Immunosuppression in Cardiac Transplantation: State of the Art and New Drugs
Summary
Introduction
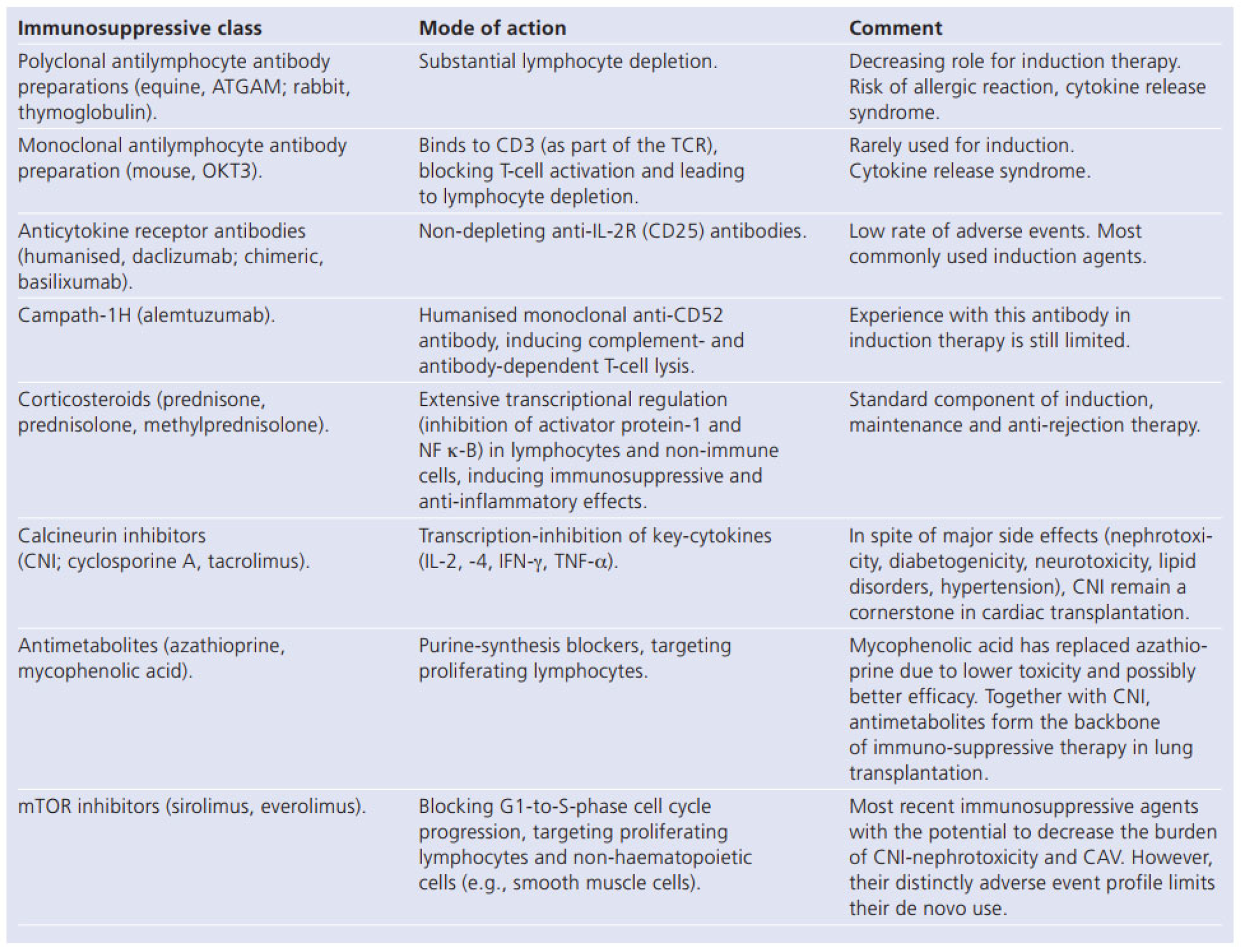
Immunosuppression in Cardiac Transplantation: State of the Art
Corticosteroid Withdrawal and CNI Minimisation
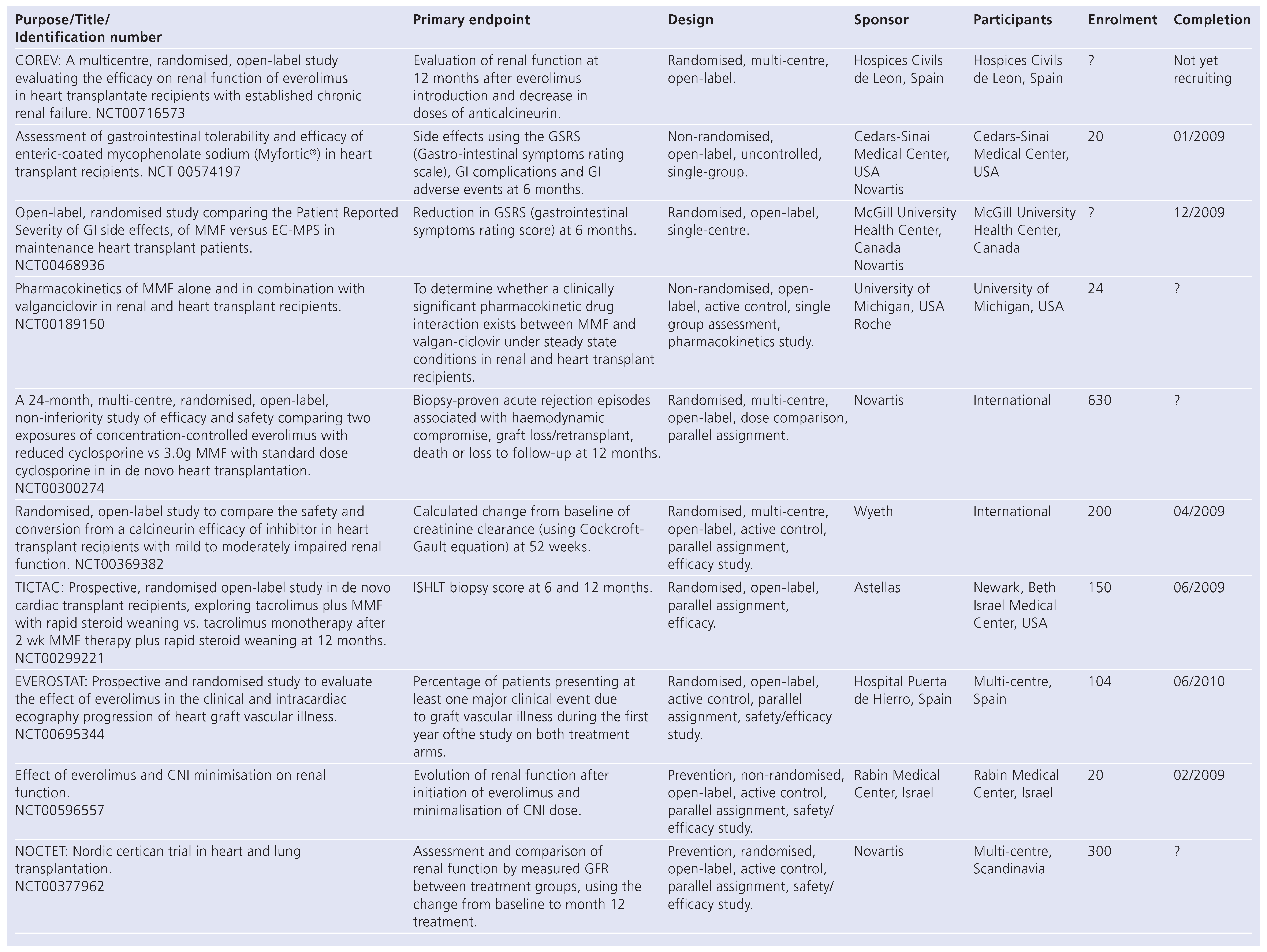
New Immunosuppressive Drugs in Solid Organ Transplantation
Small Molecules
Biological Agents
Conclusion
Conflicts of Interest
References
- Barnard, C.N. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967, 41, 1257–1274. [Google Scholar]
- Taylor, D.O.; Edwards, L.B.; Aurora, P.; et al. Registry of the international society of heart and lung transplantation: twenty-fourth official adult heart transplant report-2008. J Heart Lung Transplant. 2008, 27, 7943–7956. [Google Scholar] [CrossRef]
- Jessup, M.; Brozena, S.C. State-of-the-art strategies for immunosuppression. Curr Opin Cardiol. 2007, 12, 536–542. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Page, R.L.; Zolty, R.; et al. Drug therapy in the heart transplant recipient: part III: common medical problems. Circulation 2005, 111, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Chan, M.C.; Gao, S.Z.; et al. Glucose intolerance, as reflected by hemoglobulin A1c level, is associated with the incidence and severity of transplant coronary artery disease. J Am Coll Cardiol. 2004, 43, 1034–1041. [Google Scholar] [CrossRef][Green Version]
- Taylor, D.O.; Edwards, L.B.; Boucek, M.M.; et al. The registry of the international society of heart and lung transplantation: twenty-fourth official adult heart transplant report-2004. J Heart Lung Transplant. 2004, 23, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Al-khaldi, A.; Robbins, R.C. New directions in cardiac transplantation. Annu Rev Med. 2006, 57, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Cantarovich, M. Renal protective strategies in heart transplant patients. Curr Opin Cardiol. 2007, 22, 133–138. [Google Scholar] [CrossRef]
- Ojo, A.O.; Held, P.J.; Port, F.K. Chronic renal failure after transplantation of a non-renal organ. N Engl J Med. 2003, 349, 931–490. [Google Scholar] [CrossRef]
