Chronic Ischaemic Mitral Regurgitation: A Diagnostic and Therapeutic Challenge
Summary
Zusammenfassung
Background
Echocardiographic evaluation
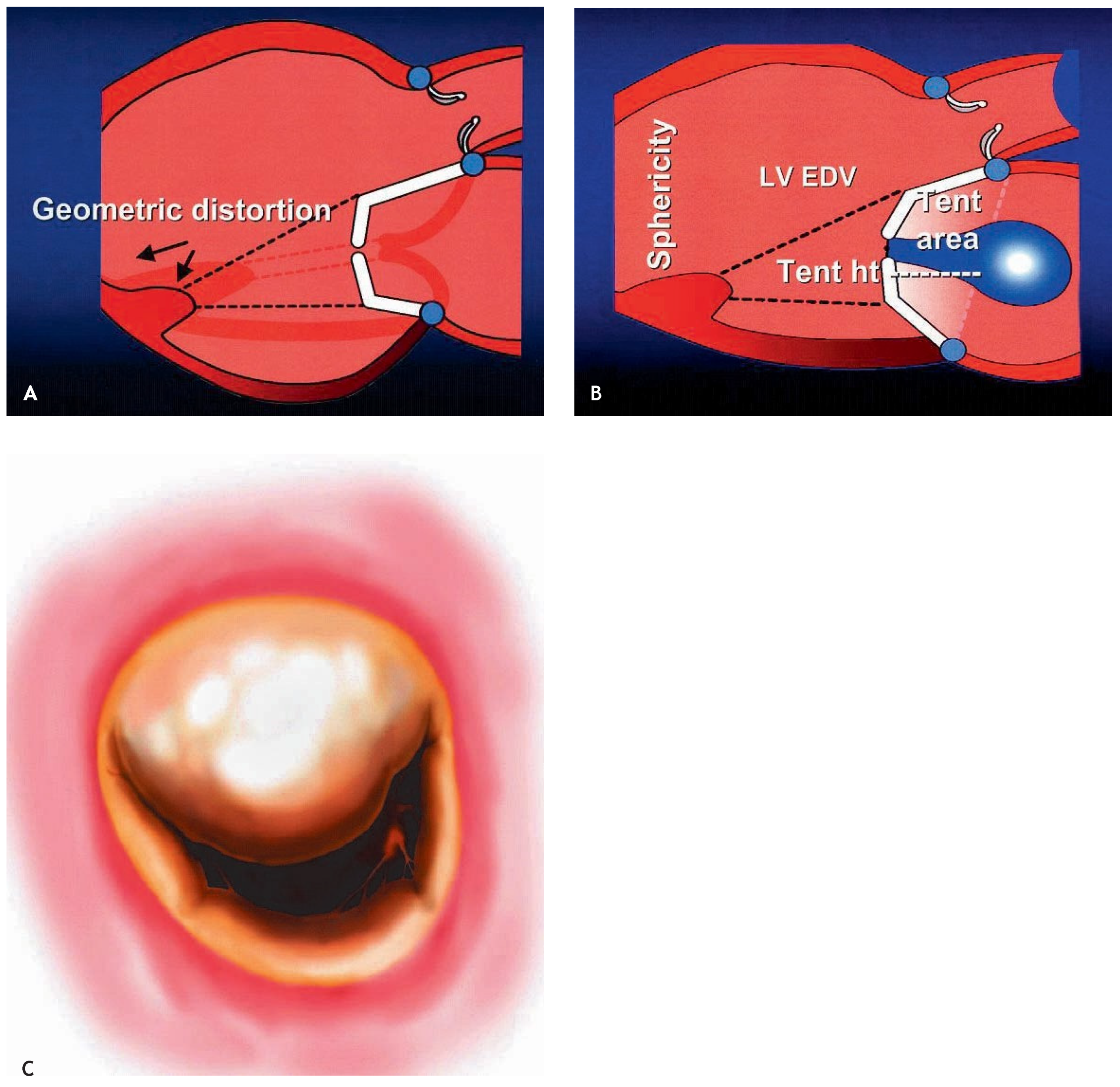
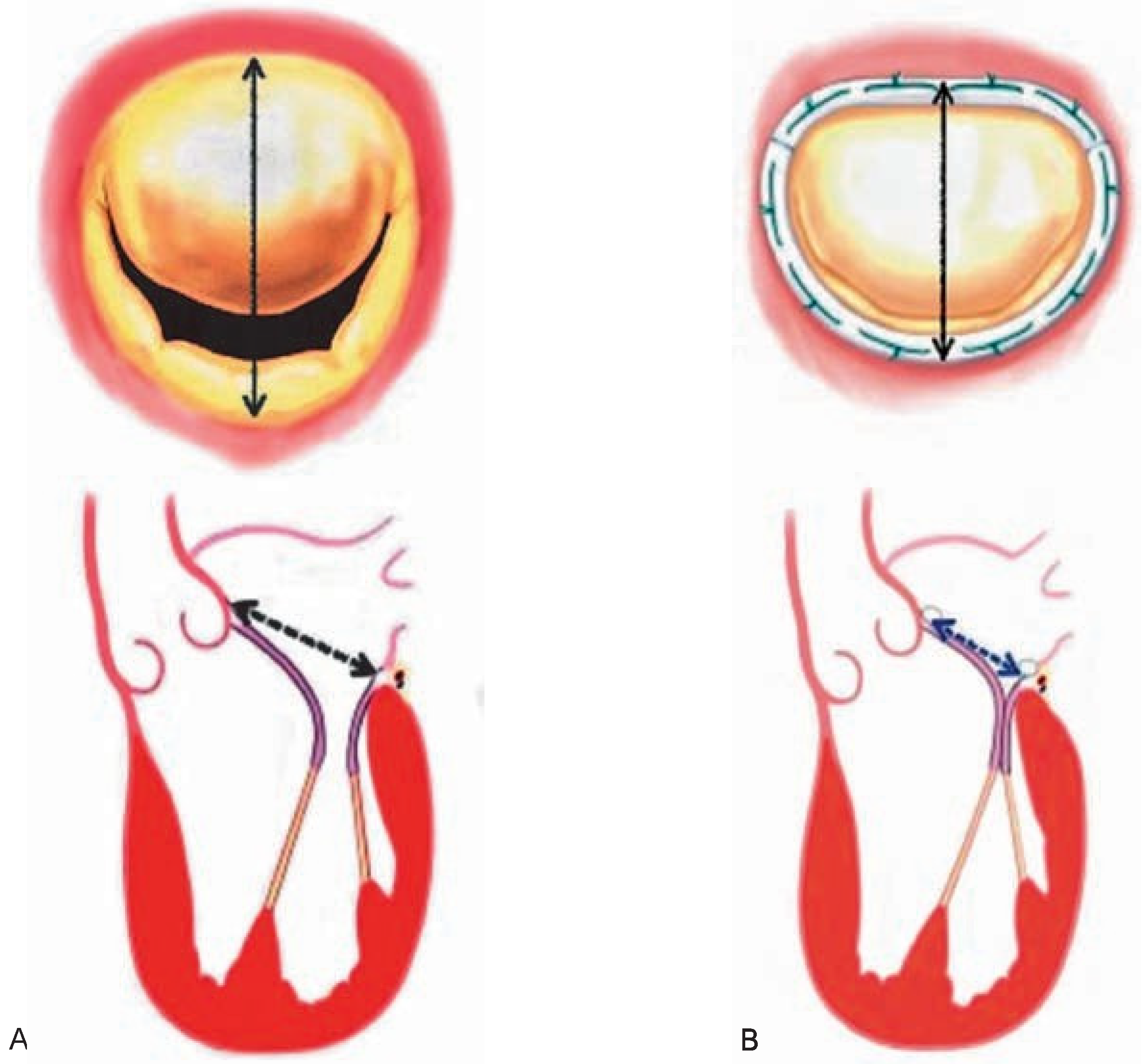
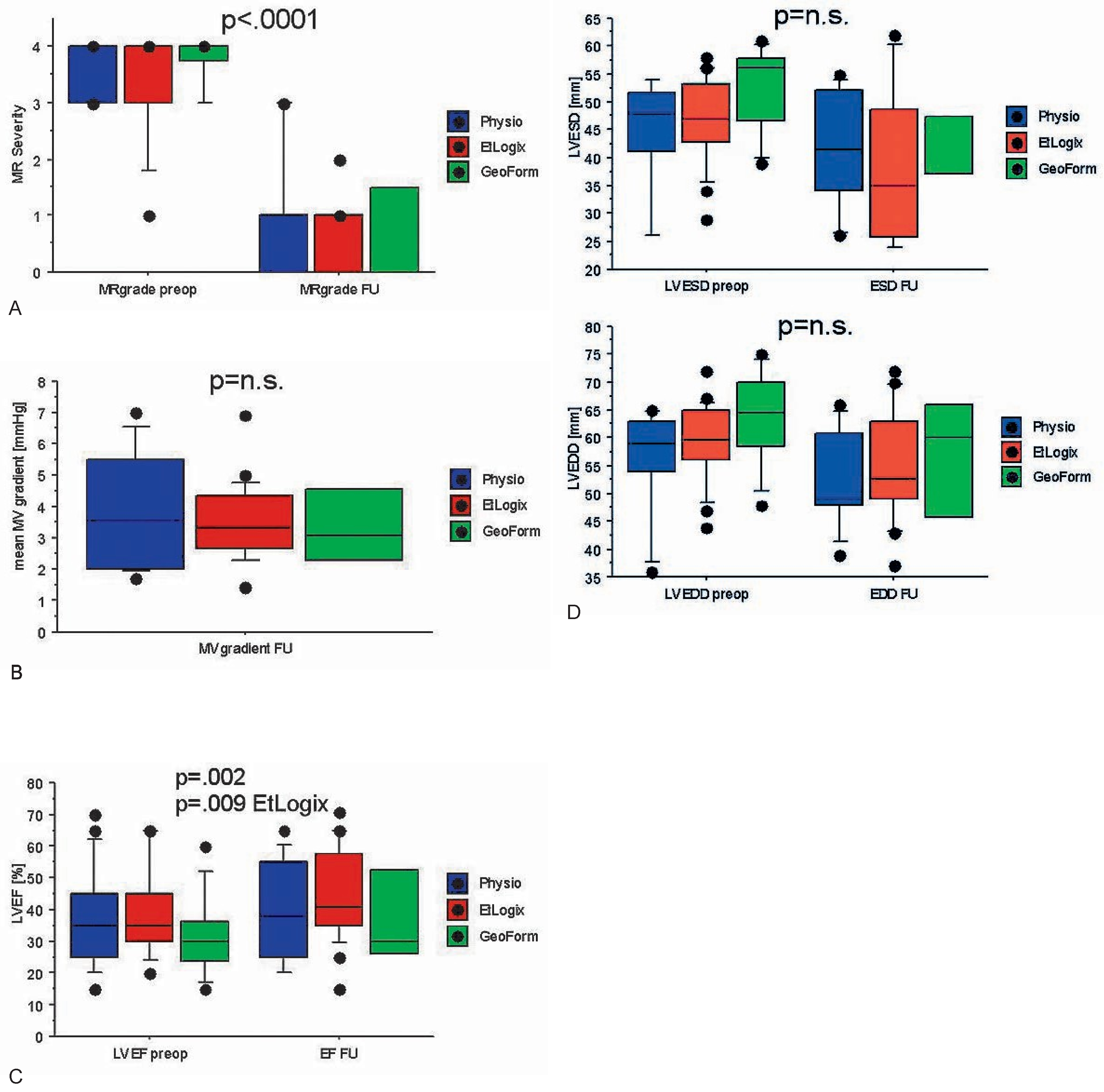
Indications and surgical techniques for correction of ischaemic MR
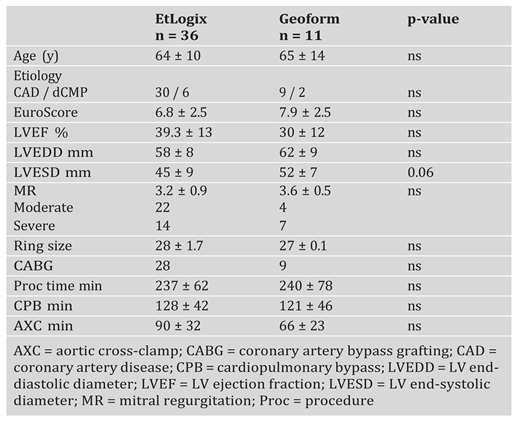 |
Alternative approaches to IMR
Informed Consent Statement
Conflicts of Interest
References
- Bursi, F.; Enriquez-Sarano, M.; Nkomo, V.T.; Jacobsen, S.J.; Weston, S.A.; Meverden, R.A.; et al. Heart failure and death after myocardial infarction in the community: The emerging role of mitral regurgitation. Circulation 2005, 111, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.K.; Gillinov, A.M.; Blackstone, E.H.; Rajeswaran, J.; Yuh, B.; Bhudia, S.K.; et al. Importance of moderate ischemic mitral regurgitation. Ann Thorac Surg. 2005, 79, 462–470; discussion–470. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, F.; Enriquez-Sarano, M.; Zehr, K.J.; Bailey, K.R.; Tajik, A.J. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001, 103, 1759–1764. [Google Scholar] [CrossRef]
- Lancellotti, P.; Lebrun, F.; Pierard, L.A. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2003, 42, 1921–1928. [Google Scholar] [CrossRef]
- Simpson, I.A.; Shiota, T.; Gharib, M.; Sahn, D.J. Current status of flow convergence for clinical applications: Is it a leaning tower of «PISA»? J Am Coll Cardio. 1996, 27, 504–509. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with twodimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef] [PubMed]
- Grewal, K.S.; Malkowski, M.J.; Piracha, A.R.; Astbury, J.C.; Kramer, C.M.; Dianzumba, S.; et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol. 2000, 85, 199–203. [Google Scholar] [CrossRef]
