Deltamethrin and Its Nanoformulations Induce Behavioral Alteration and Toxicity in Rat Brain through Oxidative Stress and JAK2/STAT3 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan Nps
2.2. Preparation of Deltamethrin Loaded Chitosan Nanoparticles
2.3. Preparation of Silica Nanoparticles
2.4. Loading Silica Nanoparticles with Deltamethrin
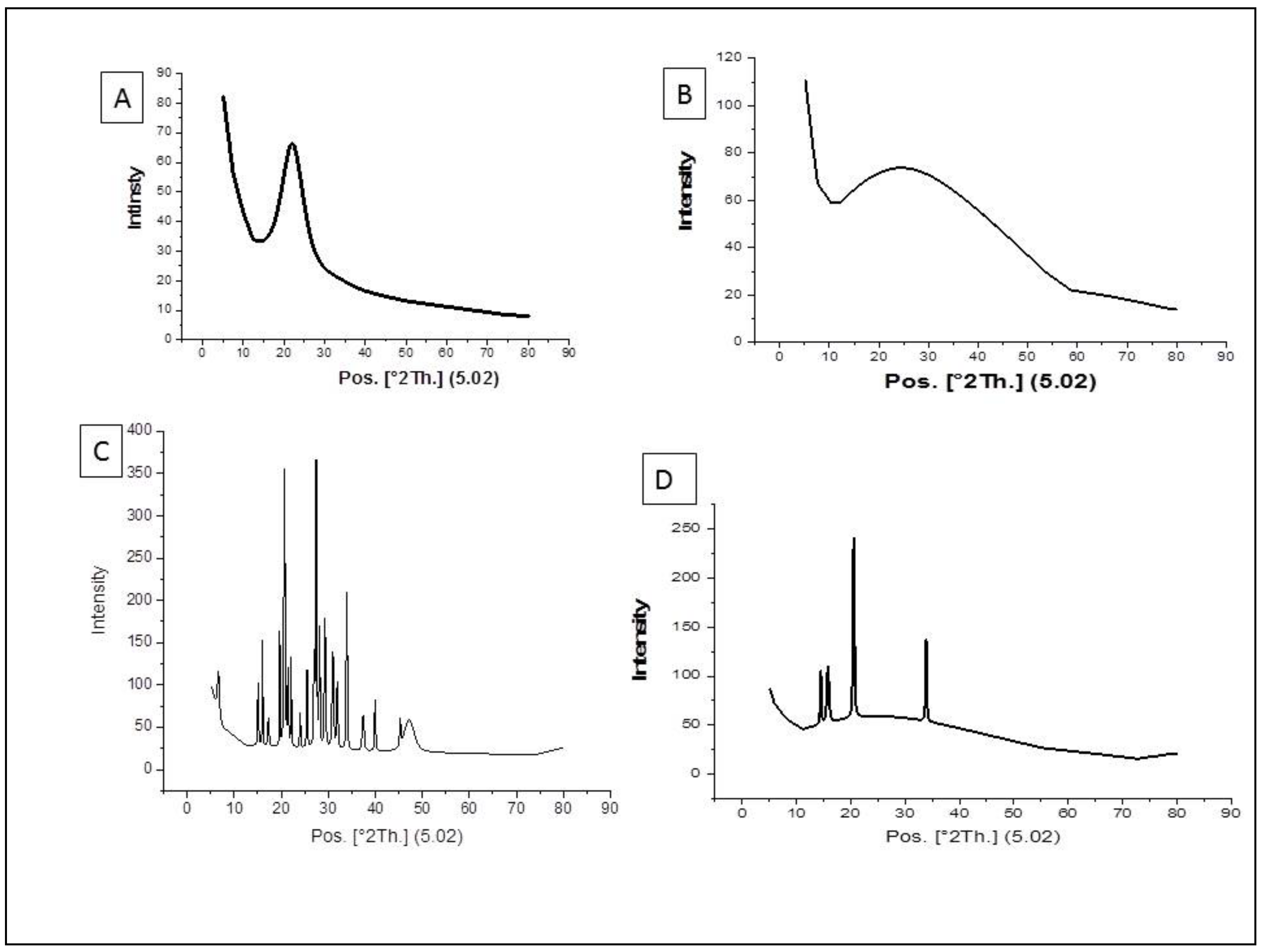
2.5. Characterization of the Nanoparticles Using XRD Method
2.6. The Subchronic Toxicity Study of Deltamethrin, S/DM Nps, and CS/DM Nps
2.7. The Effect of Deltamethrin, S/DM Nps, and CS/DM Nps on Bbehavior and Memory of Rats
2.7.1. Open Field Test
2.7.2. Y-Maze Test
2.8. Biochemical Analysis in Brain Homogenate
2.8.1. Activity of Superoxide Dismutase (SOD)
2.8.2. Lipid Peroxidation (MDA)
2.8.3. Glutathione (GSH) Content
2.8.4. Activity of Glutathione-S-Transferase
2.8.5. Activity of Glutathione Peroxidase (GPx)
2.9. RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.10. Western Blotting Analysis
2.11. Determination of Acetylcholinesterase
2.12. Determination of Monoamine Oxidase
2.13. Determination of Vascular Endothelial Growth Factor (VEGF)
2.14. Histological Preparations
2.15. Statistical Analysis
3. Results
3.1. Characterization of the Nanoparticles by Using XRD Method
3.2. The Effect of Deltamethrin, S/DM Nps, and CS/DM Nps on Behavior and Memory of Rats
3.3. Antioxidant/Oxidant Level
3.4. Acetylcholinesterase Serum Level
3.5. Monoamine Oxidase Level
3.6. Effect on the Serum Level of the Vascular Endothelial Growth Factor
3.7. Effects of Deltamethrin, S/DM Nps, and CS/DM Nps on JAK2 and STAT3 Gene Expression
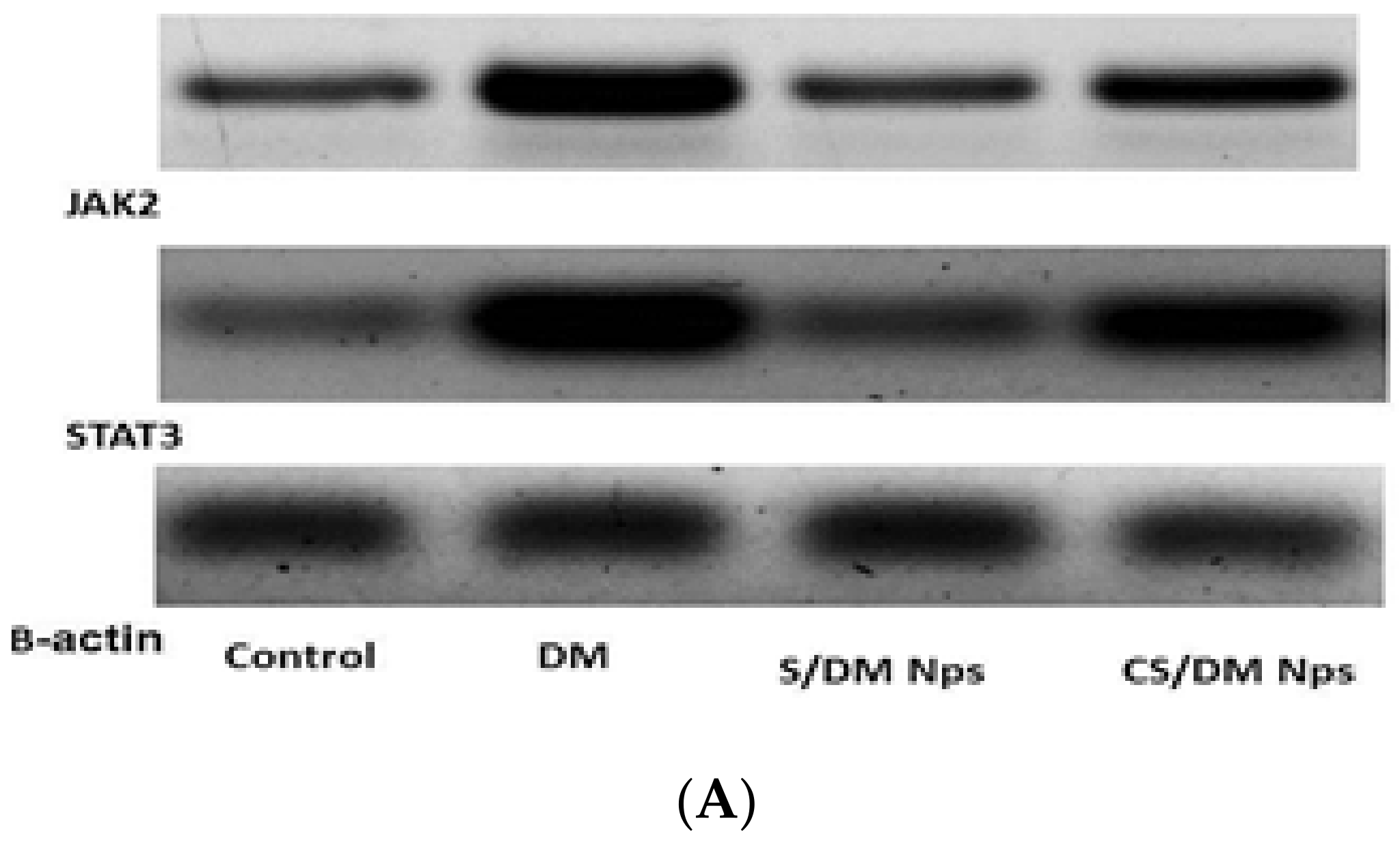
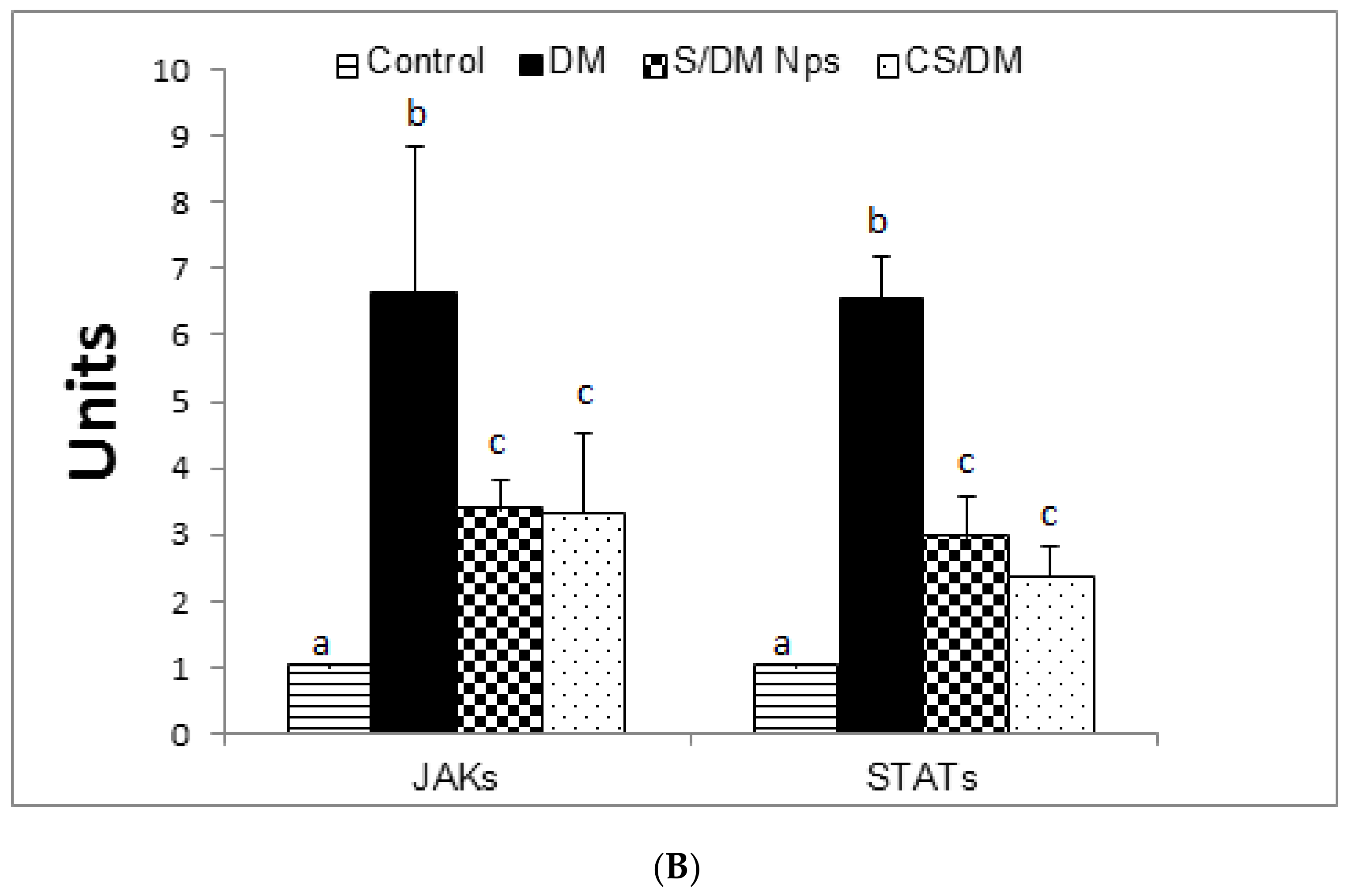
3.8. Effects of Deltamethrin, S/DM Nps, and CS/DM Nps on JAK2 and STAT3 Protein Expression
3.9. Histopathological Examination
- (1)
- Cerebral cortex
- (2)
- Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neri-Badang, M.C.; Chakraborty, S. Carbohydrate Polymers as Controlled Release Devices for Pesticides. J. Carbohydr. Chem. 2019, 38, 67–85. [Google Scholar] [CrossRef]
- Tiryaki, O.; Temur, O. The Fate of Pesticide in the Environment. J. Biol. Environ. Sci. 2010, 4, 29–38. [Google Scholar]
