Introduction Survival into adulthood
Congenital heart disease (CHD) is found in 1% of live births. Survival of children with CHD has improved dramatically since the second half of the 20th century. Historically, several factors can be found that have contributed to this progress: open heart surgery and cardiopulmonary bypass first allowed palliation of complex defects; earlier interventions even in the newborn became possible due to technical progress; new procedures have been designed continuously, so that the consequences of longstanding cyanosis and pulmonary hypertension can increasingly be avoided; ongoing progress in imaging techniques ensures assessment of defects is more accurate and early diagnosis of complications is possible [
1]; improvements in electrophysiological treatment, valve replacement and pharmacology protect patients from complications that would have otherwise been fatal.
Death in the first year of life still accounts for 65% of deaths related to CHD [
2], but once that sensitive period is passed, survival into adulthood is common and prognosis, in the least complex defects, comes close to that of the general population [
3]. Patients with more complex defects often experience late complications [
2,
3] and undergo repeat heart surgery [
2,
3,
4,
5,
6].
Prognosis remains limited in many defects depending on their complexity [
3,
7]. The actuarial survival after an observation period of 45 years is 78% in adults with CHD compared to the general population [
3], and survival beyond 50 years is rare in the most complex CHD conditions [
5].
Demand for end of life care
Adults with congenital heart disease (ACHD) generally are aware of their limited life expectancy. Adolescents may lack this awareness [
8], but it develops as decisions about career, insurance and family planning must be made [
6,
9,
10] and complications occur [
4].
In the Swiss ACHD patient organisation CUORE MATTO, a general awareness of mortality and limited prognosis is expressed in many ways:
Attendance at meetings on advance directives is above average; numerous members attend the funerals of fellow members; and mothers with CHD express relief when their children reach adulthood without being orphaned. Dealing with a limited prognosis is very individual and is more easily shared with peers than with “healthy” persons, including health care professionals.
The difference in awareness between patients and physicians is summarised by Hockley et al. 1988, “… patients cannot avoid the issue of dying even if we (health care professionals) do” [
11]. There need be nothing morbid in this and it does not preclude efforts to live as normal a life as possible. In his book
“What is your expiry date” [
12], a Canadian ACHD describes how the on-going awareness of one’s mortality can lead to a deep appreciation for life.
Research on patients with congestive heart failure (CHF) shows that prognostic information is a priority [
13,
14], patients want to know the likely manner of their death [
13] and they often work out for themselves that they are dying [
11,
15]. As death approaches, the preference to forego resuscitation becomes more common [
16]. Having lived with the ever-present threat of mortality, however, does not exclude ACHD from having the same needs for information as those with “adult-onset” cardiac disease.
Various organisations encourage ACHD to participate in decision making by completing advance directives [
17,
18]. In order to make the choices required in advance directives, such as resuscitation preferences, proxy spokesperson, and care at the end of life, patients need to understand treatment alternatives. The recommendations on management of ACHD published in 2001 under the auspices of the Canadian Cardiovascular Society explicitly state that alternatives to invasive procedures, such as those associated with palliative care (PC), are a treatment option [
17].
The WHO definition of palliative care is shown in
Table 1 [
19]. In short, PC is an approach that improves the quality of life of dying patients and their families, through the prevention and relief of suffering.
The Supply
Steady progress in cardiology may explain many cardiologists’ death-denying attitude, as illustrated by research on end of life decisions. Cardiologists rarely write “do not resuscitate” (DNR) orders, regardless of prognosis and patient preference [
20,
21]. Studies show that they tend to delay giving DNR orders until 3 days or less before death, and don’t systematically make comfort care plans [
20].
The distress of dying from heart disease is well known. As death approaches, disability and symptoms become more frequent [
16] and patients commonly prefer not to be resuscitated [
16,
21]. If the sole focus of healthcare professionals is curative and providing life prolonging treatment, frustration for both the healthcare professional, and the patient and the family, will be common and quality of care will be poor [
22,
23].
In the UK, the national service framework requires cardiologists to work with PC staff, but recent estimates show that only 4% of dying heart patients receive PC [
14].
