Introduction
Plaque rupture with intracoronary thrombosis is the underlying mechanism in the majority of patients with acute coronary syndromes (ACS). Thrombosis is the result of platelet activation and aggregation, in addition to release of tissue factor and several coagulation factors. A critical step in thrombus formation is thrombin because it engenders (1.) the conversion of fibrinogen to fibrin, (2.) self-amplification via activation of the intrinsic clotting pathway, and (3.) direct platelet activation. Unfortunately, thrombin activity persists when bound to fibrin, and therefore, thrombin-rich coronary clot may be a nidus for ongoing thrombus formation and can cause recurrent myocardial infarction and cardiovascular death.
![Cardiovascmed 11 00007 g005 Cardiovascmed 11 00007 g005]()
The European [
1] and North American [
2] consensus guidelines on antithrombotic treatment in the ACS setting are updated on a regular basis, and provide rather general than practical recommendations for the various drug regimens. The authors have reviewed the available evidence of antithrombin treatment regimens during a recent expert consensus conference. This article provides practical dose schemes of various antithrombin treatment strategies in ACS patients who are managed with an early invasive strategy, with special attention to the therapy that has been initiated at presentation prior to referral to the cardiac catheterisation laboratory.
Unfractionated Heparin (UFH)
Mechanism of action
UFH is a thrombin inhibitor of animal origin and acts primarily by forming a ternary complex with thrombin and antithrombin. Therefore, its action depends on antithrombin levels. It also binds to factor Xa with an anti-Xa/anti-IIa activity of 1:1. Only a minority of the heparin chains contain the active pentasaccharide sequence that binds to antithrombin. Heparin also exhibits prothrombogenic properties by binding thrombin and fibrin at the same time. These UFH-thrombinfibrin complexes cannot be inactivated by heparin and potentially increase the affinity of thrombin for fibrin. UFH