- Meiser, B.; Reichart, B.; Adamidis, I.; et al. First experience with de novo calcineurin-inhibitor-free immunosuppression following cardiac transplantation. Am J Transplant. 2005, 1, 827–831. [Google Scholar] [CrossRef]
- Vazque de Prada, J.A.; Vilchez, F.G.; Cobo, M.; et al. Sirolimus in de novo heart transplant recipients with severe renal impairment. Transpl Int. 2006, 19, 245–248. [Google Scholar]
- Zuckermann, A.O.; Aliabadi, A.Z. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2008. Oct 13: Epub ahead of print. [Google Scholar]
- Meiser, B.; Kaczmarek, I.; et al. Low-dose tacrolimus/sirolimus and steroid withdrawal in heart recipients is highly efficacious. J Heart Lung Transplant. 2007, 26, 598–603. [Google Scholar] [PubMed]
- Lehmkuhl, H.B.; Mai, D.; Dandel, M.; et al. Observational study with everolimus (Certican) in combination with low-dose cyclosporine in de novo heart transplant recipients. J Heart Lung Transplant. 2007, 26, 700–704. [Google Scholar] [CrossRef]
- Snell, G.; Levvey, B.J.; Chin, W.; et al. Sirolimus allows renal recovery in lung and heart transplant recipients with chronic renal impairment. J Heart Lung Transplant. 2002, 21, 540–546. [Google Scholar] [CrossRef]
- Zakliczynski, M.; Nozynski, J.; Zakliczynska, P.; et al. Deterioration of renal function after replacement of cyclosporine with sirolimus in five patients with severe renal impairment late after heart transplantation. Transplant Proc. 2003, 35, 2331–2332. [Google Scholar] [CrossRef]
- Gustafsson, F.; Ross, H.; Delgado, M.S.; et al. Sirolimus-based immunosuppression after cardiac transplantation: predictors of recovery from calcineurin inhibitor-induced renal dysfunction. J Heart Lung Transplant. 2007, 26, 998–1003. [Google Scholar] [CrossRef]
- Vincenti, F.; Kirk, A. What’s next in the pipeline. Am J Transplant. 2008, 8, 1972–1981. [Google Scholar] [CrossRef]
- Stalder, M.; Birsan, T.; Hubble, R.W.; et al. In vivo evaluation of the novel calcineurin inhibitor ISATX247 in non-human primates. J Heart Lung Transplant. 2003, 22, 1343–1352. [Google Scholar] [CrossRef]
