Case
In April 2008, a 40-year-old nurse with known HIV infection (no intravenous drug use) since 1991 presented with new onset of exertional dyspnea, chest tightness and dizziness for one month ago. Whilst climbing stairs, the patient had recently begun to need to pause for breath. She complained about mild allergic coryza during springtime, but so far asthma had not been observed. Fever and shivering as well as palpitations, irregular pulse and syncope were denied. No cardiovascular risk factors (smoking, diabetes, hypertension, hypercholesterolemia, myocardial infarction in family history) were present. Antiretroviral treatment for HIV had been stopped in February 2007 due to allergic reaction and toxic liver failure, and the patient currently took no medication at all.
Clinical examination showed an afebrile patient in a good general condition with a low regular pulse (44 bpm) and normal blood pressure (109/59 mm Hg). Heart and lung auscultation were normal, and there was no edema or jugular ingurgitation. Varicosis was present, but no calf tenderness, and no lymphadenopathy.
No acute onset of dyspnoea and lack of risk factors argued against pulmonary embolism. With haemoglobin level at 118 g/L, anaemia could not explain the symptoms. Inflammation markers (leucocytes 4.7 g/L, CRP 5 mg/L), cardiac enzymes and serum lactate dehydrogenase (LDH, sensitive marker for PCP [
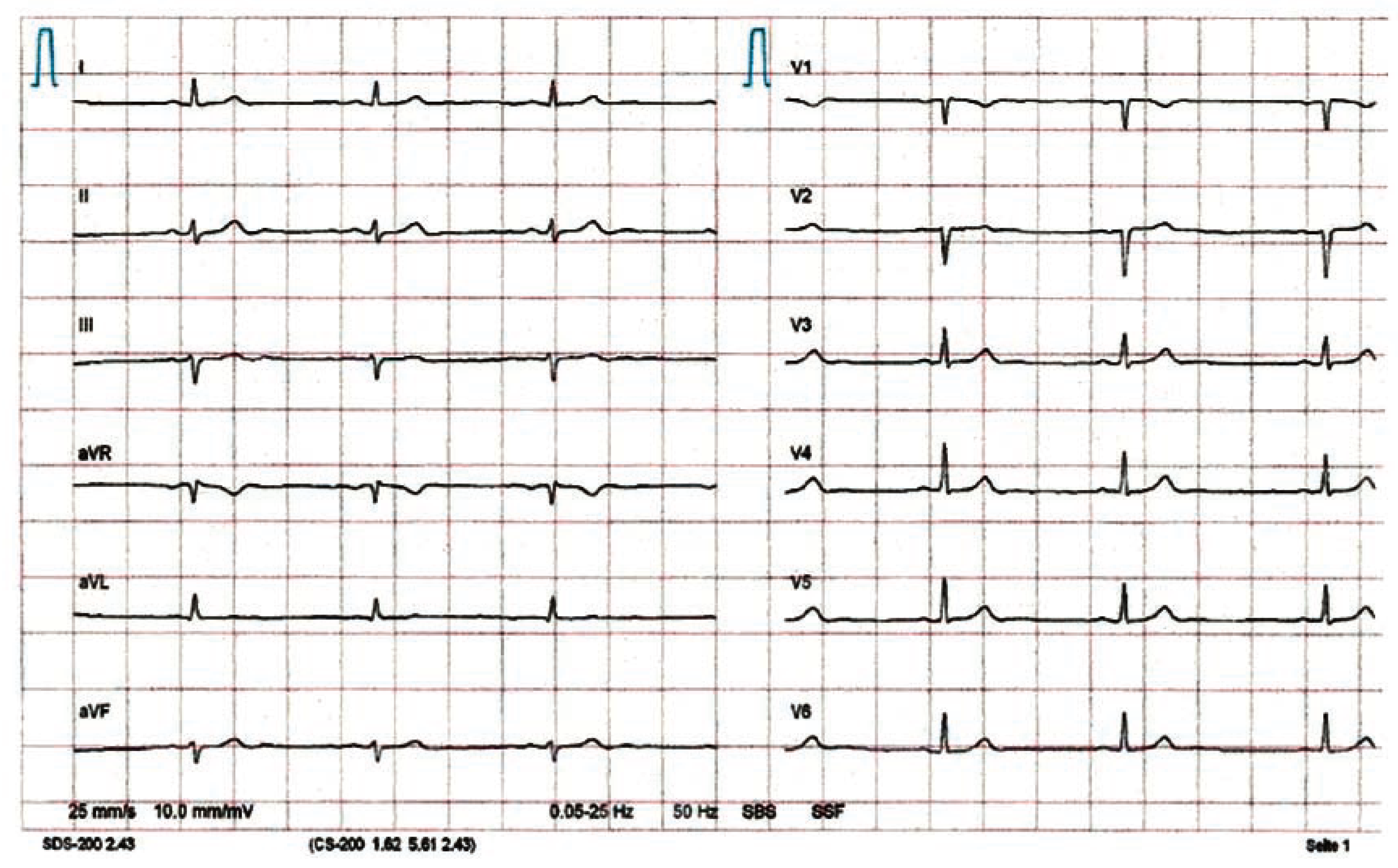
Pneumocystis jiroveci pneumonia]) were normal. Resting ECG showed sinus bradycardia, but no ischaemia (
Figure 1). No electrolyte disorders or thyroid dysfunction could be found. In the absence of antiretroviral therapy, the CD4 count had dropped to 270/μL (20%), but was still above the threshold of 200/μL, below which opportunistic infections like
Pneumocystis jiroveci pneumonia (PCP) become more likely. A chest X-ray yielded a normal result (no interstitial infiltrates, no cardiac enlargement).