- Pérez, J.J.; Williams, M.K.; Weerasekera, G.; Smith, K.; Whyatt, R.M.; Needham, L.L.; Barr, D.B. Measurement of Pyrethroid, Organophosphorus, and Carbamate Insecticides in Human Plasma Using Isotope Dilution Gas Chromatography-High Resolution Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2554–2562. [Google Scholar] [CrossRef] [Green Version]
- Mani, V.M.; Sadiq, A.M.M. Naringin Modulates the Impairment of Memory, Anxiety, Locomotor, and Emotionality Behaviors in Rats Exposed to Deltamethrin; A Possible Mechanism Association with Oxidative Stress, Acetylcholinesterase and ATPase. Biomed. Prev. Nutr. 2014, 4, 527–533. [Google Scholar] [CrossRef]
- Uchendu, C.; Ambali, S.F.; Ayo, J.O.; Esievo, K.A. The Protective Role of Alpha-Lipoic Acid on Long-Term Exposure of Rats to the Combination of Chlorpyrifos and Deltamethrin Pesticides. Toxicol. Ind. Health 2017, 33, 159–170. [Google Scholar] [CrossRef]
- Wolansky, M.J.; Harrill, J.A. Neurobehavioral Toxicology of Pyrethroid Insecticides in Adult Animals: A Critical Review. Neurotoxicol. Teratol. 2008, 30, 55–78. [Google Scholar] [CrossRef] [Green Version]
- Thatheyus, A.J.; Gnana Selvam, A.D. Synthetic Pyrethroids: Toxicity and Biodegradation. Appl. Ecol. Environ. Sci. 2013, 1, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Ferland, S.; Côté, J.; Ratelle, M.; Thuot, R.; Bouchard, M. Detailed Urinary Excretion Time Courses of Biomarkers of Exposure to Permethrin and Estimated Exposure in Workers of a Corn Production Farm in Quebec, Canada. Ann. Occup. Hyg. 2015, 59, 1152–1167. [Google Scholar] [CrossRef] [Green Version]
- Magendira Mani, V.; Asha, S.; Sadiq, A.M.M. Pyrethroid Deltamethrin-Induced Developmental Neurodegenerative Cerebral Injury and Ameliorating Effect of Dietary Glycoside Naringin in Male Wistar Rats. Biomed. Aging Pathol. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Ares, I.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Effects of Exposure to Pyrethroid Cyfluthrin on Serotonin and Dopamine Levels in Brain Regions of Male Rats. Environ. Res. 2016, 146, 388–394. [Google Scholar] [CrossRef]