- Bach, D.S.; Deeb, G.M.; Bolling, S.F. Accuracy of intraoperative transesophageal echocardiography for estimating the severity of functional mitral regurgitation. Am J Cardiol. 1995, 76, 508–512. [Google Scholar] [CrossRef]
- Byrne, J.G.; Aklog, L.; Adams, D.H. Assessment and management of functional or ischaemic mitral regurgitation. Lancet 2000, 355, 1743–1744. [Google Scholar] [CrossRef]
- Adams, D.H.; Filsoufi, F.; Aklog, L. Surgical treatment of the ischemic mitral valve. J Heart Valve Dis. 2002, 11 (Suppl 1), S21–S25. [Google Scholar]
- Dion, R.; Benetis, R.; Elias, B.; Guennaoui, T.; Raphael, D.; Van Dyck, M.; et al. Mitral valve procedures in ischemic regurgitation. J Heart Valve Dis. 1995, 4 (Suppl 2), S124–S129; discussion S9–S31. [Google Scholar]
- Bax, J.J.; Schinkel, A.F.; Boersma, E.; Elhendy, A.; Rizzello, V.; Maat, A.; et al. Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse longterm prognosis. Circulation 2004, 110 (Suppl 1), II18–22. [Google Scholar] [CrossRef]
- Braun, J.; Bax, J.J.; Versteegh, M.I.; Voigt, P.G.; Holman, E.R.; Klautz, R.J.; et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg 2005, 27, 847–853. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Wolf, I.; Mottl-Link, S.; Hoda, R.; Mikhail, B.; Sack, F.U.; et al. A clinical study of annular geometry and dynamics in patients with ischemic mitral regurgitation: New insights into asymmetrical ring annuloplasty. Eur J Cardiothorac Surg. 2006, 29, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Pellizon, G.; Grines, C.L.; Cox, D.A.; Stuckey, T.; Tcheng, J.; Garcia, E.; et al. Importance of mitral regurgitaiton in patients undergoing percutaneuous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2004, 43, 1368–1374. [Google Scholar] [CrossRef]
- Grossi, E.A.; Crooke, G.A.; DiGiorgi, P.L.; Schwartz, C.F.; Jorde, U.; Applebaum, R.M.; et al. Impact of moderate functional mitral insufficiency in patients undergoing surgical revascularization. Circulation 2006, 114 (1 Suppl), I573–I576. [Google Scholar] [CrossRef] [PubMed]