To rule out exercise-induced asthma, lung function with the methacholine challenge test was performed which showed no abnormalities. As pulmonary hypertension constitutes one of the differential diagnoses of dyspnea in HIV-patients, the next step was echocardiography—again with normal result (no valvular defect, normal ejection fraction).
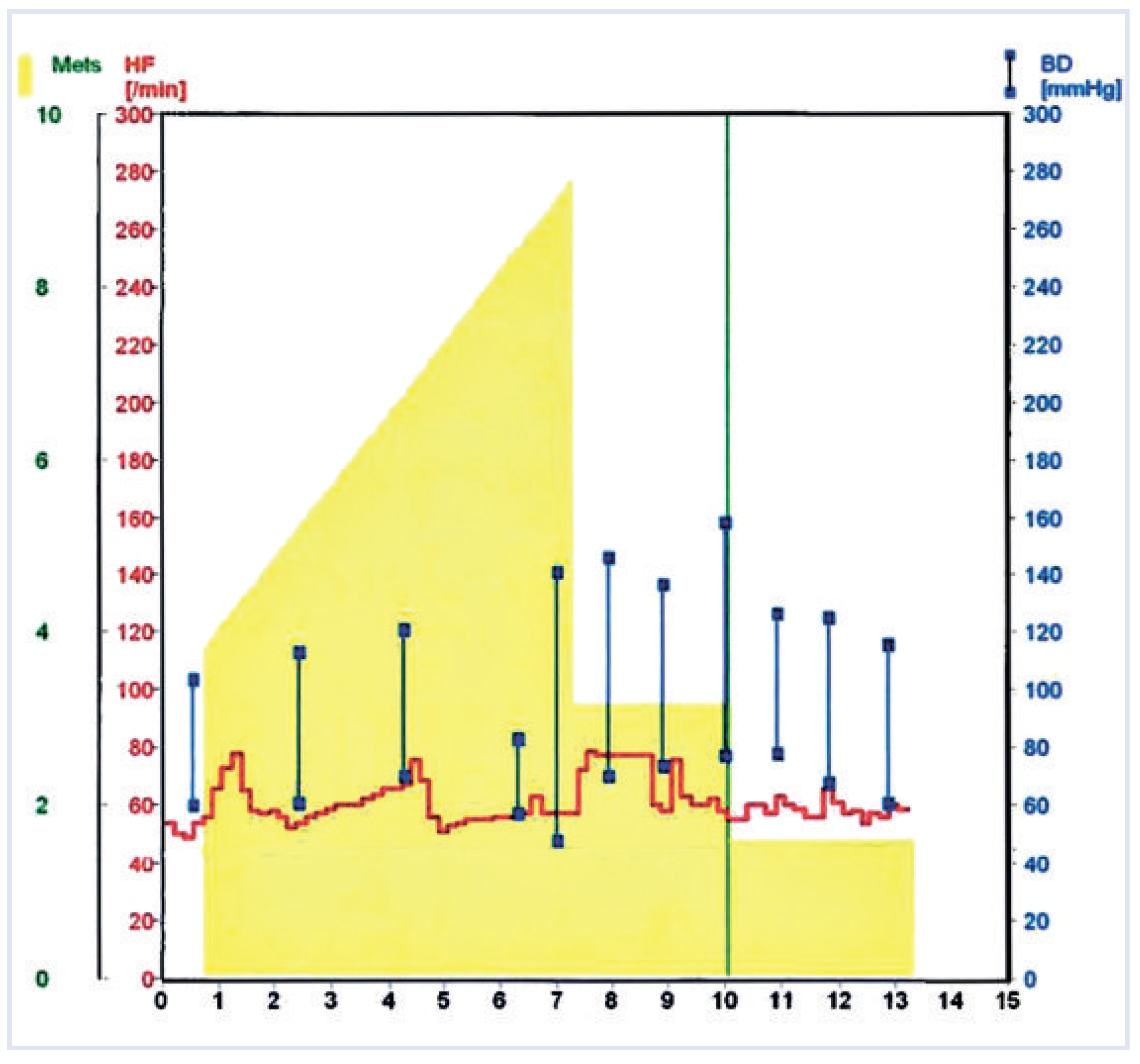
Finally, spiroergometry revealed a cardiac dysrhythmia as the cause of the patient’s symptoms. During the