- Grillo, R.; Abhilash, P.C.; Fraceto, L.F. Nanotechnology Applied to Bio-Encapsulation of Pesticides. J. Nanosci. Nanotechnol. 2016, 16, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Hui, D.; Dzantor, E.K. Characterizing Rhizodegradation of the Insecticide Bifenthrin in Two Soil Types. J. Environ. Prot. 2011, 2, 940–946. [Google Scholar] [CrossRef] [Green Version]
- Stehle, S.; Schulz, R. Agricultural Insecticides Threaten Surface Waters at the Global Scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, A.G.; Moselhy, W.A.; Mohammed, H.M.; Nabil, T.M.; Shaban, M.; Aboelhadid, S.M.; Abdou, K.H. Toxicological Evaluations of Chitosan and Silica Nanoparticles Loaded with Deltamethrin with Improved Efficiency against Culex Pipiens Larvae. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Tığlı Aydın, R.S.; Pulat, M. 5-Fluorouracil Encapsulated Chitosan Nanoparticles for PH-Stimulated Drug Delivery: Evaluation of Controlled Release Kinetics. J. Nanomater. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural Extracts into Chitosan Nanocarriers for Rosmarinic Acid Drug Delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servat-Medina, L.; González-Gómez, A.; Reyes-Ortega, F.; Sousa, I.M.O.; Queiroz, N.D.C.A.; Zago, P.M.W.; Jorge, M.P.; Monteiro, K.M.; de Carvalho, J.E.; San Román, J.; et al. Chitosan–Tripolyphosphate Nanoparticles as Arrabidaea Chica Standardized Extract Carrier: Synthesis, Characterization, Biocompatibility, and Antiulcerogenic Activity. Int. J. Nanomed. 2015, 10, 3897–3909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Cui, H.; Sun, C.; Zhao, X.; Cui, B. Construction and Evaluation of Controlled-Release Delivery System of Abamectin Using Porous Silica Nanoparticles as Carriers. Nanoscale Res. Lett. 2014, 9, 2490. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.-X.; Li, Z.-Z.; Zou, H.-K.; Liu, A.-Q.; Chen, J.-F. Controlled Release of Avermectin from Porous Hollow Silica Nanoparticles. Pest Manag. Sci. 2005, 61, 583–590. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. [Google Scholar]
