Transcatheter Closure of Perimembranous Ventricular Septal Defect in Adult Using the Amplatzer Septal Occluder: Report of Two Challenging Cases
Introduction

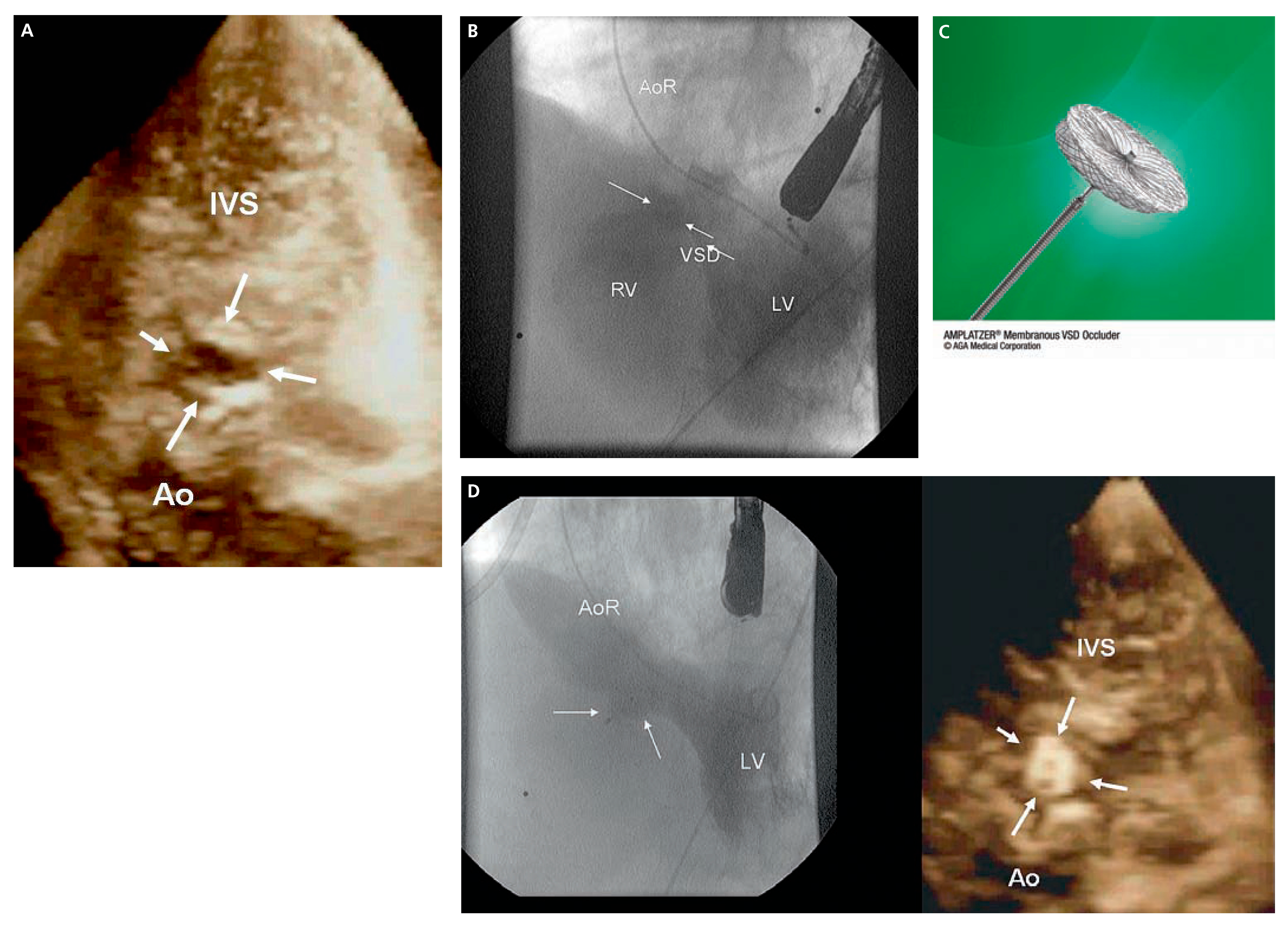
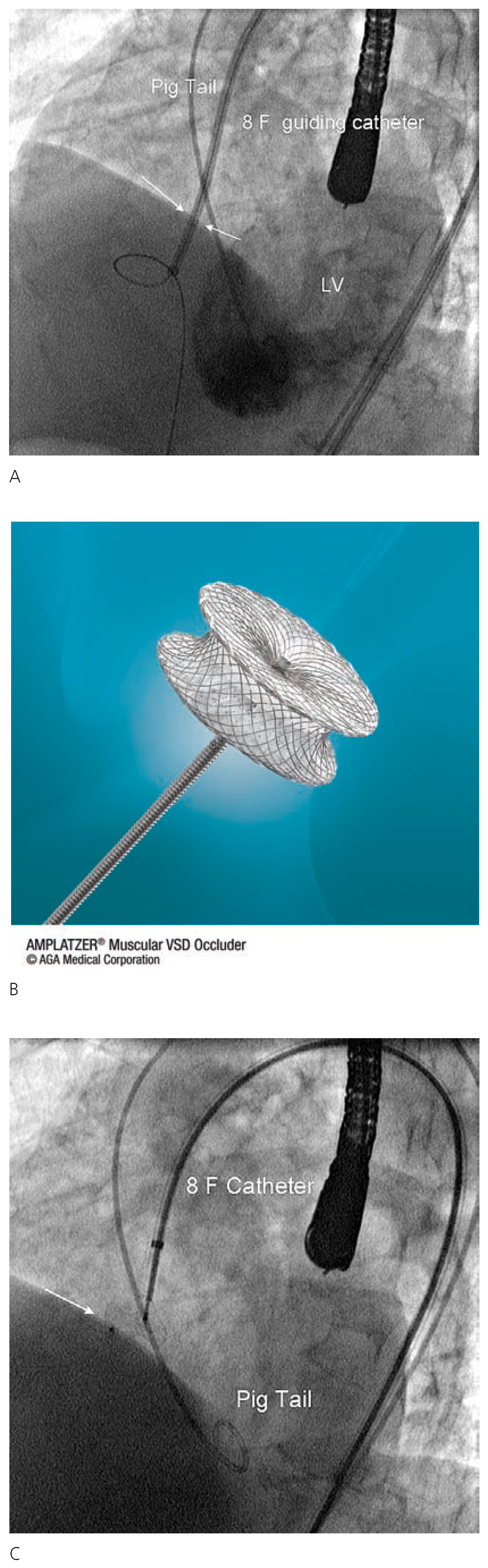
Case 1
Case 2
Discussion
References
- Ammash, N.M.; Warnes, C.A. Ventricular septal defects in adults. Ann Intern Med. 2001, 135(9), 812–824. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.E.; et al. Transcatheter closure of ventricular septal defects. Circulation. 1988, 78(2), 361–368. [Google Scholar] [CrossRef] [PubMed]
- Amin, Z.; et al. New device for closure of muscular ventricular septal defects in a canine model. Circulation. 1999, 100(3), 320–328. [Google Scholar] [PubMed]
- Holzer, R.; et al. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: immediate and mid-term results of a U.S. registry. J Am Coll Cardiol. 2004, 43(7), 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Carminati, M.; et al. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol. 2005, 96(12a), 52–58L. [Google Scholar] [CrossRef] [PubMed]
