1. Introduction
Genetically modified (GM) crops have been widely used in agricultural and food production [
1]. In 2019, 190.4 million hectares of genetically modified crops were cultivated worldwide, with herbicide tolerant crops accounting for 43% [
2]. Most commercial biotech crops planted throughout the world have one or more genes transferred into their genomes by contemporary biotechnology to gain desired traits such as herbicide tolerance as well as insect or disease resistance [
3,
4,
5,
6]. Since it is nutritious and affordable, maize is not only a staple food crop but also an essential raw ingredient for the feed industry. Despite this, weeds caused significant losses in productivity and quality [
7,
8]. Favorable climatic conditions foster weed development in widely spread crops, such as maize, resulting in yield reductions of up to 70% [
9]. In addition, weeds disrupt crop metabolism, reducing carbs, proteins, fats and cellulose levels, which ultimately lowers crop quality. When maize is affected by weeds, the amount of protein and starch in the grains is reduced [
10]. Herbicides are the most common, effective and cost-efficient technique for preventing and treating weeds. Herbicide-tolerant traits in genetically modified crops can help farmers increase crop yields by reducing weed pressure [
11,
12,
13]. Most GM crops, on the other hand, are only herbicide-tolerant to one herbicide, such as glyphosate, and continued use of herbicides with the same mechanism of action can lead to the evolution of herbicide-resistant weeds [
14,
15,
16,
17]. To prevent the emergence and spread of herbicide-resistant weeds, new genes are required to develop transgenic herbicide-tolerant crops. In China, an herbicide-tolerant maize variety (ZDAX5) was developed by inserting the
P450-N-Z1 gene isolated from
Cynodon dactylon (L.) Pers. and the
cp4 epsps gene isolated from
Agrobacterium tumefaciens strain CP4 into the maize genome. The initial analysis showed that ZDAX5 corn was highly tolerant to flazasulfuron and glyphosate under field conditions (data not published). Flazasulfuron is a sulfonylurea herbicide that controls a wide range of weeds, including both annual and perennial species [
18,
19,
20,
21]. It is a selective systematic herbicide for pre-emergence and early post-emergence weed control. Mixed or alternate use of flazasulfuron and glyphosate can effectively slow down the emergence and development of herbicide resistance in weeds due to different modes of action between the flazasulfuron and glyphosate. Currently, there is no flazasulfuron-tolerant transgenic maize in both China and overseas. Farmers will be free to choose between herbicides with two different modes of action in their weed-control plans once ZDAX5 corn is available on the market. It may delay the appearance of new herbicide-resistant weeds if flazasulfuron is used in a reasoned manner in combination with glyphosate.
The food and feed safety of GM plants must be evaluated prior to commercialization. The study of the safety of foods or feeds obtained from GM crops tackles two key sources of possible health implications: those resulting from the activity and presence of the inserted trait (typically a protein) as well as the characteristics of the resulting food or feed crop plant [
22]. Therefore, it was important to determine whether the insertion of the
P450-N-Z1 and
cp4 epsps genes into the corn genome or the presence of the P450-N-Z1 and CP4 EPSPS proteins caused any significant changes in the safety and nutrients of ZDAX5. The study of whether a GM plant is as healthy and nutritious as its counterpart plant includes several elements of evaluation, including toxicological, nutritional, microbiological and environmental consequences, in a process referred to as substantial equivalence [
23]. The concept of substantial equivalence was created by the Organization for Economic Co-operation and Development (OECD) in 1993 and was subsequently developed by FAO/WHO in 2000 and 2002; it has been used internationally for GM crops risk assessment [
24,
25]. Although the phrase substantial equivalence was coined to describe the evaluation of foods, it is also applicable to the evaluation of plants and their products used as feedstuffs. Comparing GM crops to their non-transgenic counterparts is necessary for determining substantial equivalence. In order to discover similarities and probable discrepancies between the GM crops and its counterpart plant, the compositional analysis of GM plants and derived food and feed is a primary aspect of the comparative safety evaluation strategy. The major food and feed nutrients and anti-nutrients to consider in evaluating new maize varieties are outlined in the OECD consensus document on maize [
26]. The analysis includes macronutrient proximate analysis, micronutrient proximate analysis and intrinsic toxins, allergies and anti-nutrient proximate analysis. Once a GM plant’s compositional equivalency has been demonstrated, the work can then concentrate on rodent feeding trials to validate its nutritional equivalence and gather more information on safety. In some cases, a 90-day rodent feeding study has been recommended to assess potential unintended effects of toxicological and nutritional relevance and to determine whether the GM food and feed is as safe and nutritious as its traditional comparator following long-term exposure [
27]. In China, one of the key requirements of the application for the safety certification of GM crops is a 90-day feeding study in rodents. Lots of rodent feeding studies have been designed to determine whether the diets incorporated with grains from GM crops are substantially equivalent in composition and nutritional characteristics to the non-transgenic control diets [
28,
29,
30,
31]. The results from these studies demonstrated that the GM food and feed is as safe and nutritious as its traditional comparator.