- Brown, R.E.; Corey, S.C.; Moore, A.K. Differences in Measures of Exploration and Fear in MHC-Congenic C57BL/6J and B6-H-2K Mice. Behav. Genet. 1999, 29, 263–271. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A Critical Review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef] [PubMed]
- Choleris, E.; Thomas, A.W.; Kavaliers, M.; Prato, F.S. A Detailed Ethological Analysis of the Mouse Open Field Test: Effects of Diazepam, Chlordiazepoxide and an Extremely Low Frequency Pulsed Magnetic Field. Neurosci. Biobehav. Rev. 2001, 25, 235–260. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Tuohimaa, P. Experimental Modeling of Anxiety and Depression. Acta Neurobiol. Exp. 2004, 64, 439–448. [Google Scholar]
- Nodoushan, M.J.; Roghani, M. The Effect of Nigella sativa on Learning and Memory in Male Diabetic Rats. Basic Clin. Neurosci. 2009, 1, 32–34. [Google Scholar]
- Baluchnejadmojarad, T.; Roghani, M.; Kamran, M.; Karimi, N. The Effect of Alpha-Lipoic Acid on Learning and Memory Deficit in a Rat Model of Temporal Lobe Epilepsy. Basic Clin. Neurosci. 2012, 3, 58–66. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of Product of Lipid Peroxidation (Malonyl Dialdehyde) in Biochemical Systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Mannervik, B.; Guthenberg, C. Glutathione Transferase (Human Placenta). Methods Enzymol. 1981, 77, 231–235. [Google Scholar]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M. Hesperidin Protects against Cyclophosphamide-Induced Hepatotoxicity by Upregulation of PPARγ and Abrogation of Oxidative Stress and Inflammation. Can. J. Physiol. Pharmacol. 2014, 92, 717–724. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Churchill Livingstone/Elsevier: Philadelphia, PA, USA, 2008. [Google Scholar]
- Nguyen, H.M.; Hwang, I.C.; Park, J.W.; Park, H.J. Photoprotection for Deltamethrin Using Chitosan-Coated Beeswax Solid Lipid Nanoparticles. Pest Manag. Sci. 2012, 68, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.K.; Kumar, A.; Prakash, J.; Singh, D.; Jagdale, P.; Shankar, J.; Kumar, M. Genotoxicity Evaluation of Zinc Oxide Nanoparticles in Swiss Mice after Oral Administration Using Chromosomal Aberration, Micronuclei, Semen Analysis, and RAPD Profile. Toxicol. Ind. Health 2017, 33, 821–834. [Google Scholar] [CrossRef]