Mortality of ACHD
Survival in CHD operated in childhood has improved over the last decades, and for the less complex defects survival is very close to that of the normal population [
3]. Nevertheless, Kaplan-Meyer curves that level out after a few years still mean that there are early deaths. In 25% of the cases, the deaths are sudden leaving the majority of the deaths preceded by a more or less long and distressing terminal phase [
24,
25] in which PC would be necessary and helpful.
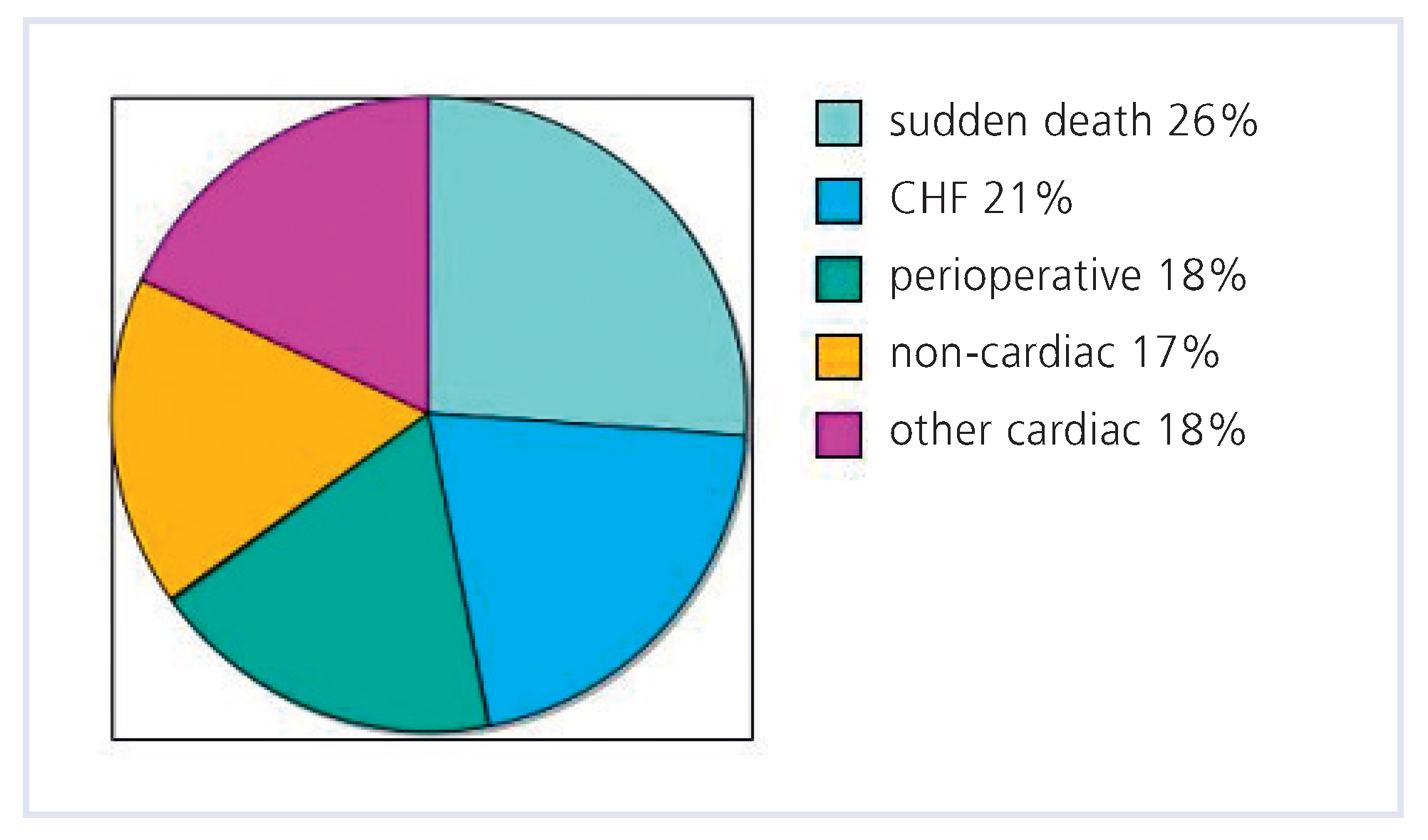
Few studies have focused on modes of death for ACHD. In a cross-sectional study in 2609 patients treated in an ACHD referral center, 197 of the 199 deaths were examined [
24]. Mean age at death was 37 ± 15 years (range 18–80). The most common causes of death were sudden death (26%), progressive heart failure (21%) and perioperative (18%) (
Figure 1). As this center primarily treats patients with complex CHD, the distribution of the causes of death may differ from patients with less complex conditions.
