Emboli Protection Devices in Cardiovascular Medicine
Abstract
Introduction
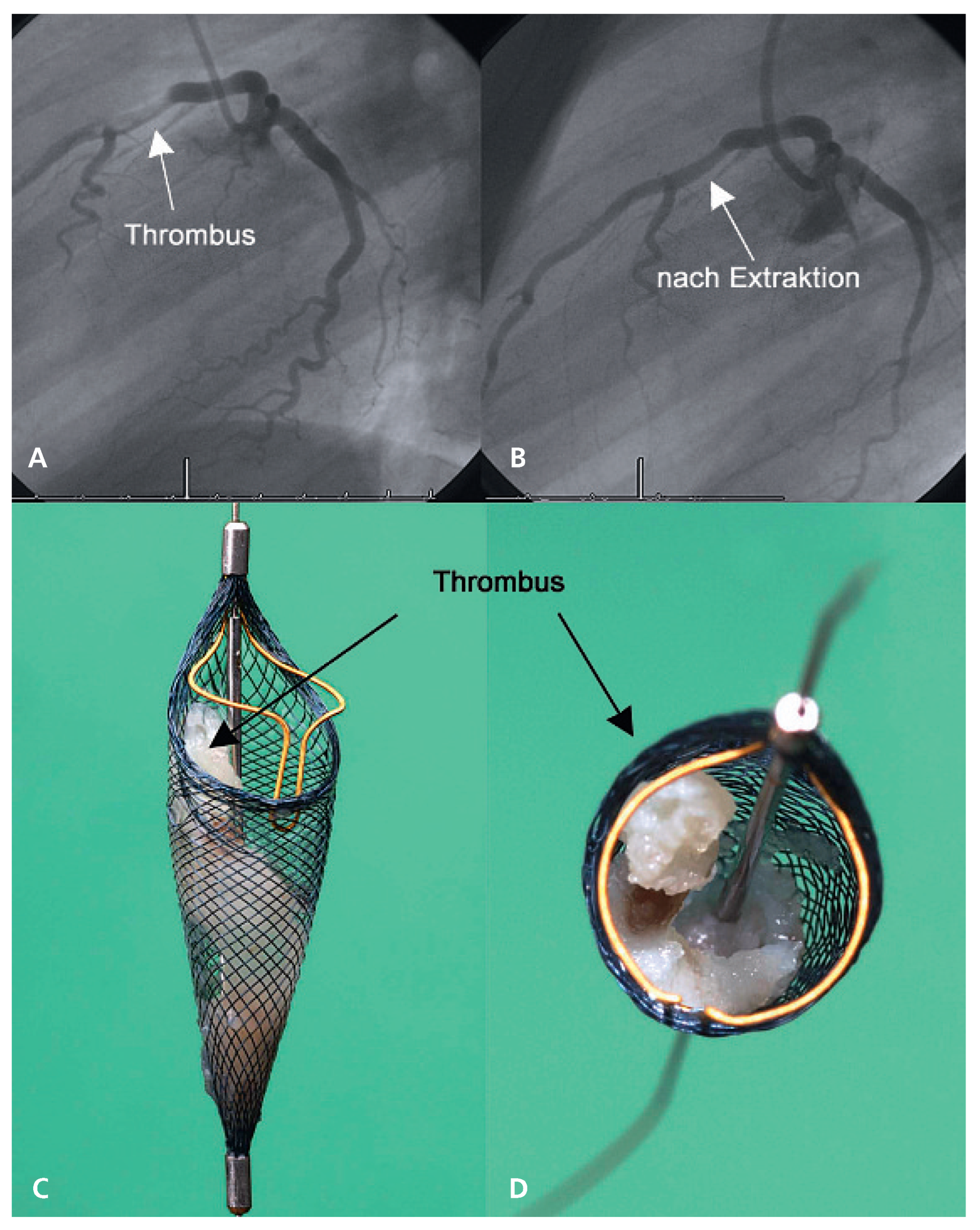
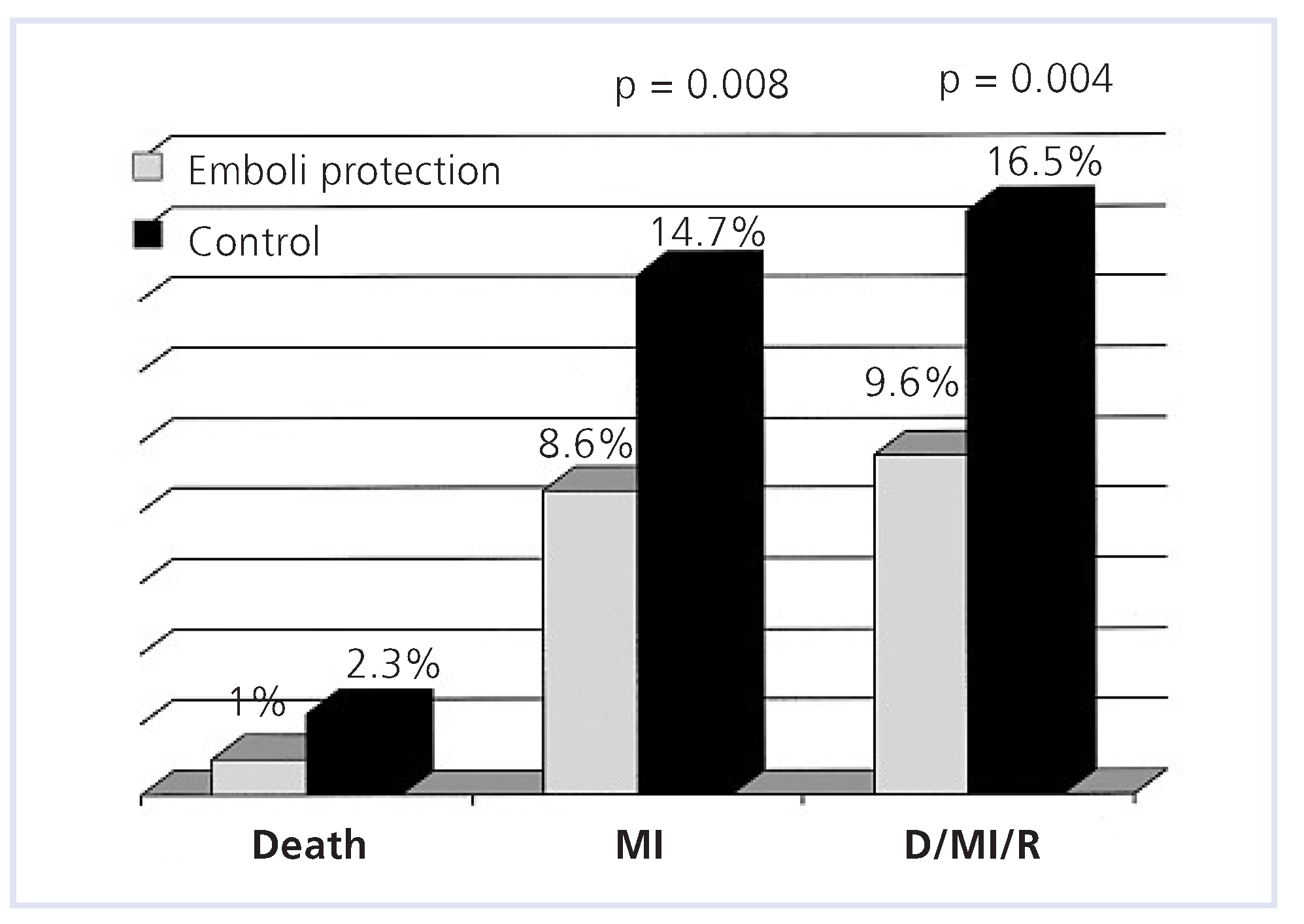
Acute myocardial infarction
Aortocoronary bypass grafts interventions
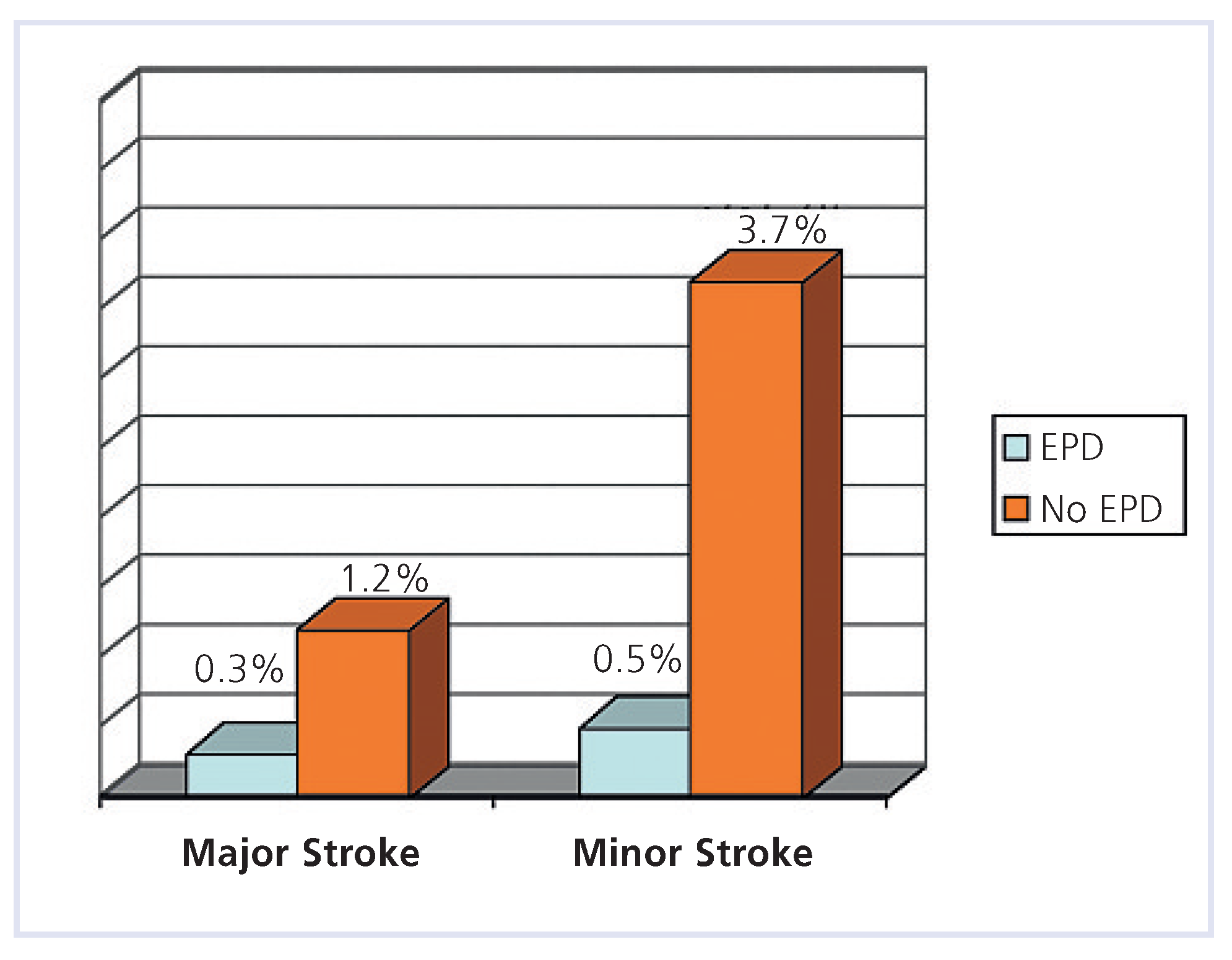
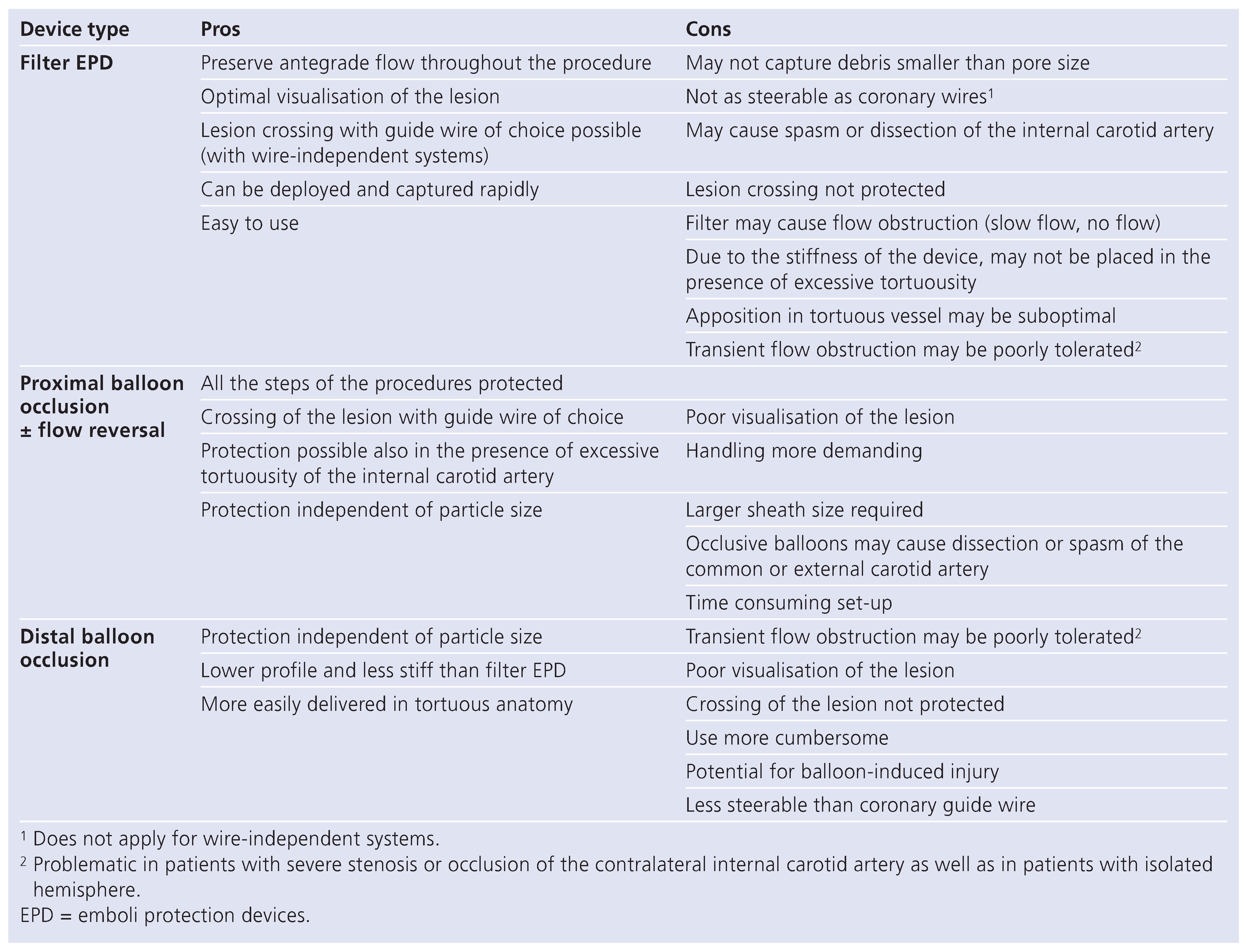
Carotid artery stenting
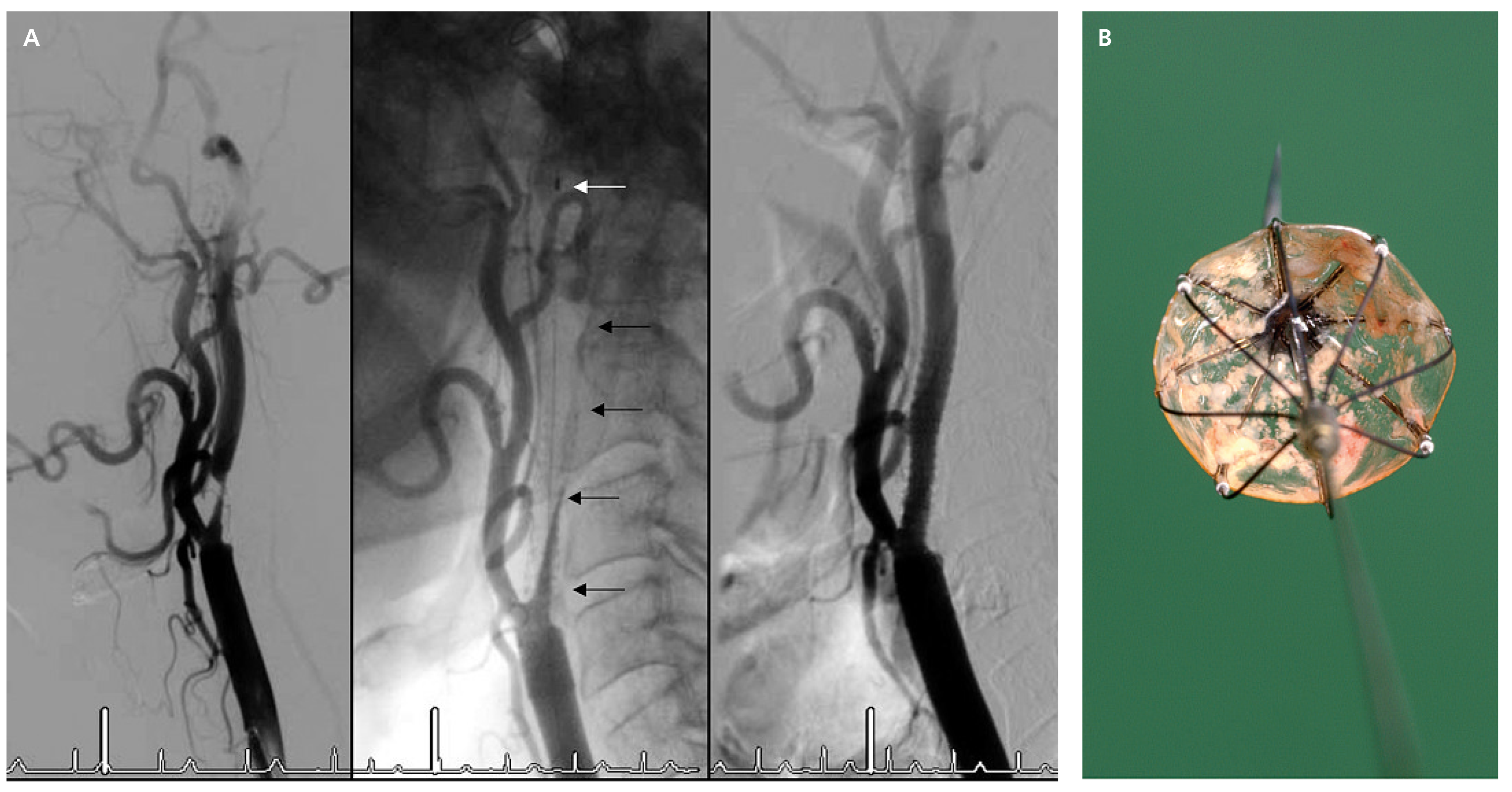
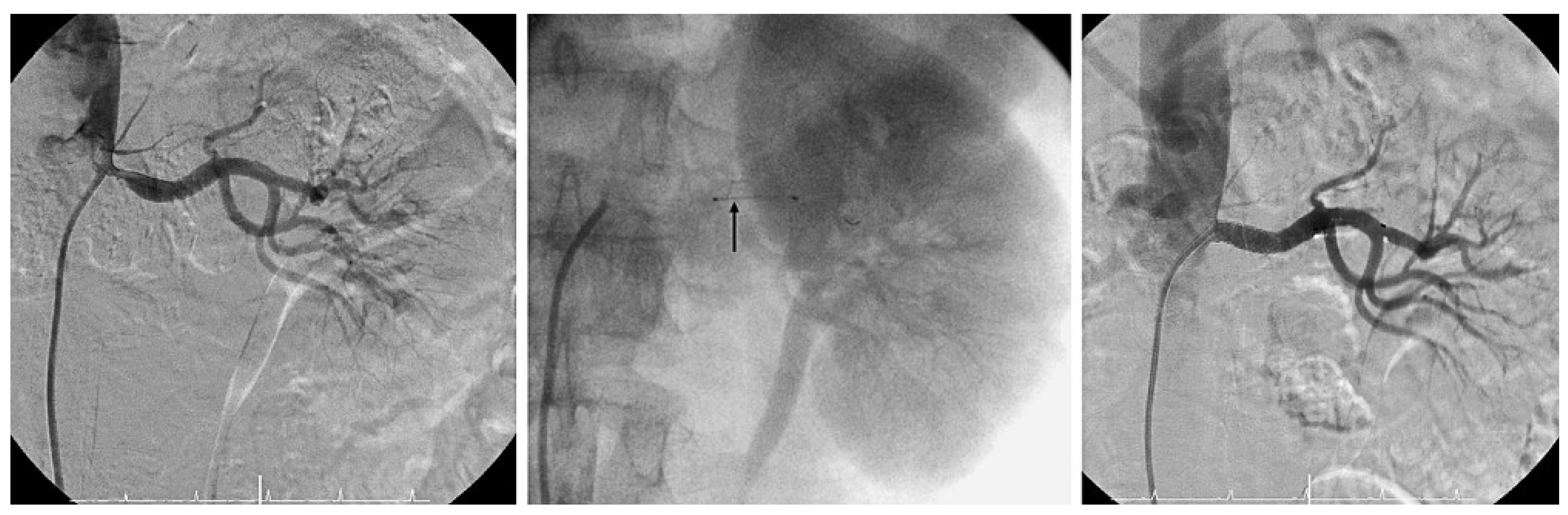
Renal stenting
Conclusions
Conflicts of Interest
References
- Stone, G.W.; Webb, J.; Cox, D.A.; Brodie, B.R.; Qureshi, M.; Kalynych, A.; et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA. 2005, 293, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Suryapranata, H.; Stone, G.W.; Antoniucci, D.; Neumann, F.J.; Chiariello, M. Adjunctive mechanical devices to prevent distal embolization in patients undergoing mechanical revascularization for acute myocardial infarction: a meta-analysis of randomized trials. Am Heart J. 2007, 153, 343–353. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Hasselblad, V.; Tcheng, J.E.; Stone, G.W.; Califf, R.M.; Kastrati, A.; et al. Improved clinical outcomes with abciximab therapy in acute myocardial infarction: a systematic overview of randomized clinical trials. Am Heart J. 2004, 147, 457–462. [Google Scholar]
- Motwani, J.G.; Topol, E.J. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998, 97, 916–931. [Google Scholar] [CrossRef]
- Baim, D.S.; Wahr, D.; George, B.; Leon, M.B.; Greenberg, J.; Cutlip, D.E.; et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002, 105, 1285–1290. [Google Scholar]
- Stone, G.W.; Rogers, C.; Hermiller, J.; Feldman, R.; Hall, P.; Haber, R.; et al. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts. Circulation. 2003, 108, 548–553. [Google Scholar] [PubMed]
- Mauri, L.; Cox, D.; Hermiller, J.; Massaro, J.; Wahr, J.; Tay, S.W.; et al. The PROXIMAL trial: proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: a randomized, prospective, multicenter clinical trial. J Am Coll Cardiol. 2007, 50, 1442–1449. [Google Scholar]
- Roffi, M.; Mukherjee, D.; Chew, D.P.; Bhatt, D.L.; Cho, L.; Robbins, M.A.; et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation. 2002, 106, 3063–3067. [Google Scholar] [PubMed]
- Mehta, S.K.; Frutkin, A.D.; Milford-Beland, S.; Klein, L.W.; Shaw, R.E.; Weintraub, W.S.; et al. Utilization of distal embolic protection in saphenous vein graft interventions (an analysis of 19,546 patients in the American College of Cardiology-National Cardiovascular Data Registry). Am J Cardiol. 2007, 100, 1114–1118. [Google Scholar]
- Roffi, M.; Abou-Chebl, A.; Mukherjee, D. Principles of Carotid Artery Stenting, 1st ed.; Uni-Med: Bremen, Germany, 2008; In Print. [Google Scholar]