- Bax, J.J.; Braun, J.; Somer, S.T.; Klautz, R.; Holman, E.R.; Versteegh, M.I.; et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation 2004, 110 (11 Suppl 1), II103–II108. [Google Scholar] [CrossRef]
- Kim, Y.H.; Czer, L.S.; Soukiasian, H.J.; De Robertis, M.; Magliato, K.E.; Blanche, C.; et al. Ischemic mitral regurgitation: Revascularization alone versus revascularization and mitral valve repair. Ann Thorac Surg. 2005, 79, 1895–1901. [Google Scholar] [CrossRef]
- Prifti, E.; Bonacchi, M.; Frati, G.; Giunti, G.; Babatasi, G.; Sani, G. Ischemic mitral valve regurgitation grade II–III: Correction in patients with impaired left ventricular function undergoing simultaneous coronary revascularization. J Heart Valve Dis. 2001, 10, 754–762. [Google Scholar]
- Paparella, D.; Mickleborough, L.L.; Carson, S.; Ivanov, J. Mild to moderate mitral regurgitation in patients undergoing coronary bypass grafting: Effects on operative mortality and longterm significance. Ann Thorac Surg. 2003, 76, 1094–1100. [Google Scholar] [CrossRef]
- Wu, A.H.; Aaronson, K.D.; Bolling, S.F.; Pagani, F.D.; Welch, K.; Koelling, T.M. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005, 45, 381–387. [Google Scholar] [CrossRef]
- Trichon, B.H.; Glower, D.D.; Shaw, L.K.; Cabell, C.H.; Anstrom, K.J.; Felker, G.M.; et al. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation 2003, 108 (Suppl 1), II103–II110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonow, R.O.; Carabello, B.A.; Kanu, C.; de Leon, A.C., Jr.; Faxon, D.P.; Freed, M.D.; et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Developed in collaboration with the Society of Cardiovascular Anesthesiologists: Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006, 114, e84–e231. [Google Scholar] [PubMed][Green Version]