From the comprehensive national registry for CHD in Finland, recent results (2007) show somewhat different figures [
25]. Of the 6024 patients who survived their first operation, 592 (9%) died during the 45-year follow-up period. CHD was the cause of death in the majority of patients (397 [67%]). The main mode for CHD-related deaths was heart failure (40%). Other modes included perioperative (26%), sudden (22%), and other cardiovascular (12%) deaths. The number of deaths caused by neurological and respiratory diseases was higher and the number of accidental deaths was lower than expected.
These data show that most ACHD will experience progressive disease and a terminal stage before dying, in which they could benefit from treatment according to PC standards.
As death in young patients is unusual in most cardiologists’ professional experience, ACHD are at risk of receiving more aggressive care than they would wish for [
26].
Medical challenges Diagnosing dying
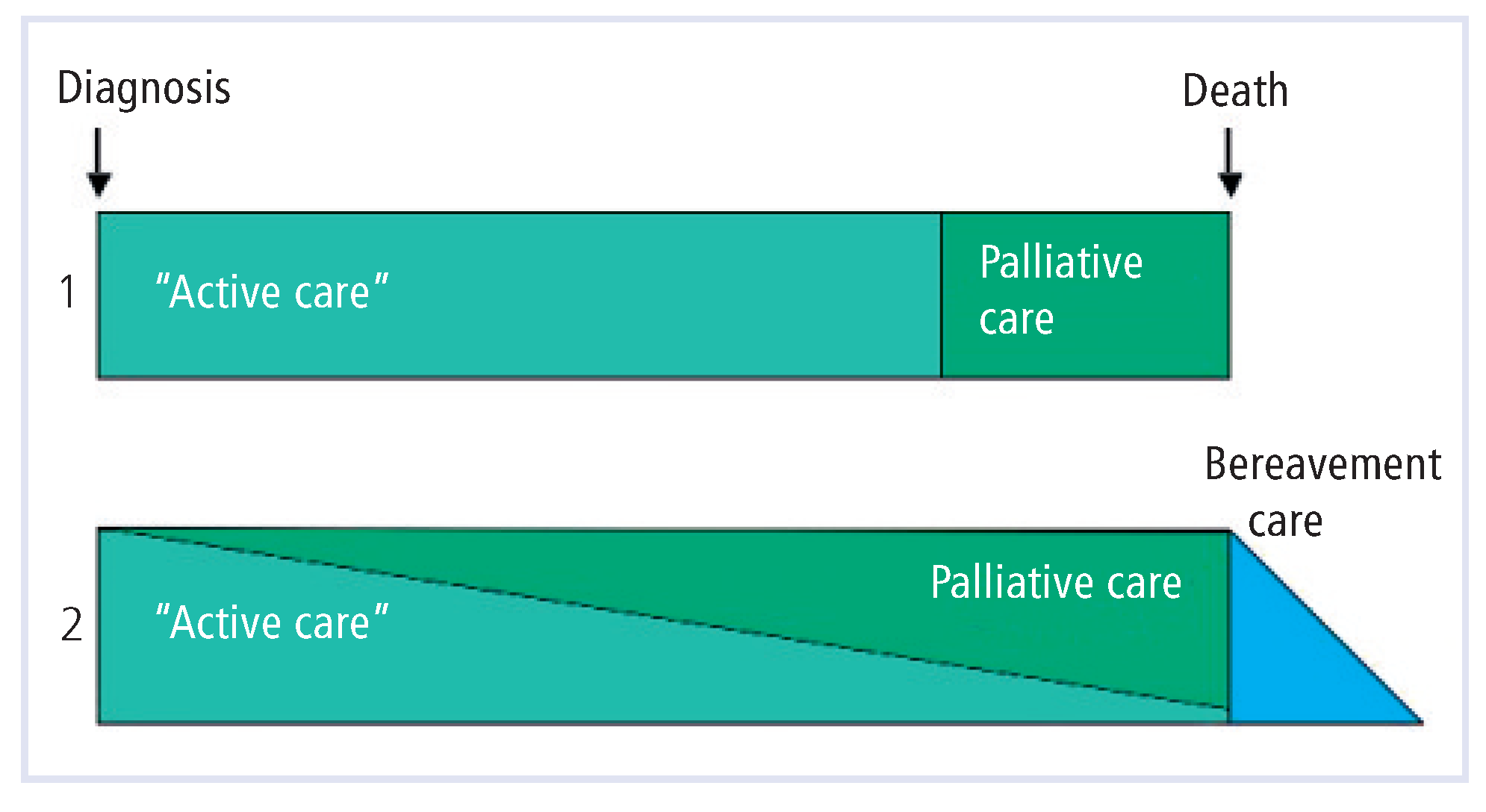
In the oncology world, PC can be provided either as an alternative to “active” treatment, or along with life-sustaining treatment, whenever the patient’s distress justifies it.
Figure 2 shows these two models as the WHO has defined them [
14].
In cardiac patients, there is little difference between disease specific versus symptomatic treatment [
14,
27]. However, thorough symptom control goes far beyond control of congestive heart failure, and includes support by a multiprofessional team as described in the WHO definition of PC. The second model in Figure 2, where disease specific treatment and palliative care overlap, is then appropriate, as long phases of stability at a low level always warrant a comprehensive effort to improve quality of life. The approaching end of life, or dying, still needs to be diagnosed in order to shift the treatment paradigm from more aggressive to supportive care. Futile aggressive treatment in the last days of life happens far too often [
26].
In the USA, inclusion in a hospice program under Medicare requires the prognosis to be 6 months or less (see the first model in Figure 2) [