- Krapf, H.; Nagele, T.; Kastrup, A.; Buhring, U.; Gronewaller, E.; Skalej, M.; et al. Risk factors for periprocedural complications in carotid artery stenting without filter protection: A serial diffusion-weighted MRI study. J Neurol. 2006, 253, 364–371. [Google Scholar]
- Kastrup, A.; Groschel, K.; Krapf, H.; Brehm, B.R.; Dichgans, J.; Schulz, J.B. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003, 34, 813–819. [Google Scholar]
- Zahn, R.; Mark, B.; Niedermaier, N.; Zeymer, U.; Limbourg, P.; Ischinger, T.; et al. Embolic protection devices for carotid artery stenting: better results than stenting without protection? Eur Heart J. 2004, 25, 1550–1558. [Google Scholar] [CrossRef]
- Ouriel, K.; Wholey, M.H.; Fayad, P.; Katzen, B.T.; Whitlow, P.; Frentzko, M.; et al. Feasibility trial of carotid stenting with and without an embolus protection device. J Endovasc Ther. 2005, 12, 525–537. [Google Scholar] [CrossRef]
- Roffi, M.; Greutmann, M.; Eberli, F.R.; Rainoni, L.; Luscher, T.F.; Amann-Vesti, B.; et al. Starting a carotid artery stenting program is safe. Catheter Cardiovasc Interv. 2008, 71, 469–473. [Google Scholar] [PubMed]