© 2008 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Pedrazzini, G.B.; Carminati, M.; Faletra, F.; Moccetti, T. Transcatheter Closure of Perimembranous Ventricular Septal Defect in Adult Using the Amplatzer Septal Occluder: Report of Two Challenging Cases. Cardiovasc. Med. 2008, 11, 16. https://doi.org/10.4414/cvm.2008.01298
Pedrazzini GB, Carminati M, Faletra F, Moccetti T. Transcatheter Closure of Perimembranous Ventricular Septal Defect in Adult Using the Amplatzer Septal Occluder: Report of Two Challenging Cases. Cardiovascular Medicine. 2008; 11(1):16. https://doi.org/10.4414/cvm.2008.01298
Chicago/Turabian StylePedrazzini, Giovanni B., Mario Carminati, Franco Faletra, and Tiziano Moccetti. 2008. "Transcatheter Closure of Perimembranous Ventricular Septal Defect in Adult Using the Amplatzer Septal Occluder: Report of Two Challenging Cases" Cardiovascular Medicine 11, no. 1: 16. https://doi.org/10.4414/cvm.2008.01298
APA StylePedrazzini, G. B., Carminati, M., Faletra, F., & Moccetti, T. (2008). Transcatheter Closure of Perimembranous Ventricular Septal Defect in Adult Using the Amplatzer Septal Occluder: Report of Two Challenging Cases. Cardiovascular Medicine, 11(1), 16. https://doi.org/10.4414/cvm.2008.01298



