What Is an Optimal Stent? Biological Requirements of Drug-Eluting Stents
Summary
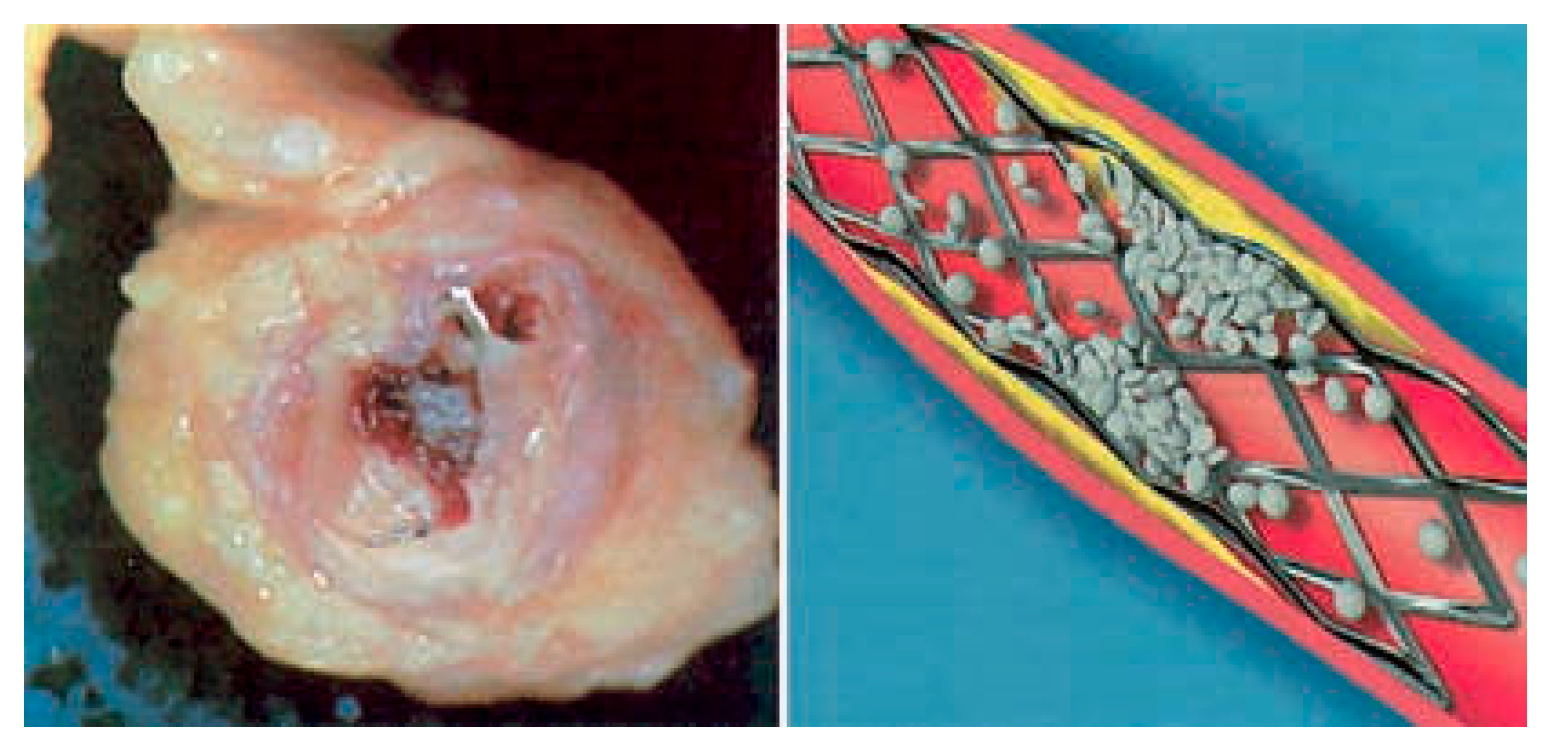
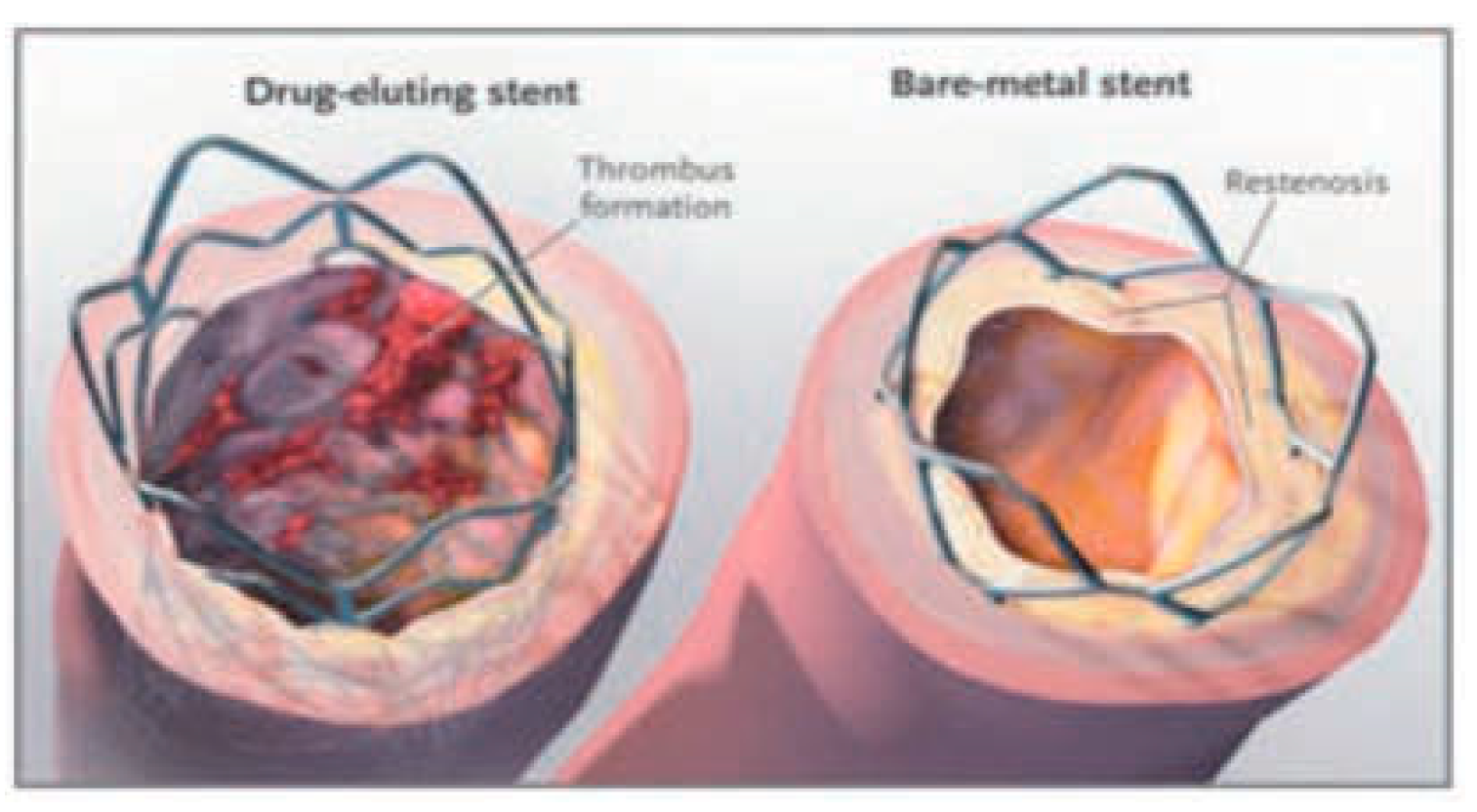
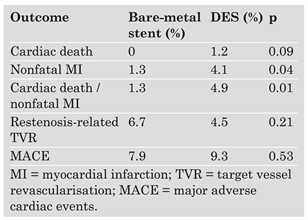
Introduction

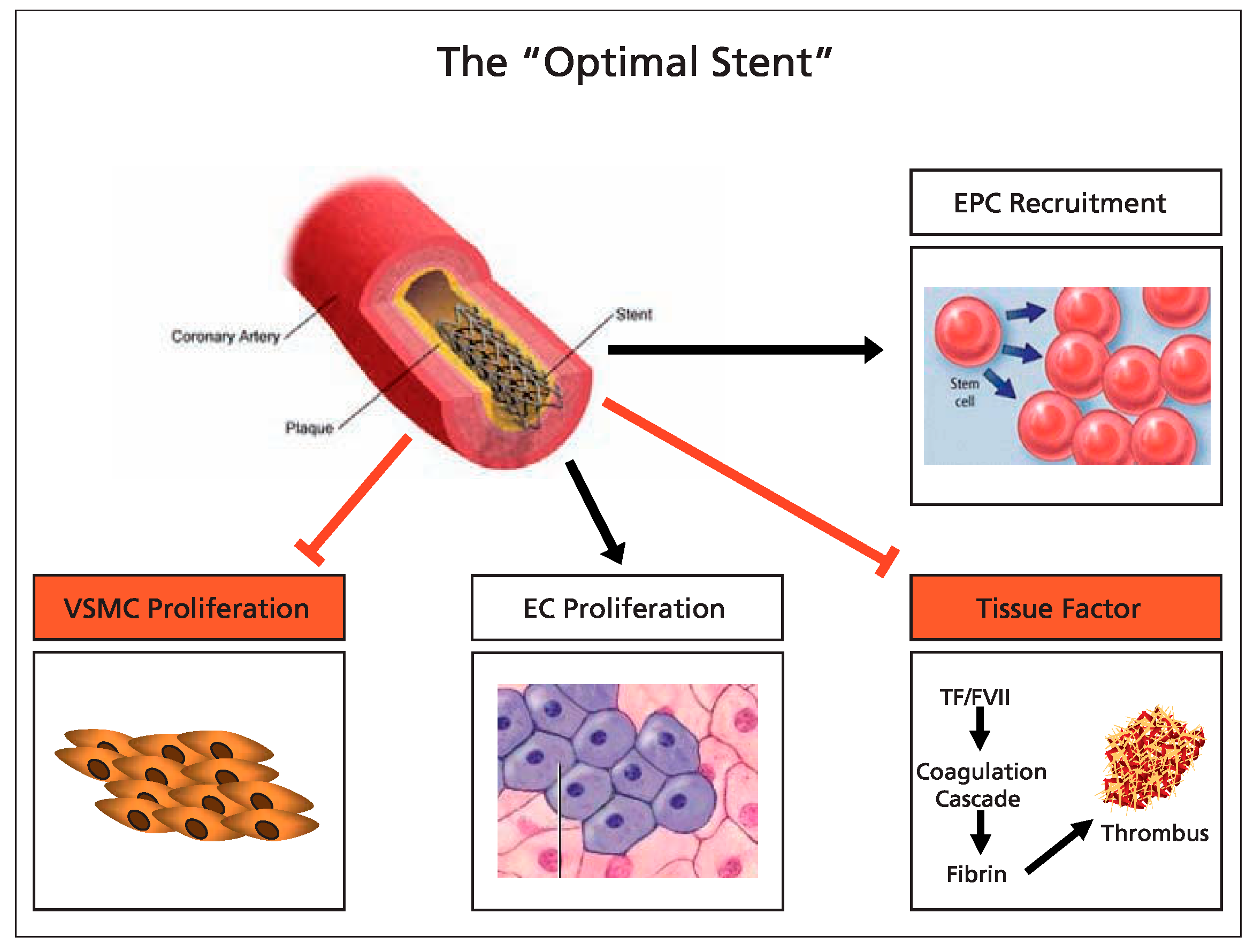
Conclusion
References
- Steffel, J.; Tanner, F.C. Biological effects of drug-eluting stents in the coronary circulation. Herz. 2007, 32, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Iakovou, I.; Schmidt, T.; Bonizzoni, E.; Ge, L.; Sangiorgi, G.M.; Stankovic, G.; et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005, 293, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, E.; Steg, P.G.; Wijns, W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007, 115, 1440–1455, discussion 1455. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Latini, R.A.; Akhmedov, A.; Zimmermann, D.; Zimmerling, P.; Luscher, T.F.; et al. Rapamycin, but not FK-506, increases endothelial tissue factor expression: implications for drug-eluting stent design. Circulation. 2005, 112, 2002–2011. [Google Scholar] [CrossRef]
- Stahli, B.E.; Camici, G.G.; Steffel, J.; Akhmedov, A.; Shojaati, K.; Graber, M.; et al. Paclitaxel enhances thrombin-induced endothelial tissue factor expression via c-Jun terminal NH2 kinase activation. Circ Res. 2006, 99, 149–155. [Google Scholar] [CrossRef]