In this study, the food safety of GM maize ZDAX5 with P450-N-Z1 and cp4 epsps was assessed in a compositional analysis and 90-day feeding study and compared with its non-transgenic counterpart to determine whether GM maize ZDAX5 is as nutritious and safe as its traditional comparator. This study will provide important data for the safety assessment of GM maize ZDAX5 in China. Furthermore, once ZDAX5 is available on the market, farmers will be free to choose between glyphosate and flazasulfuron in their weed-control plans, which may delay the evolution of herbicide-resistant weeds.
4. Discussion
Population increase, climate change, weeds, pests and lack of fertile land are key issues that contemporary agriculture faces, and genetically modified plants have great potential for facing these challenges [
8,
45,
46]. Genetically modified plants and derived food and feed that are already on the market were modified by inserting a single or a few genes that confer traits such as herbicide tolerance [
27]. These GM plants produce new proteins that are not found in their wildtypes. Therefore, regardless of advancements in transgenic technology, the safety of food and feed derived from GM crops, including herbicide-tolerant plants, has always been a source of public worry [
47]. The biosafety or toxicity safety assessment of GM crops is critical for their successful adoption because maize (
Zea mays L.) is an important staple food and raw ingredient for food. Thus, this study was conducted to evaluate the safety of transgenic maize ZDAX5 expressing the P450-N-Z1 and CP4 EPSPS proteins using well-established methods.
Transgenic herbicide-tolerant crops developed with novel genes with clear intellectual property rights is of critical importance. ZDAX5 is a novel variety of herbicide-tolerant maize that exhibits high tolerance to flazasulfuron and glyphosate and co-expresses the
P450-N-Z1 and
cp4 epsps genes. The
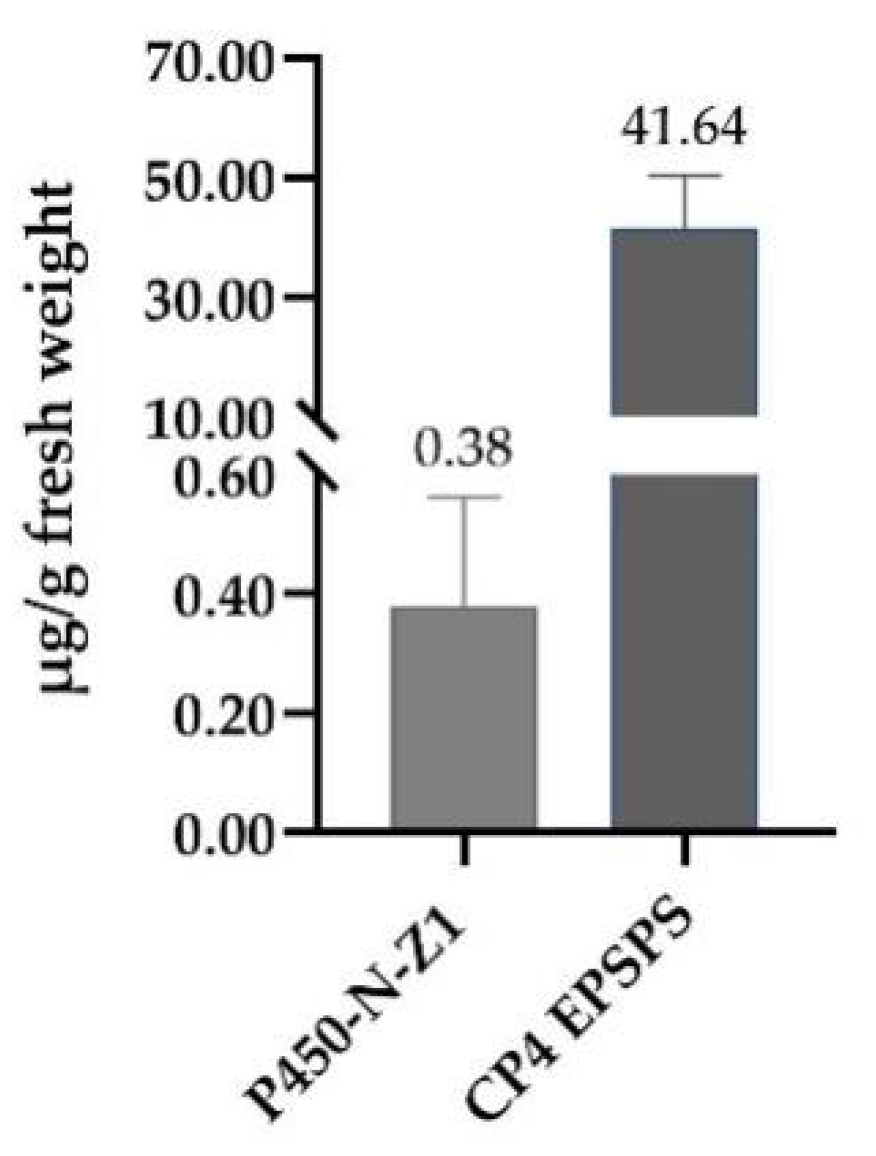
P450-N-Z1 gene was cloned from Bermuda grass in our laboratory previously, and this gene was patented in the United States [
48]. Both China and the rest of the world currently lack flazasulfuron-tolerant transgenic maize. Once ZDAX5 corn is available on the market, it will be more competitive than similar herbicide-resistant products abroad. The P450-N-Z1 and CP4 EPSPS proteins in ZDAX5 are detectable and quantifiable in ZDAX5 grain. The biosafety assessment of the introduced CP4 EPSPS protein was previously documented [
49,
50,
51,
52]. The CP4 EPSPS protein has a history of safe usage in agriculture and has no known allergenic or toxic qualities [
53]. The acute oral toxicity of CP4 EPSPS was assessed in mice, showing no adverse effects [
53]. In addition, the CP4 EPSPS protein has demonstrated no adverse effects in sub-chronic toxicity trials [
49,
50]. For P450-N-Z1, the amino acid sequence of P450-N-Z1 shares high similarity to numerous plant P450s, including CYP81A6 and Nsf1, derived from rice and maize, respectively [