- Grossi, E.A.; Bizekis, C.S.; LaPietra, A.; Derivaux, C.C.; Galloway, A.C.; Ribakove, G.H.; et al. Late results of isolated mitral annuloplasty for «functional» ischemic mitral insufficiency. J Card Surg. 2001, 16, 328–332. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.C.; Gillinov, A.M.; Blackstone, E.H.; Rajeswaran, J.; Cohen, G.; Najam, F.; et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004, 128, 916–924. [Google Scholar] [CrossRef]
- Messas, E.; Guerrero, J.L.; Handschumacher, M.D.; Conrad, C.; Chow, C.M.; Sullivan, S.; et al. Chordal cutting: A new therapeutic approach for ischemic mitral regurgitation. Circulation 2001, 104, 1958–1963. [Google Scholar] [CrossRef]
- Kron, I.L.; Green, G.R.; Cope, J.T. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002, 74, 600–601. [Google Scholar] [CrossRef]
- Alfieri, O.; Maisano, F.; De Bonis, M.; Stefano, P.L.; Torracca, L.; Oppizzi, M.; et al. The double-orifice technique in mitral valve repair: A simple solution for complex problems. J Thorac Cardiovasc Surg. 2001, 122, 674–681. [Google Scholar] [CrossRef]
- Bolling, S.F. Mitral valve reconstruction in the patient with heart failure. Heart Fail Rev. 2001, 6, 177–185. [Google Scholar] [CrossRef]
- Daimon, M.; Fukuda, S.; Adams, D.H.; McCarthy, P.M.; Gillinov, A.M.; Carpentier, A.; et al. Mitral valve repair with Carpentier-McCarthy-Adams IMR ETlogix annuloplasty ring for ischemic mitral regurgitation: Early echocardiographic results from a multi-center study. Circulation 2006, 114 (Suppl), I588–I593. [Google Scholar] [CrossRef]
- Dor, V. Left ventricular reconstruction for ischemic cardiomyopathy. J Card Surg. 2002, 17, 180–187. [Google Scholar] [CrossRef]
- Oz, M.C.; Konertz, W.F.; Kleber, F.X.; Mohr, F.W.; Gummert, J.F.; Ostermeyer, J.; et al. Global surgical experience with the Acorn cardiac support device. J Thorac Cardiovasc Surg. 2003, 126, 983–991. [Google Scholar] [CrossRef]