- Satpute, R.; Pawar, P.; Puttewar, S.; Sawale, S.; Ambhore, P. Effect of Resveratrol and Tetracycline on the Subacute Paraquat Toxicity in Mice. Hum. Exp. Toxicol. 2017, 36, 1303–1314. [Google Scholar] [CrossRef]
- Delibas, N.; Ozcankaya, R.; Altuntas, I.; Sutcu, R. Effect of Cigarette Smoke on Lipid Peroxidation, Antioxidant Enzymes and NMDA Receptor Subunits 2A and 2B Concentration in Rat Hippocampus. Cell Biochem. Funct. 2003, 21, 69–73. [Google Scholar] [CrossRef]
- Ogaly, H.A.; Khalaf, A.A.; Ibrahim, M.A.; Galal, M.K.; Abd-Elsalam, R.M. Influence of Green Tea Extract on Oxidative Damage and Apoptosis Induced by Deltamethrin in Rat Brain. Neurotoxicol. Teratol. 2015, 50, 23–31. [Google Scholar] [CrossRef]
- Saoudi, M.; Badraoui, R.; Bouhajja, H.; Ncir, M.; Rahmouni, F.; Grati, M.; Jamoussi, K.; Feki, A.E. Deltamethrin Induced Oxidative Stress in Kidney and Brain of Rats: Protective Effect of Artemisia Campestris Essential Oil. Biomed. Pharmacother. 2017, 94, 955–963. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of Pyrethroid Neurotoxicity: Implications for Cumulative Risk Assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- Ray, D.E.; Fry, J.R. A Reassessment of the Neurotoxicity of Pyrethroid Insecticides. Pharmacol. Ther. 2006, 111, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Nieradko-Iwanicka, B.; Borzęcki, A. Subacute Poisoning of Mice with Deltamethrin Produces Memory Impairment, Reduced Locomotor Activity, Liver Damage and Changes in Blood Morphology in the Mechanism of Oxidative Stress. Pharmacol. Rep. 2015, 67, 535–541. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, R. Protective Role of Curcumin in Deltamethrin Induced System Toxicity in Wistar Rats. Planta Med. 2013, 79, PB43. [Google Scholar] [CrossRef]
- Floyd, R.A.; Carney, J.M. Free Radical Damage to Protein and DNA: Mechanisms Involved and Relevant Observations on Brain Undergoing Oxidative Stress. Ann. Neurol. 1992, 32, S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.; Coleman, M.D. The Blood-Brain Barrier: In Vitro Methods and Toxicological Applications. Toxicol. In Vitro 1995, 9, 191–204. [Google Scholar] [CrossRef]