14]. It has been shown that doctors’ estimates of prognosis for cardiac patients are often grossly inaccurate and usually overly optimistic [
16,
28]. Objective criteria aren’t easy to define [
29].
Research on prognostication has been done for a long time in view of heart transplantation [
30]. Some of the clinical prognostic factors found in these studies might help to identify when end of life decision making is necessary.
Ideally, burdensome tests should be avoided. For diagnosing dying, dynamic prognostic criteria are proposed such as increasing frequency of hospital admissions, severe symptoms (NYHA III–IV) in spite of optimal therapy, increasing difficulties to control exacerbations, no identifiable reversible precipitant, severe impairment of functional capacity, objective evidence of severe cardiac dysfunction, and deteriorating renal function [
29,
31,
32]. The Liverpool Care Pathway for the Care of the Dying [
33] is currently being adapted for cardiology patients and will define pragmatic criteria for diagnosing dying. Its application in ACHD may require further adaptation.
Sudden death remains more or less unpredictable, but its high incidence would justify addressing it with patients and their families as they may wish to be prepared [
14]. Issues need to be discussed such as preference for resuscitation (yes or no) and subsequent training of family members in CPR, options for implantable cardio defibrillator placement, and written advance directives in case patients are no longer able to discuss decisions regarding their care.
Symptom control
Patients’ use of words to describe their symptoms may be very different from medical terminology [
34]. Patients are often unsure which symptoms are caused by heart disease versus drug side effects or comorbidity [
14]. Therefore, history taking in irreversibly advancing disease needs to be done patiently, picking up clues, encouraging patients to explain what they are experiencing and showing interest in symptoms not belonging specifically to cardiac disease.