48]. To date, no reports have demonstrated or speculated that these proteins are toxic or allergenic in humans or animals. The P450-N-Z1 protein is expressed in Bermuda grass, which is used as a feed and has not been found to have any toxicity in animals [
54,
55]. When the P450-N-Z1 protein is incubated with a digestive enzyme in vitro, the proteins are degraded within 30 min and do not resemble any known allergens or toxins structurally or functionally. Furthermore, the biosafety of P450-N-Z1 was tested in mice by giving them a large oral dosage of pure P450-N-Z1 protein; no negative effects or toxicity were observed (data not shown).
In the nutritional composition analysis, key nutritional components from the herbicide-tolerant maize ZDAX5 and its non-transgenic counterpart Ruifeng-1 were measured. All mean values calculated for the transgenic maize ZDAX5 samples, except for fiber, glycine, methionine, C17:0, C18:3 and magnesium, were within the ranges reported by the OECD (2002) or the ILSI Crop Composition Database (2014). The levels of C17:0, glycine and magnesium were higher than those provided by the OECD (2002) or the ILSI Crop Composition Database (2014) but equal to those of Ruifeng-1. The C18:3, methionine, and vitamin B1 levels were lower than those reported by the OECD (2002) or the ILSI Crop Composition Database (2014) but comparable to those of Ruifeng-1. These results demonstrated that the nutritional quality of transgenic maize grain was substantially equivalent to that of the non-transgenic counterpart. Compositional equivalence between ZDAX5 and its non-transgenic counterpart, Ruifeng-1, was clearly demonstrated. Even though some differences were statistically significant, the values fell within the normal variation range for the considered parameters and thus had no biological or toxicological significance. These results indicated that expressions of P450-N-Z1 and CP4 EPSPS in grain have no impact on the measured components. As previously stated, the introduction of well-characterized genes into a genome is unlikely to have a unexpected effect on crop composition [
56]. In a crop such as maize, which has a long history of safe use, random genetic perturbations are unlikely to have a unexpected effect on food safety [
57].
Though long-term feeding of transgenic products to animals shows no evidence of adverse effects on the human health, some researchers have still raised possibilities that these plants may cause unintended effects via different pathways, which needs to be strictly evaluated [
58,
59]. In particular, products from the exogenous genes that might cause the production of toxins that hurt human health should be evaluated. However, there is no evidence that products from the genes introduced to the plant genome are considered to be toxic. To solve public concerns regarding food safety, any transgenic plant should undergo a strict and systematic evaluation before entering the market [
60,
61].