- Hazarika, A.; Sarkar, S.N.; Hajare, S.; Kataria, M.; Malik, J.K. Influence of Malathion Pretreatment on the Toxicity of Anilofos in Male Rats: A Biochemical Interaction Study. Toxicology 2003, 185, 1–8. [Google Scholar] [CrossRef]
- Yousef, M.I.; Awad, T.I.; Mohamed, E.H. Deltamethrin-Induced Oxidative Damage and Biochemical Alterations in Rat and Its Attenuation by Vitamin, E. Toxicology 2006, 227, 240–247. [Google Scholar] [CrossRef]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and Biochemistry of Lipid Peroxidation Products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.S.; Ramana, K.V. Regulation of NF-ΚB-Induced Inflammatory Signaling by Lipid Peroxidation-Derived Aldehydes. Oxid. Med. Cell. Longev. 2013, 2013, 690545. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sasmal, D.; Sharma, N. An Insight into Deltamethrin Induced Apoptotic Calcium, P53 and Oxidative Stress Signalling Pathways. Toxicol. Environ. Health Sci. 2015, 7, 25–34. [Google Scholar] [CrossRef]
- Pimpão, C.T.; Zampronio, A.R.; Silva de Assis, H.C. Effects of Deltamethrin on Hematological Parameters and Enzymatic Activity in Ancistrus multispinis (Pisces, Teleostei). Pestic. Biochem. Physiol. 2007, 88, 122–127. [Google Scholar] [CrossRef]
- Yu, S.J.; Nguyen, S.N. Insecticide Susceptibility and Detoxication Enzyme Activities in Permethrin-Selected Diamondback Moths. Pestic. Biochem. Physiol. 1996, 56, 69–77. [Google Scholar] [CrossRef]
- Kostaropoulos, I.; Papadopoulos, A.I.; Metaxakis, A.; Boukouvala, E.; Papadopoulou-Mourkidou, E. Glutathione S-Transferase in the Defence against Pyrethroids in Insects. Insect Biochem. Mol. Biol. 2001, 31, 313–319. [Google Scholar] [CrossRef]
- Suresh Kumar, R.S.; Shiny, P.J.; Anjali, C.H.; Jerobin, J.; Goshen, K.M.; Magdassi, S.; Mukherjee, A.; Chandrasekaran, N. Distinctive Effects of Nano-Sized Permethrin in the Environment. Environ. Sci. Pollut. Res. 2013, 20, 2593–2602. [Google Scholar] [CrossRef]
- Edmondson, D.E.; Mattevi, A.; Binda, C.; Li, M.; Hubalek, F. Structure and Mechanism of Monoamine Oxidase. Curr. Med. Chem. 2012, 11, 1983–1993. [Google Scholar] [CrossRef]