shows variable and non-linear dose response, and its effect cannot be well predicted in the individual patient. The main reasons for its inconsistent action include (1.) poor bioavailability, (2.) unspecific protease and cell binding, complex formation with platelet factor-4, (3.) GP IIb/IIIa receptor activation, and (4.) rebound of thrombin formation after drug discontinuation. The inconsistent action of UFH mandates regular dose adjustments and close anticoagulant monitoring by measuring the thrombin time during upstream treatment or monitoring of the activated clotting time (ACT) during PCI in the catheterisation laboratory.
Antithrombotic strategy
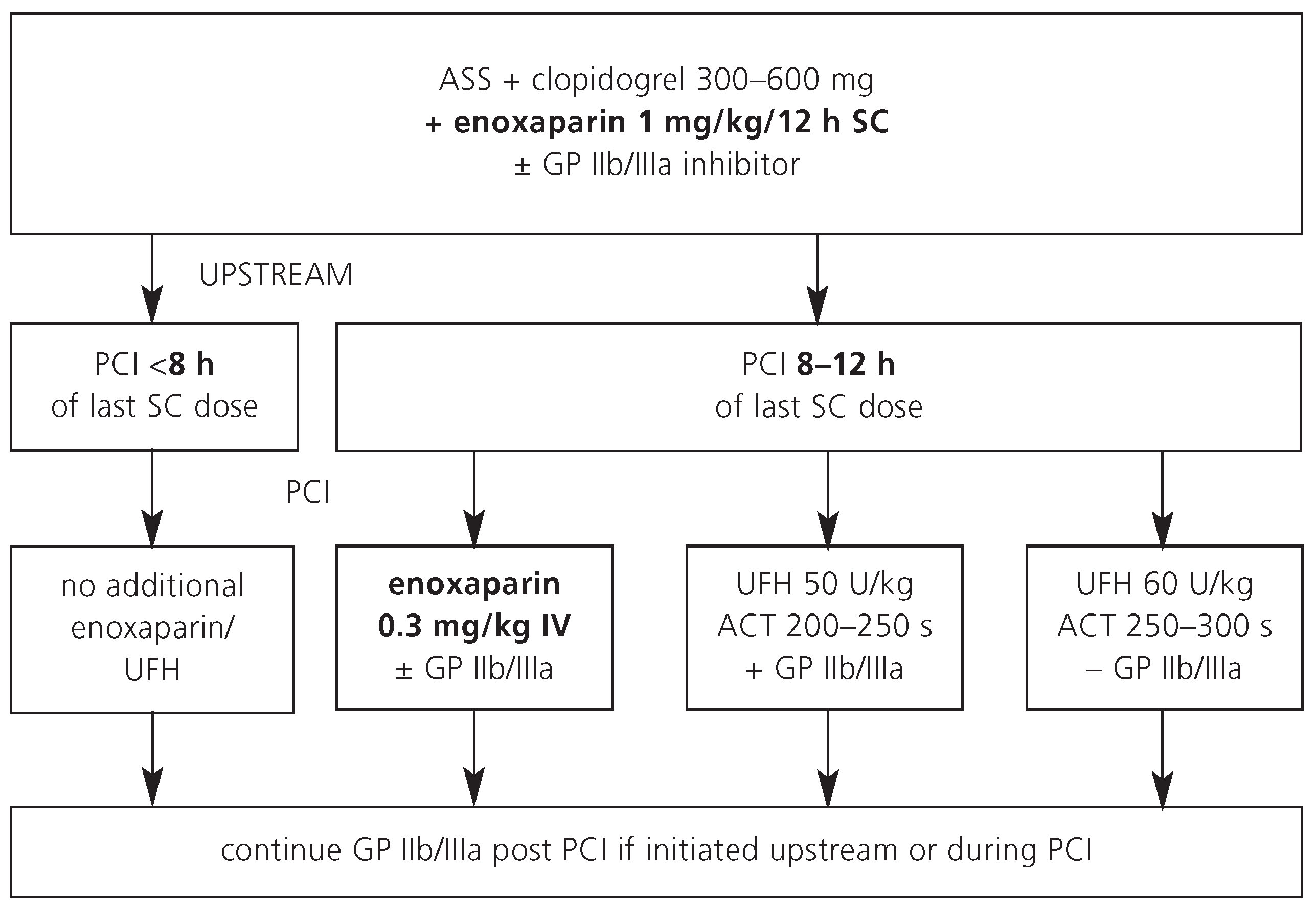
In Switzerland, UFH is still the most commonly used antithrombotic agent during PCI. Advantages and disadvantages of UFH are listed in
Table 1. A typical dose regimen for patients who are referred for an early invasive strategy is shown in
Figure 1.
Glycoprotein IIb/IIIa inhibitors
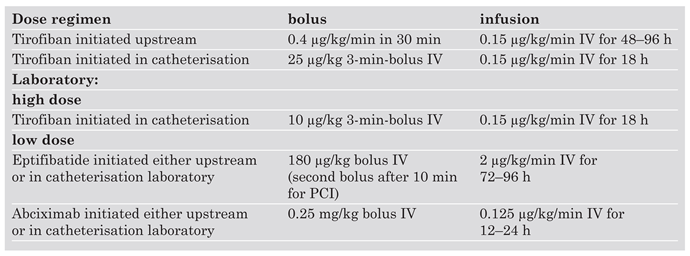
GP IIb/IIIa inhibitors are established for the management of high-risk ACS patients. Debates continue whether GP IIb/IIIa inhibitors should be initiated upstream or at initiation of PCI in patients with high-risk ACS. Dose regimens for the GP IIb/IIIa inhibitors tirofiban, eptifibatide and abciximab are given in
Table 2. Note that tirofiban requires dose-halving with a creatinin clearance of less than 30 ml per minute while abciximab does not need dose adjustment with renal insufficiency.
Enoxaparin
Mechanism of action
Low molecular weight heparins (LMWH) are of animal origin and contain depolymerised UFH chains. Because LMWH contain more pentasaccharides than UFH, they exhibit greater anti-Xa activity. Among the LMWH, the anti-Xa/anti-IIa ratio is highest for enoxaparin (3.8:1). In contrast to UFH, the favorable pharmacodynamic profile of LMWH is responsible for a consistent action in individual patients. Monitoring of the anti-Xa activity is therefore recommended only in obese or elderly patients as well as in those with renal insufficiency.