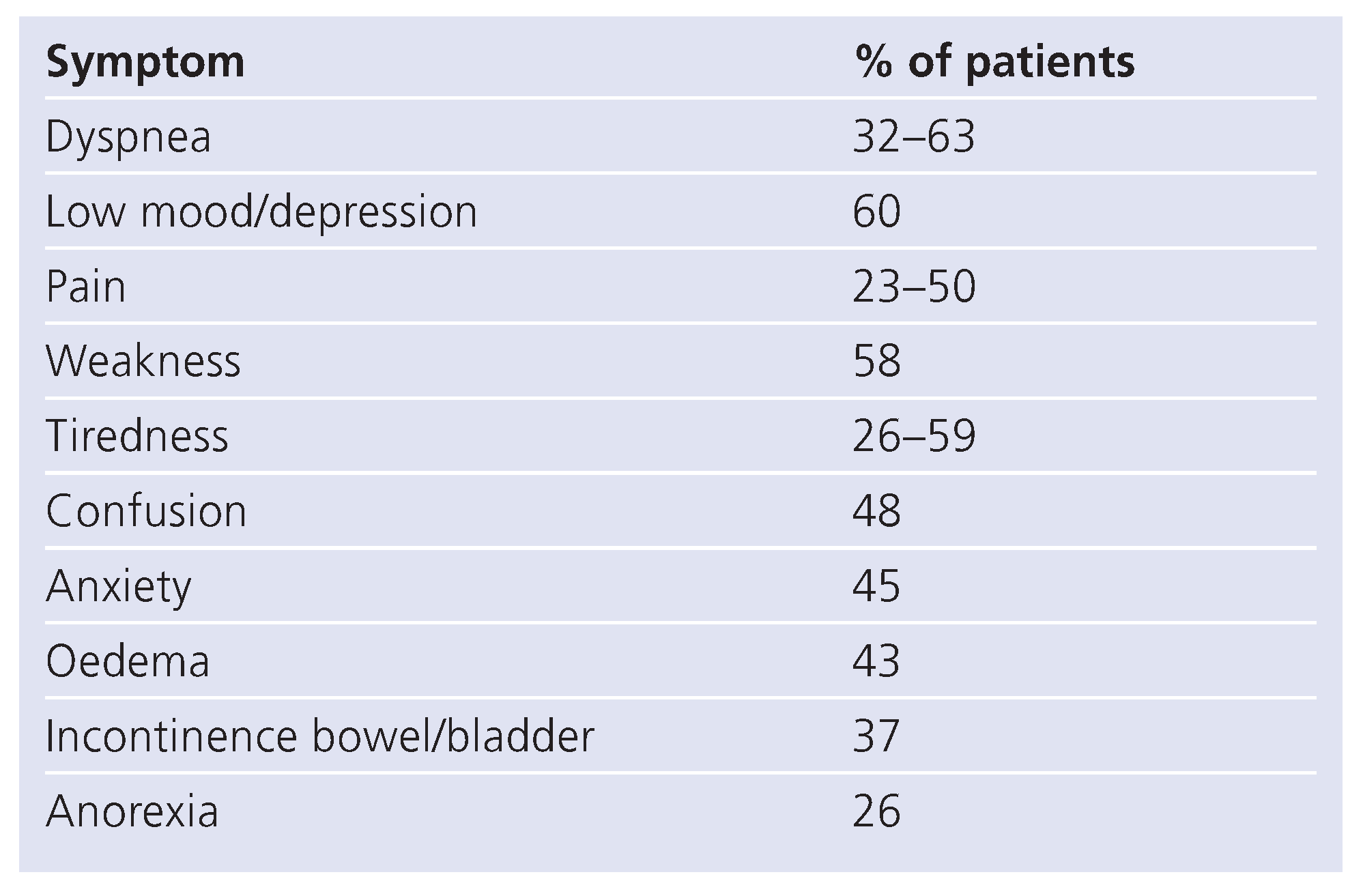
Several studies of symptom prevalence in CHF have been conducted in elderly patients and have shown a list of symptoms that is very similar to that in oncology PC patients (
Table 2) [
22,
35,
36]. In particular there is a high incidence of pain. Causes of pain in congestive heart failure include oedema [
14], muscle and joint pain due to increasing weakness, discomfort related to pressure sores, angina pectoris, and other unspecific causes of pain. Pain needs careful evaluation and judicious treatment. Dame Cicely Saunders defined the pain experienced in the end of life as “total pain” which encompasses physical, spiritual, psychological, and social (including financial and bureaucratic) pain [
37].