bicycle stress test with sufficient work load, dyspnoea, chest tightness and dizziness were associated with chronotropic incompetence (no appropriate increase of heart rate) (
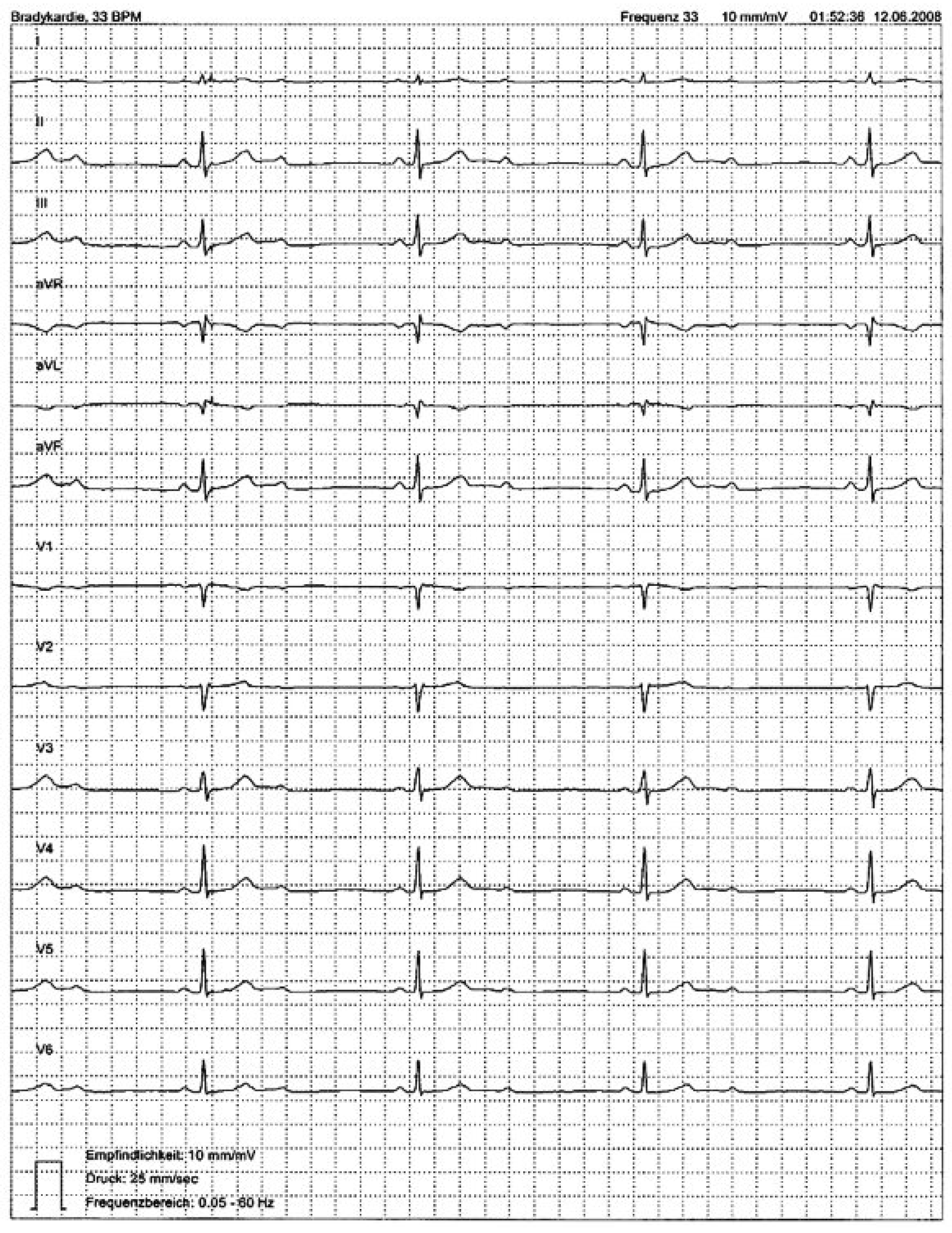
Figure 2). The corresponding ECG showed exercise-induced second degree 3:1 AV block (
Figure 3). Intermittent second degree 2:1 AV block was documented with 24-h Holter monitoring a couple of days later (mean heart rate 48 bpm, minimum 33 bpm, maximum 73 bpm) (
Figure 4).