- Roffi, M.; Baumgartner, R.W.; Eberli, F.R. Images in cardiology. No reflow during carotid stenting. Heart (British Cardiac Society). 2006, 92, 538. [Google Scholar]
- Roffi, M.; Greutmann, M.; Schwarz, U.; Luscher, T.F.; Eberli, F.R.; AmannVesti, B. Flow impairment during protected carotid artery stenting: impact of filter device design. J Endovasc Ther. 2008, 15, 103–109. [Google Scholar] [PubMed]
- Zahn, R.; Ischinger, T.; Mark, B.; Gass, S.; Zeymer, U.; Schmalz, W.; et al. Embolic protection devices for carotid artery stenting: is there a difference between filter and distal occlusive devices? J Am Coll Cardiol. 2005, 45, 1769–1774. [Google Scholar] [CrossRef]
- Cremonesi, A.; Manetti, R.; Setacci, F.; Setacci, C.; Castriota, F. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003, 34, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M. Angioplasty for renal artery stenosis: technical improvements and results. In Electronic Update of the 7th edition of the Textbook Braunwald’s Heart Disease; Zipes, D.P., Libby, P., Bonow, R.O., Braunwald, E., Eds.; Saunders: Philadelphia, 2006. [Google Scholar]
- Henry, M.; Klonaris, C.; Henry, I.; Tzetanov, K.; Le Borgne, E.; Foliguet, B.; et al. Protected renal stenting with the PercuSurge GuardWire device: a pilot study. J Endovasc Ther. 2001, 8, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Holden, A.; Hill, A.; Jaff, M.R.; Pilmore, H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int. 2006, 70, 948–955. [Google Scholar] [CrossRef][Green Version]
- Roffi, M.; Abou-Chebl, A.; Mukherjee, D. Principles of Carotid Artery Stenting, 1st ed.Uni-Med: Bremen, Germany, in Print.[Green Version]
- Roffi, M.; Mukherjee, D. Carotid artery disease management. In Manual of Vascular Diseases, 1st ed.; Rajagopalan, S., Mohler, E., Mukherjee, D., Eds.; Lippincott William and Wilkins: New York, NY, USA, 2005. [Google Scholar][Green Version]
- Surder, D.; Kucher, N.; Eberli, F.R.; Roffi, M. Intracoronary thrombus in a 26-year-old man. Eur Heart J. 2006, 27, 2631. [Google Scholar] [CrossRef] [PubMed]

 |
© 2009 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Roffi, M. Emboli Protection Devices in Cardiovascular Medicine. Cardiovasc. Med. 2009, 12, 45. https://doi.org/10.4414/cvm.2009.01390
Roffi M. Emboli Protection Devices in Cardiovascular Medicine. Cardiovascular Medicine. 2009; 12(2):45. https://doi.org/10.4414/cvm.2009.01390
Chicago/Turabian StyleRoffi, Marco. 2009. "Emboli Protection Devices in Cardiovascular Medicine" Cardiovascular Medicine 12, no. 2: 45. https://doi.org/10.4414/cvm.2009.01390
APA StyleRoffi, M. (2009). Emboli Protection Devices in Cardiovascular Medicine. Cardiovascular Medicine, 12(2), 45. https://doi.org/10.4414/cvm.2009.01390