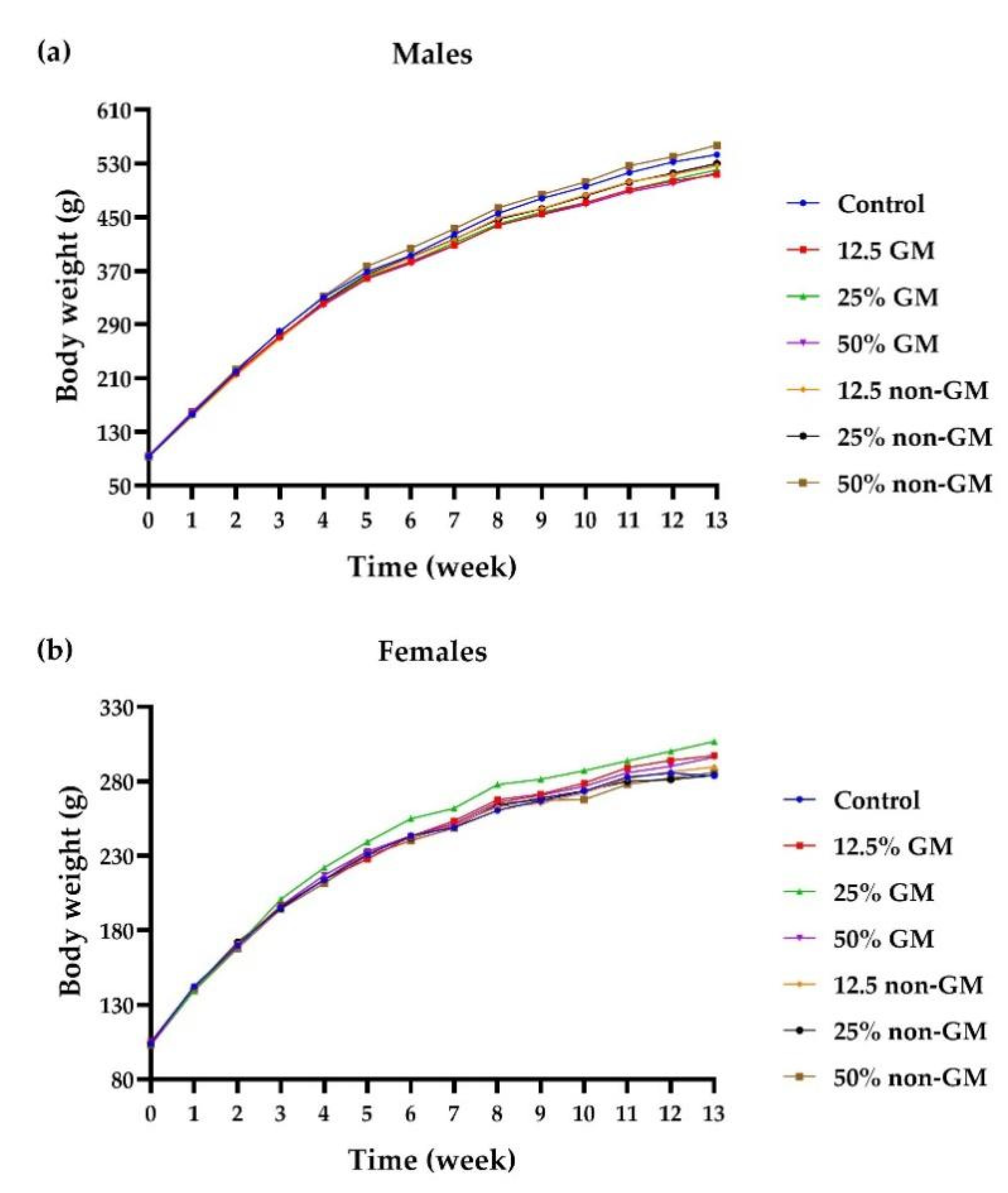
In the 90-day feeding trial, standard toxicological response variables in rats were used to assess the safety of long-term dietary exposure to grain from GM maize. During the 90-day toxicity study, all animals survived and stayed healthy. Rats fed transgenic maize in their diet showed identical body weight to rats fed regular food and diet containing non-transgenic maize. When the rats feeding on diets containing ZDAX5 maize grain were compared to those feeding on non-transgenic maize and control diets, there were several significant differences in the mean values of the response parameters (hematology and serum chemistry and organ weight). However, they were not thought to be negative and were not connected to the addition of GM maize. After further investigation, no changes were considered to be group-related. These findings matched those of prior 90-day rat feeding experiments [
62,
63,
64]. For example, Zhu et al. [
62] conducted a 90-day feeding study in rats with grain from glyphosate-tolerant maize that included the
G2-aroA gene. The results suggested that G2-aroA maize grain has no adverse effects in Sprague Dawley rats, and it is as safe and wholesome as maize grain obtained from non-GM crops. Consumption of GM maize ZDAX5 showed no observed toxicological effects on animal health based on our 90-day feeding study. Therefore, the transgenic maize ZDAX5 carrying the exogenous
P450-N-Z1 and
cp4 epsps genes is as safe as the non-transgenic maize Ruifeng-1. This will provide important data for the safety assessment of GM maize ZDAX5 at home and abroad. Once ZDAX5 is available on the market, farmers will be free to choose between glyphosate and flazasulfuron in their weed-control plans, which may delay the evolution of herbicide-resistant weeds. This will bring great social value and economic value to human society.