- Pilla, J.J.; Blom, A.S.; Brockman, D.J.; Bowen, F.; Yuan, Q.; Giammarco, J.; et al. Ventricular constraint using the acorn cardiac support device reduces myocardial akinetic area in an ovine model of acute infarction. Circulation 2002, 106 (Suppl 1), I207–I211. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.A.; Goldberg, J.D.; LaPietra, A.; Ye, X.; Zakow, P.; Sussman, M.; et al. Ischemic mitral valve reconstruction and replacement: Comparison of long-term survival and complications. J Thorac Cardiovasc Surg. 2001, 122, 1107–1124. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, M.J.; Kang, S.J.; Song, J.M.; Song, H.; Hong, M.K.; et al. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation 2006, 114 (Suppl), I499–I503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukamachi, K.; Inoue, M.; Doi, K.; Schenk, S.; Nemeh, H.; Faber, C.; et al. Reduction of mitral regurgitation using the Coapsys device: A novel ex vivo method using excised recipients’ hearts. Asaio J. 2005, 51, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Tibayan, F.A.; Rodriguez, F.; Langer, F.; Zasio, M.K.; Bailey, L.; Liang, D.; et al. Does septal-lateral annular cinching work for chronic ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2004, 127, 654–663. [Google Scholar] [CrossRef][Green Version]
- Mishra, Y.K.; Mittal, S.; Jaguri, P.; Trehan, N. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation: 1-year results. Ann Thorac Surg. 2006, 81, 42–46. [Google Scholar] [CrossRef]
- Palacios, I.F.; Condado, J.A.; Brandi, S.; Rodriguez, V.; Bosch, F.; Silva, G.; et al. Safety and feasibility of acute percutaneous septal sinus shortening: First-in-human experience. Catheter Cardiovasc Interv. 2007, 69, 513–518. [Google Scholar] [CrossRef]
- Rogers, J.H.; Macoviak, J.A.; Rahdert, D.A.; Takeda, P.A.; Palacios, I.F.; Low, R.I. Percutaneous septal sinus shortening: A novel procedure for the treatment of functional mitral regurgitation. Circulation 2006, 113, 2329–2334. [Google Scholar] [CrossRef]