The focus of palliative care is maximising the patient’s experienced quality of life. Therefore, both subjective distress and objective findings are assessed and treated. The subjective benefit of pharmaceutical therapies needs to be evaluated, and the non-essential medications need to be discontinued toward the end of life.
Palliative care in oncology, neurology, AIDS and geriatrics has developed effective means to control symptoms according to their pathophysiology by an unconventional and sometimes off-label use of medications [
36]. It also has a wealth of unspecific ways to relieve distress. It will be necessary to determine their value in all heart patients including the younger ACHD.
Communication issues
Oncology controversies about truth-telling concluded that sharing prognostic information with patients was helpful [
38,
39,
40].
When talking to ACHD and their parents, it appears that the possibility of early death is a frequent topic of family conversation preceding any worsening of the patient’s health. Communication between patients and healthy relatives can become strained when the reality of impending death becomes more concrete. Patients tend to wish for more openness than their families can deal with [
10,
11].
Heart patients often perceive the nearing of death [
13,
15] and would like to talk about it with health care professionals. In particular, they try to know about their likely manner of death. There are barriers to communication on both the patients’ and the professionals’ sides (
Table 3) [
13].
Relatives also tend to perceive that their family member is dying, and discussions about this take place within families. However, they have even less opportunities than patients have, to discuss their observations and fears with health care professionals. Their communication needs include information as well as support [
15].
Early discussion of issues around death has the following advantages:
A dual approach combining life prolonging treatment and envisaging possible death feels less defeatist; discussing uncertain recovery is seen as a strength and builds trust [
31]; patient choices (place of death, resuscitation, etc.) are known; and addressing issues of sudden death may decrease trauma to families [
14].
Communication about end of life also needs to take place within healthcare teams. Disagreements cause conflicting messages to be given to patients and families, increasing their distress, and the many issues around end of life need to be dealt with in a multiprofessional team approach [
31].
Team challenges Psychosocial issues
An important goal of PC is to give patients a chance to bring to closure issues of their own life. Good symptom control frees energies for attending to other issues such as unfinished business, material and moral legacies, goodbyes, and to address needs of all involved [
41].
The patients’ need for prognostic information has been discussed earlier. It can help them to make choices about treatment, to write advance directives and to express their preferences regarding place of death and level of care or procedures at the end of life [
14]. They may seek support in sorting out relationship problems and attending to cultural and spiritual requirements [
36,
41]. The team needs to take care to provide some form of continuity to the patients [
23]. Deepening relationships between patients, families and healthcare professionals allow for continued discussions and empower the patients to express their wishes as they evolve.
Depression needs to be evaluated and treated, as it is found in up to 50% of elderly CHF patients [
14]. Its prevalence in ACHD has been estimated in one small pilot study to be about 36% [
42] but has not been studied in ACHD who are at the end of their lives.
Patients need very concrete support as functional impairments increase and mobility is reduced [
16].
Families need support to adjust to the situation of impending death of their loved one, discuss their fears and avoid exhaustion [
11,
36]. In retrospect, many wish to have been at the patient’s bedside at the moment of death [
15]. This means not only a need for ongoing information but also for support when facing those frightening last moments when their loved one is dying.
Some of those issues may be more difficult for young ACHD whose family systems are very complex. Much of what follows is based on the experiences of the authors and has not been addressed scientifically:
As ACHD join together in organisations, many will marry someone who has a similar congenital heart disease. ACHD often have children, most of whom are healthy. The ACHD’s death will often leave young children behind, along with parents and siblings with a lifelong history of having a loved one with a life threatening illness, and inlaws who may have a similar history.
Financial difficulties often add to the distress of dying [
37]. In many countries an ACHD’s financial situation may be difficult due to low income, limited insurability and the lack of possibilities to make provisions for loved ones.