- Gaber, O.A.; Busque, S.; Mulgaonkar, S.; et al. A phase IIB multicenter, open label, concentration-controlled trial in de novo renal transplantation. Am J Transplant. 2008, 8 (Suppl. S2), 336. [Google Scholar]
- Evenou, J.P.; Brinkmann, V.; Towbin, H.; et al. Enzymatic and cellular characteristization of NVP-AEB071 (AEB), a novel and selective protein kinase C (PKC) inhibitor that blocks early T cell activation, and its use to define the role of PKC in T cells. Am J Transplant. 2006, 6 (Suppl. S2), 1026. [Google Scholar]
- Evenou, J.P.; Thille, N.; Cottens, C.; et al. NVP-AEB071 (AEB), a novel protein kinase C inhibitor, abrogates early mouse T cell activation without affecting activation induced cell death. Am J Transplant. 2006, 6 (Suppl. S2), 1029. [Google Scholar]
- Podder, H.; Kahan, B.D. A novel target for selective transplant immunosuppression. Expert Opin Ther Targets. 2004, 8, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Mendez, R.; Pescovitz, M. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant. 2007, 7, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.J.; Brickelmaier, M.; Majeau, G.R.; et al. Alefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and Cd2/Cd16-dependent apoptosis of CD2+ cells. J Immunol. 2002, 168, 4462–4471. [Google Scholar] [CrossRef]
- Vincenti, F. Costimulation blockade in autoimmunity and transplantation. J Allergy Clin immunol. 2008, 121, 299–306. [Google Scholar] [CrossRef]
 |
 |
© 2009 by the author. Attribution - Non-Commercial - NoDerivatives 4.0
Share and Cite
Korom, S. Immunosuppression in Cardiac Transplantation: State of the Art and New Drugs. Cardiovasc. Med. 2009, 12, 272. https://doi.org/10.4414/cvm.2009.01454
Korom S. Immunosuppression in Cardiac Transplantation: State of the Art and New Drugs. Cardiovascular Medicine. 2009; 12(10):272. https://doi.org/10.4414/cvm.2009.01454
Chicago/Turabian StyleKorom, Stephan. 2009. "Immunosuppression in Cardiac Transplantation: State of the Art and New Drugs" Cardiovascular Medicine 12, no. 10: 272. https://doi.org/10.4414/cvm.2009.01454
APA StyleKorom, S. (2009). Immunosuppression in Cardiac Transplantation: State of the Art and New Drugs. Cardiovascular Medicine, 12(10), 272. https://doi.org/10.4414/cvm.2009.01454