Antithrombotic strategy
Intravenous enoxaparin is currently used in several Swiss tertiary care hospitals during PCI if it was given subcutaneously as upstream antithrombotic agent. Enoxaparin is the only low molecular weight heparin that covers the entire spectrum of antithrombin therapy in the ACS setting, regardless of the treatment strategy. The other available LMWH preparations have not been fully studied in ACS patients with an early invasive strategy and are therefore not recommended from current European and North American consensus guidelines. Many physicians use enoxaparin as initial anticoagulant in patients with ACS due to its ease of administration (subcutaneous injections) without the need of monitoring. In contrast to other LMWH, established dosing recommendations (
Figure 2) for the intravenous administration of enoxaparin in the catheterisation laboratory during PCI are available, without the need for switching anticoagulant regimens. Around 60% anti-Xa activity of enoxaparin may be neutralised by protamine.
In a systematic review of available data, enoxaparin is more effective than UFH in ACS patients who are managed conservatively. In patients who are managed with an early invasive strategy, enoxaparin is as effective and safe as UFH. As with UFH, enoxaparin can safely be used in combination with GP IIb/IIIa inhibitors without an increase in major bleeding complications. In contrast to UFH, no dose adjustment of enoxaparin is necessary with concomitant therapy with GP IIb/IIIa inhibitors. During initial upstream therapy, a dose reduction of enoxaparin is warranted in patients with a creatinin clearance of less than 30 ml per minute.
Bivalirudin
Mechanism of action
Bivalirudin is a direct thrombin inhibitor and exhibits reversible and bivalent binding of thrombin (active site and exosite 1). Therefore, bivalirudin displaces thrombin from its fibrin substrate. Bivalirudin has an excellent pharmacodynamic profile with consistent action in individual patients. It is currently the only approved agent that is independent of antithrombin levels. In contrast to UFH, it also inhibits clot-bound fibrin. In addition, it is the only agent with strong inhibition of thrombin-mediated platelet activation. There is no binding to non-specific protease or platelet factor-4. The short elimination half-life of 25 minutes makes bivalirudin an attractive antithrombotic agent for PCI on one side but requires an infusion if prolonged activity is desired on the other side.