- Zhu, S.; Viswambharan, H.; Gajanayake, T.; Ming, X.F.; Yang, Z. Sirolimus increases tissue factor expression but not activity in cultured human vascular smooth muscle cells. BMC Cardiovasc Disord. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holmes, D.R.; Jr, Kereiakes, D.J.; Laskey, W.K.; Colombo, A.; Ellis, S.G.; Henry, T.D.; et al. Thrombosis and drug-eluting stents: an objective appraisal. J Am Coll Cardiol. 2007, 50, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Hsieh, W.H.; Massaro, J.M.; Ho, K.K.; D’Agostino, R.; Cutlip, D.E. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007, 356, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Moses, J.W.; Ellis, S.G.; Schofer, J.; Dawkins, K.D.; Morice, M.C.; et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007, 356, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Parry, T.J.; Brosius, R.; Thyagarajan, R.; Carter, D.; Argentieri, D.; Falotico, R.; et al. Drug-eluting stents: sirolimus and paclitaxel differentially affect cultured cells and injured arteries. Eur J Pharmacol. 2005, 524, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Matter, C.M.; Rozenberg, I.; Jaschko, A.; Greutert, H.; Kurz, D.J.; Wnendt, S.; Kuttler, B.; et al. Effects of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2006, 48, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Simon, R.; Lins, M.; Klauss, V.; Eberli, F.R.; Roffi, M.; et al. Randomized comparison of a titanium-nitrideoxide-coated stent with a stainless steel stent for coronary revascularization: the TiNOX trial. Circulation. 2005, 111, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Griese, D.P.; Ehsan, A.; Melo, L.G.; Kong, D.; Zhang, L.; Mann, M.J.; et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003, 108, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Urao, N.; Okigaki, M.; Yamada, H.; Aadachi, Y.; Matsuno, K.; Matsui, A.; et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006, 98, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.G.; Chen, J.Z.; Wang, X.X. Effects of rapamycin on number activity and eNOS of endothelial progenitor cells from peripheral blood. Cell Prolif. 2006, 39, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Butzal, M.; Loges, S.; Schweizer, M.; Fischer, U.; Gehling, U.M.; Hossfeld, D.K.; et al. Rapamycin inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Exp Cell Res. 2004, 300, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Blindt, R.; Vogt, F.; Astafieva, I.; Fach, C.; Hristov, M.; Krott, N.; et al. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006, 47, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Dujovny, M.; Rozario, R.; Kossovsky, N.; Diaz, F.G.; Segal, R. Antiplatelet effect of dimethyl sulfoxide, barbiturates, and methyl prednisolone. Ann N Y Acad Sci. 1983, 411, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Serruys, P.W.; van Beusekom, H.; Ong, A.T.; McFadden, E.P.; Sianos, G.; et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALINGFIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005, 45, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
 |
© 2008 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Camici, G.G. What Is an Optimal Stent? Biological Requirements of Drug-Eluting Stents. Cardiovasc. Med. 2008, 11, 22. https://doi.org/10.4414/cvm.2008.01301
Camici GG. What Is an Optimal Stent? Biological Requirements of Drug-Eluting Stents. Cardiovascular Medicine. 2008; 11(1):22. https://doi.org/10.4414/cvm.2008.01301
Chicago/Turabian StyleCamici, Giovanni G. 2008. "What Is an Optimal Stent? Biological Requirements of Drug-Eluting Stents" Cardiovascular Medicine 11, no. 1: 22. https://doi.org/10.4414/cvm.2008.01301
APA StyleCamici, G. G. (2008). What Is an Optimal Stent? Biological Requirements of Drug-Eluting Stents. Cardiovascular Medicine, 11(1), 22. https://doi.org/10.4414/cvm.2008.01301