Another source of distress is the perceptible insecurity of healthcare professionals, caused by a number of factors. ACHD confront professional carers with unimaginable anatomy, physiology and haemodynamics. Death seems to come as a surprise even to ACHD specialists [
43] and ACHD are “The Rare Patient” for the majority of healthcare professionals. Given that ACHD will normally have a shorter life expectancy than most cardiac patients, healthcare professionals may have a tendency to use futile aggressive treatments to fight against death. These professionals should be encouraged to seek advice from both ACHD and PC specialists.
Bereavement
In the complex family systems of ACHD, some of the known risk factors for complicated grief are likely to be overrepresented and cumulated. These are listed in
Table 4 [
44].
Complicated grief is a syndrome that includes a wide range of symptoms (physical, emotional, psychosocial, including strong aggression) which can pose a threat to self or others [
45].
To minimise pathological bereavement following an ACHD’s death, it is imperative that families have prognostic information and feel prepared [
11]. PC must be provided, focusing on the whole spectrum of needs of families, given that “the last days permanently color memories” [
46].
Sudden death is an independent risk factor for complicated grief. In addition, if healthcare professionals recognise that death is imminent but do not prepare the family, the family will experience the loss of their loved one like a sudden death.
Dependent relationships are the natural consequence of having physical restrictions, but dependency can also exist at a psychological level. Siblings are often ambivalent about a chronically ill brother or sister, given the many ways that the illness affects their own childhood. Parents (especially mothers) may cling to their handicapped child regardless of its adult age.
As ACHD are tending to intermarry, there already is an increasing number of ACHD who have lost their spouse. Heart disease and widowhood in a bereaved person is an accumulation of risk factors that might be even more serious if the couple had similar congenital defects.
Children aged 15 years or less can be either a sibling or child of the ACHD. In both cases attention needs to be paid to the child’s bereavement, especially if the other relatives are also at risk for bereavement problems.
An ongoing assessment of each family member’s risks for complicated grief should be part of any case history, and be updated as families change and grow.
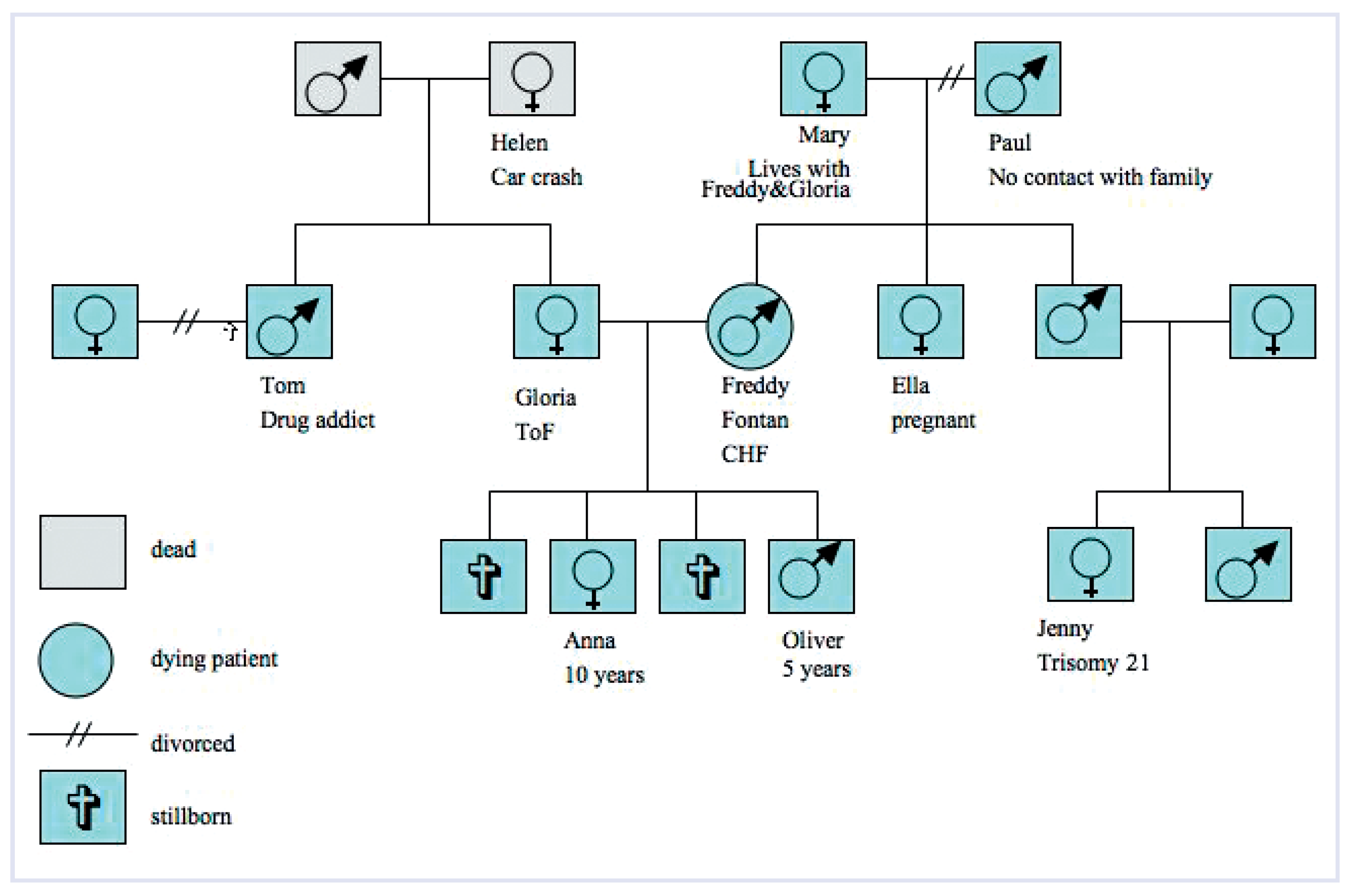
Tools for this are well established in PC, namely genograms with a note on each person stating potential risk factors (