Antithrombotic strategy
Bivalirudin was just recently approved by the Swiss authorities, and a few PCI centers have started to gain experience with this antithrombotic agent. Among intermediate-risk ACS patients, a bivalirudin-alone strategy appears as effective as but safer than a strategy with UFH/LMWH + GP IIb/IIIa inhibitors. The risk of severe bleeding complications appears to be lower with bivalirudin as compared to with UFH + GP IIb/IIIa inhibitors. More data are required to prove that bivalirudin can replace GP IIb/IIIa inhibitors with high-risk non-ST elevation myocardial infarction. Of note, bivalirudin can be combined with GP IIb/IIIa inhibitors (bail-out indication), without an increase in major bleeding complications.
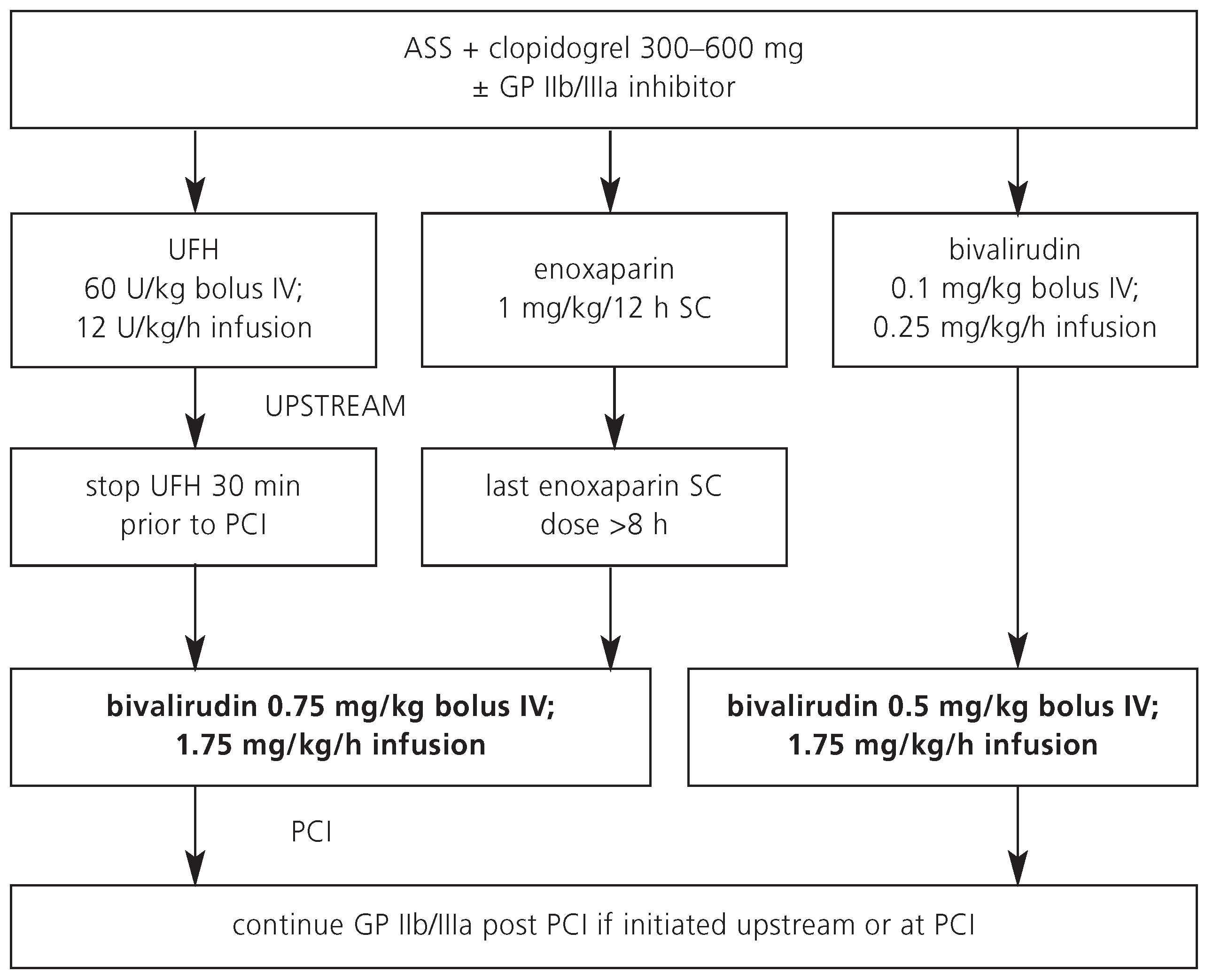
Because of its recent introduction and cost, bivalirudin is not yet commonly used as upstream treatment in patients with ACS. Most patients receive UFH or enoxaparin as initial antithrombin prior to referral to the catheterisation laboratory (
Figure 3). A wash-out period of these upstream agents is required (UFH 30 minutes, enoxaparin 8 hours) before bivalirudin can be safely administered for PCI. If these wash-out periods are respected by the interventional cardiologist, bivalirudin potentially has a role in the cardiac catheterisation laboratory, particularly in elderly ACS patients or in those with an increased risk of bleeding.
Fondaparinux
Mechanism of action
Fondaparinux is a synthetic pentasaccharide, which selectively binds and inhibits factor Xa. Similar to UFH and LMWH, inhibition of factor Xa is achieved via binding to antithrombin. Similar to enoxaparin, fondaparinux also inhibits clot-bound fibrin and there is partial inhibition of thrombin-mediated platelet activation. In contrast to the short half-life of enoxaparin (270 minutes) that mandates a twice-daily regimen, fondaparinux has a halflife of approximately 18 hours. Therefore, a once-daily regimen of fondaparinux in a dose of 2.5 mg subcutaneously is sufficient. No specific antidote is available for fondaparinux. In patients with creatinin clearance of less than 30 ml per minute, fondaparinux should not be used.