- Kaye, D.M.; Byrne, M.; Alferness, C.; Power, J. Feasibility and short-term efficacy of percutaneous mitral annular reduction for the therapy of heart failure-induced mitral regurgitation. Circulation 2003, 108, 1795–1797. [Google Scholar] [CrossRef][Green Version]
- Webb, J.G.; Harnek, J.; Munt, B.I.; Kimblad, P.O.; Chandavimol, M.; Thompson, C.R.; et al. Percutaneous transvenous mitral annuloplasty: Initial human experience with device implantation in the coronary sinus. Circulation 2006, 113, 851–855. [Google Scholar] [CrossRef]
- Feldman, T.; Wasserman, H.S.; Herrmann, H.C.; Gray, W.; Block, P.C.; Whitlow, P.; et al. Percutaneous mitral valve repair using the edge-to-edge technique: Six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol. 2005, 46, 2134–2140. [Google Scholar] [CrossRef]
- Maisano, F.; Caldarola, A.; Blasio, A.; De Bonis, M.; La Canna, G.; Alfieri, O. Midterm results of edge-to-edge mitral valve repair without annuloplasty. J Thorac Cardiovasc Surg. 2003, 126, 1987–1997. [Google Scholar] [CrossRef]


© 2007 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Berdat, P.A. Chronic Ischaemic Mitral Regurgitation: A Diagnostic and Therapeutic Challenge. Cardiovasc. Med. 2007, 10, 271. https://doi.org/10.4414/cvm.2007.01267
Berdat PA. Chronic Ischaemic Mitral Regurgitation: A Diagnostic and Therapeutic Challenge. Cardiovascular Medicine. 2007; 10(9):271. https://doi.org/10.4414/cvm.2007.01267
Chicago/Turabian StyleBerdat, Pascal A. 2007. "Chronic Ischaemic Mitral Regurgitation: A Diagnostic and Therapeutic Challenge" Cardiovascular Medicine 10, no. 9: 271. https://doi.org/10.4414/cvm.2007.01267
APA StyleBerdat, P. A. (2007). Chronic Ischaemic Mitral Regurgitation: A Diagnostic and Therapeutic Challenge. Cardiovascular Medicine, 10(9), 271. https://doi.org/10.4414/cvm.2007.01267