Figure 3) [
45] or checklists of risks, to be filled for each family member.
Immediately after a patient’s death, the “180 seconds treatment” is the minimum each physician should be capable of providing [
47]. This includes extending sympathy to the bereaved, expressing the importance of their contribution to the patient’s care, including those who are absent at that moment, and offering opportunities for discussing “left-over” medical questions at a future time.
ACHD centers should work with existing bereavement services of local PC teams or join them in an effort to build up bereavement follow-up and counseling. Bereavement counseling is effective in at-risk persons [
44].
Conclusions
There is a rich knowledge base relevant to PC in the cardiology literature. Studies have been published on symptom distress from CHF in elderly patients, and on communication needs of dying heart patients. A scientific approach to prognostication in advanced heart disease is being sought. The national strategy in the UK to further a dual, curative and palliative approach gives rise to more research and the preparation of guidelines to end of life care in cardiology.
In most European countries, PC exists to some degree. Although it is mostly organised around cancer, AIDS and neurologic diseases, heart patients could benefit from specialised PC support teams in hospitals, home care, hospices and palliative care units. Their families could benefit from existing bereavement follow-up and counseling, which should be available in a supportive advisory capacity for existing heart failure programs as well as for ACHD centres.
Congenital cardiology has an approach that is rather close to that of PC as it cares for the whole person, offering a support system to help patients live as actively as possible, using a team approach to address the needs of patients and their families, and enhancing quality of life, while positively influencing the course of illness.
Our hope for ACHD is that their dying will soon be seen as a normal process, so that decisions of beginning, withholding or withdrawing treatments are made with a view to enhance their quality of life until the end.
However, much still needs to be done in the next years (
Table 5). Palliative care specialists can work together with multiprofessional cardiology teams to ensure that the principles of total care are available for the cardiac patients and their families throughout the course of the illness and through death to bereavement care.
Therefore, networking with existing PC institutions is the one thing you can do and need to do immediately! A good life needs a good ending, and that is a challenge for our health care teams.