A.; Wilkoff, B.L. Safe performance of magnetic resonance imaging on five patients with permanent cardiac pacemakers. Pacing Clin Electrophysiol. 1996, 19, 913–919. [Google Scholar] [CrossRef]
- Coman, J.A.; Martin, E.T.; Ramza, B.M.; et al. Pacemaker safety during magnetic resonance imaging at 1.5 Tesla (Abstract). JAm Coll Cardiol. 2001, 37 (Suppl. A), 436A. [Google Scholar]
- Schwitter, J. (Ed.) CMR Update, 1st ed.; Zuerich, 2008. [Google Scholar]
- Hayes, D.L.; Vlietstra, R.E. Pacemaker malfunction. Ann Intern Med. 1993, 119, 828–835. [Google Scholar] [CrossRef]
- Luechinger, R.; Duru, F.; Scheidegger, M.B.; Boesiger, P.; Candinas, R. Force and torque effects of a 1.5-Tesla MRI scanner on cardiac pacemakers and ICDs. Pacing Clin Electrophysiol. 2001, 24, 199–205. [Google Scholar] [CrossRef]
- Shellock, F.G.; Tkach, J.A.; Ruggieri, P.M.; Masaryk, T.J. Cardiac pacemakers, ICDs, and loop recorder: evaluation of translational attraction using conventional (“long-bore”) and “short-bore” 1.5- and 3.0-Tesla MR systems. J Cardiovasc Magn Reson. 2003, 5, 387–397. [Google Scholar] [CrossRef]
- Luechinger, R.; Duru, F.; Zeijlemaker, V.A.; Scheidegger, M.B.; Boesiger, P.; Candinas, R. Pacemaker reed switch behavior in 0.5, 1.5, and 3.0 Tesla magnetic resonance imaging units: are reed switches always closed in strong magnetic fields? Pacing Clin Electrophysiol. 2002, 25, 1419–23. [Google Scholar] [CrossRef]
- Sommer, T.; Naehle, C.P.; Yang, A.; Zeijlemaker, V.; Hackenbroch, M.; Schmiedel, A.; et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006, 114, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Nordbeck, P.; Weiss, I.; Ehses, P.; Ritter, O.; Warmuth, M.; Fidler, F.; et al. Measuring RF-induced currents inside implants: Impact of device configuration on MRI safety of cardiac pacemaker leads. Magn Reson Med. 2009, 61, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Roguin, A.; Zviman, M.M.; Meininger, G.R.; Rodrigues, E.R.; Dickfeld, T.M.; Bluemke, D.A.; Lardo, A.; Berger, R.D.; Calkins, H.; Halperin, H.R. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004, 110, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Luechinger, R.; Zeijlemaker, V.A.; Pedersen, E.M.; Mortensen, P.; Falk, E.; Duru, F.; et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005, 26, 376–383; discussion 325–377. [Google Scholar] [CrossRef]
- Pohost, G.M.; Blackwell, G.G.; Shellock, F.G. Safety of patients with medical devices during application of magnetic resonance methods. Ann N Y Acad Sci. 1992, 649, 302–312. [Google Scholar] [CrossRef]
- Roguin, A.; Donahue, J.K.; Bomma, C.S.; Bluemke, D.A.; Halperin, H.R. Cardiac magnetic resonance imaging in a patient with implantable cardioverter-defibrillator. Pacing Clin Electrophysiol. 2005, 28, 336–338. [Google Scholar] [CrossRef]
- Martin, E.T.; Coman, J.A.; Shellock, F.G.; Pulling, C.C.; Fair, R.; Jenkins, K. Magnetic resonance imaging and cardiac pacemaker safety at 1.5Tesla. J Am Coll Cardiol. 2004, 43, 1315–1324. [Google Scholar] [CrossRef]
- Nazarian, S.; Roguin, A.; Zviman, M.M.; Lardo, A.C.; Dickfeld, T.L.; Calkins, H.; Weiss, R.G.; Berger, R.D.; Bluemke, D.A.; Halperin, H.R. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006, 114, 1277–1284. [Google Scholar] [CrossRef]
- Nazarian, S.; Halperin, H.R. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm. 2009, 6, 138–143. [Google Scholar] [CrossRef]
- Kanal, E.; Barkovich, A.J.; Bell, C.; Borgstede, J.P.; Bradley, W.G., Jr; Froelich, J.W.; et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007, 188, 1447–1474. [Google Scholar] [CrossRef]
- Roguin, A.; Schwitter, J.; Vahlhaus, C.; Lombardi, M.; Brugada, J.; Vardas, P.; et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace. 2008, 10, 336–346. [Google Scholar] [CrossRef]
- Levine, G.N.; Gomes, A.S.; Arai, A.E.; Bluemke, D.A.; Flamm, S.D.; Kanal, E.; et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: anAmerican HeartAssociation scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007, 116, 2878–2891. [Google Scholar]
 |
© 2010 by the authors. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Luechinger, R.; Duru, F. Do We Need MR Conditional Pacemakers? Cardiovasc. Med. 2010, 13, 70. https://doi.org/10.4414/cvm.2010.01477
Luechinger R, Duru F. Do We Need MR Conditional Pacemakers? Cardiovascular Medicine. 2010; 13(2):70. https://doi.org/10.4414/cvm.2010.01477
Chicago/Turabian StyleLuechinger, Roger, and Firat Duru. 2010. "Do We Need MR Conditional Pacemakers?" Cardiovascular Medicine 13, no. 2: 70. https://doi.org/10.4414/cvm.2010.01477
APA StyleLuechinger, R., & Duru, F. (2010). Do We Need MR Conditional Pacemakers? Cardiovascular Medicine, 13(2), 70. https://doi.org/10.4414/cvm.2010.01477