Antithrombotic strategy
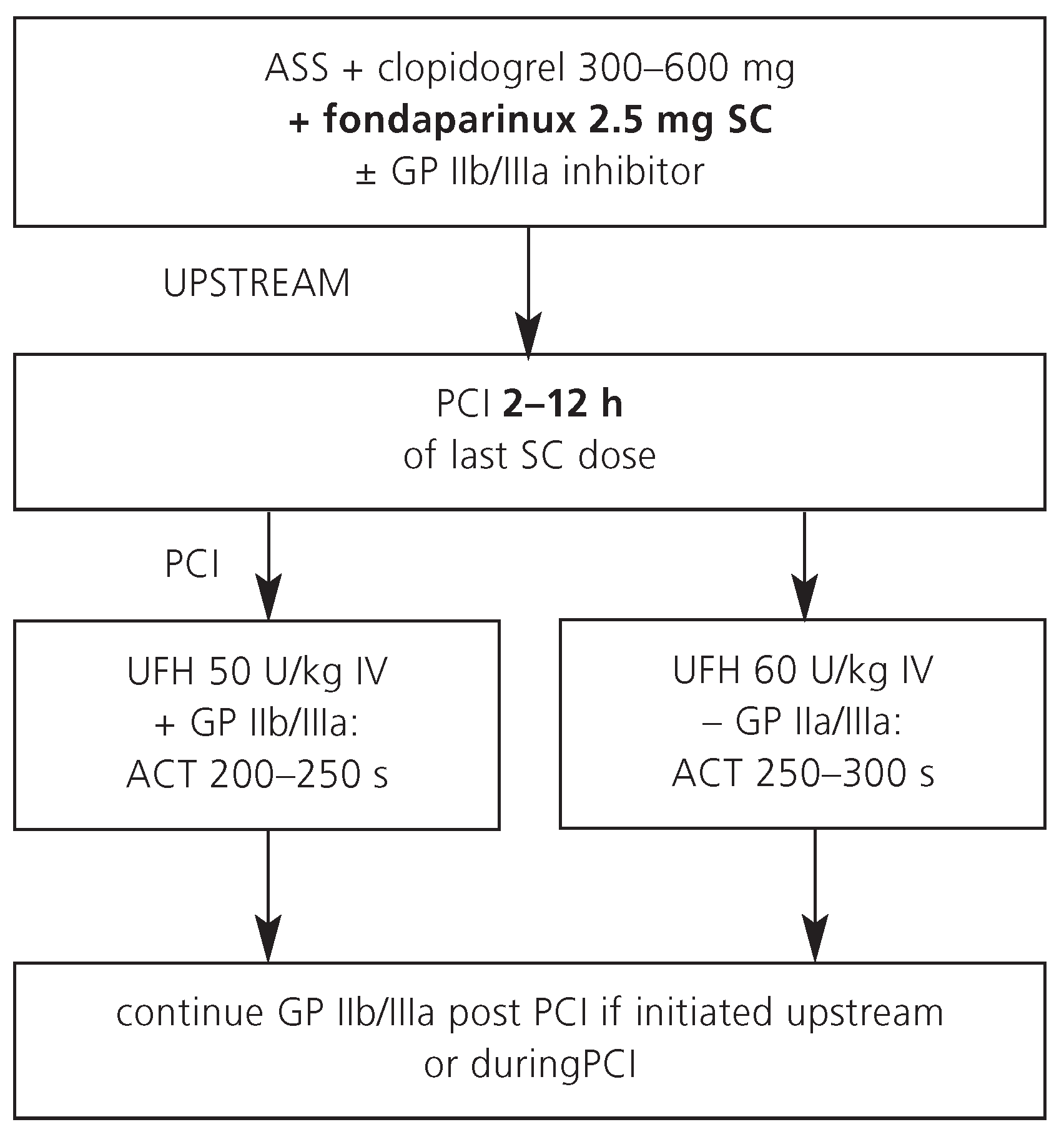
The reduction in bleeding complications with fondaparinux among intermediate-risk and high-risk ACS patients translated into a significant reduction in mortality in the clinical trials. The convincing safety profile makes fondaparinux a promising anticoagulant choice for patients with ACS, who are primarily managed conservatively. However, among patients who were managed with PCI, an increased rate of catheter thrombosis was reported with the use of fondaparinux compared to patients treated with enoxaparin or unfractioned heparin. Thus, fondaparinux alone is not recommended as antithrombotic agent for PCI. Afull dose of UFH iv on top of fondaparinux 2.5 mg sc may avoid this potentially severe complication during PCI without an increase in bleeding, but further studies are needed to confirm the safety and efficacy of such combination (
Figure 4). Fondaparinux is not yet approved for patients with ACS by the Swiss authorities.
Perspective
Many novel anticoagulant drugs are in development. The majority of these drugs target factor Xa or thrombin and are currently in phase II or III programs. Some of these drugs are administered orally, such as rivaroxaban, a factor Xa inhibitor, or dabigatran, an oral direct thrombin inhibitor. Whether these new drugs are useful in the ACS setting has to be investigated.
There are also new drugs in development that inhibit platelets, such as the P2Y12 inhibitor prasugrel, or the first reversible ADP receptor antagonist AZD6140. These drugs may exhibit more consistent action on platelets than existing medications for the management of patients with ACS.