Borreliosis as a treatable cause of atrioventricular block could be excluded (negative serology). In June 2008 (i.e., three months after the onset of dyspnoea), a DDD pacemaker (SJM Zephyr XL DR) was implanted (stimulation, whenever heart rate <50 bpm). Thereafter, the patient was free of complaints. The proportion of ventricle stimulation increased over time, from 25% in October 2008 to 81% in February 2010 (progression to third degree atrioventricular block). With regard to the HIV, in August 2008, another antiretroviral therapy was initiated which is currently effective and well tolerated.
Discussion
Extensive diagnostic work-up including anamnesis, clinical examination, laboratory analyses, rest ECG, chest X-ray, lung function with methacholine challenge test and echocardiography was frustrating for this middle-aged HIV-infected woman with a new onset of effort dyspnoea, chest tightness and dizziness. It was not until a bicycle stress test was performed that the underlying exercise-induced second degree 3:1 AV block could be diagnosed.
Major causes of AV block are increased vagal tone (e.g., due to athletic training, sleep), fibrosis and sclerosis of the conduction system (50%) [
1] (Lev’s disease in older and Lenegre’s disease in younger patients), ischaemic heart disease (40%) [
1], cardiomyopathy and myocarditis (including borreliosis [
2,
3]), congenital heart disease, familial AV block, hyperkalaemia, infiltrative malignancies (e.g., lymphoma), neonatal lupus syndrome, severe hypo- or hyperthyreoidism, trauma, degenerative neuromuscular diseases, antiarrhythmic drugs, cardiac surgery or catheter ablation of arrhythmias.
The AV node is relatively resistant to permanent injury by infarction or infection, and normal function can be recovered over time. Permanent damage occurs more readily to the bundle of His [
4]. Second degree AV block Mobitz type I (Wenckebach block) is mostly localised above the His bundle and often reversible, whereas second degree AV block Mobitz type II is mostly intra- or infra-Hissian and thus irreversible. In the case of 2:1 conduction (
Figure 4), when prolongation of the PR interval before the block cannot be assessed, atropine test or exercise ECG testing can help to differentiate between second degree AV block type I and II. In type I, AV conduction improves with a prolongation of the Wenckebach periodic (or transition to first degree AV block). In type II, AV conduction deteriorates with transition to 3:1 or 4:1 block, as observed in our patient (
Figure 3).
Since symptomatic AV block is a recognised indication for pacemaker implantation irrespective of the anatomical localisation [
5], further examinations specifying the site of the AV block (e.g., electrophysiology study with His bundle ECG) would have been only of academic value. After ruling out reversible/treatable causes (negative borreliosis serology, normal electrolytes and TSH, normal inflammation markers and cardiac enzymes, no medication, no prior cardiac invasive procedure) a dual chamber pacemaker was implanted, resolving the patient’s symptoms. During the second year of follow-up, progression to third degree AV block was observed.
Given the young age of the patient and the course of the disease, Lenegre’s disease (progressive, fibrotic, sclerodegenerative affliction of the conduction system in younger individuals) [
6] seems the most probable diagnosis. Basically, familial AV block [
7] must be considered too, but the patient’s family history was not suggestive of a hereditary aetiology.
However, cardiac dysrhythmia is not the first thing to consider in an untreated HIV-positive patient with dyspnoea on exertion. Particularly, in the case of advanced immunosuppression (i.e., CD4 count <200/μL),
Pneumocystis jiroveci pneumonia (PCP) forms a major differential diagnosis [
8].
Nevertheless, patients with advanced HIV infection can also have a variety of cardiovascular manifestations including pericarditis, myocarditis, cardiomyopathy, pulmonary vascular disease and pulmonary hypertension, valvular disease (e.g., due to endocarditis in intravenous drug users) and an increased incidence of vascular disease including premature coronary atherosclerosis [
9]. Besides, a 24-h Holter monitoring study in 21 HIV-infected patients revealed an unexpectedly high incidence of conduction disturbances: one patient (4.8%) with intermittent Mobitz type I second degree AV block, two patients (9.5%) with paroxistic 2:1 AV block and one patient (4.8%) with a bifascicular block [
10]. Electrocardiogramm abnormalities including new onset atrioventricular block and bundle branch block resulted in premature termination of a study of concurrent atazanavir- and lopinavir/ritonavir-treatment in HIV patients with extensive antiretroviral resistance [
11]. However, the patient depicted in this case report had not taken antiretroviral drugs for more than one year, when her symptoms appeared.
Whether the progressive atrioventricular conduction disturbance in the presented case is idiopathic (Lenegre’s disease) or associated with HIV-infection remains unclear (no biopsy or MRI was performed). Irrespective of HIV infection, spiroergometry has proven to be a useful second-stage diagnostic tool to differentiate between respiratory and cardiovascular system effort dyspnoea, if the initial evaluation with standard tests is non-diagnostic.