- Nebbioso, M.; Pascarella, A.; Cavallotti, C.; Pescosolido, N. Monoamine Oxidase Enzymes and Oxidative Stress in the Rat Optic Nerve: Age-Related Changes. Int. J. Exp. Pathol. 2012, 93, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Husain, R.; Husain, R.; Adhami, V.M.; Seth, P.K. Behavioral, neurochemical, and neuromorphological effects of deltamethrin in adult rats. J. Toxicol. Environ. Health 1996, 48, 515–516. [Google Scholar] [PubMed]
- Pitzer, E.M.; Sugimoto, C.; Gudelsky, G.A.; Huff Adams, C.L.; Williams, M.T.; Vorhees, C.V. Deltamethrin Exposure Daily from Postnatal Day 3–20 in Sprague-Dawley Rats Causes Long-Term Cognitive and Behavioral Deficits. Toxicol. Sci. 2019, 169, 511–523. [Google Scholar] [CrossRef]
- Lange, C.; Storkebaum, E.; De Almodóvar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular Endothelial Growth Factor: A Neurovascular Target in Neurological Diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef]
- Kovács, Z.; Ikezaki, K.; Samoto, K.; Inamura, T.; Fukui, M. VEGF and Flt: Expression Time Kinetics in Rat Brain Infarct. Stroke 1996, 27, 1865–1873. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Van Bruggen, N.; Chopp, M. VEGF Enhances Angiogenesis and Promotes Blood-Brain Barrier Leakage in the Ischemic Brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Magavi, S.S.; Leavitt, B.R.; Macklis, J.D. Induction of Neurogenesis in the Neocertex of Adult Mice. Nature 2000, 405, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular Endothelial Growth Factor (VEGF) Stimulates Neurogenesis in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef] [Green Version]
- Acosta, L.; Morcuende, S.; Silva-Hucha, S.; Pastor, A.M.; de la Cruz, R.R. Vascular Endothelial Growth Factor (VEGF) Prevents the Downregulation of the Cholinergic Phenotype in Axotomized Motoneurons of the Adult Rat. Front. Mol. Neurosci. 2018, 11, 241. [Google Scholar] [CrossRef]
- Renauld, J.C. Class II Cytokine Receptors and Their Ligands: Key Antiviral and Inflammatory Modulators. Nat. Rev. Immunol. 2003, 3, 667–676. [Google Scholar] [CrossRef]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The Role of JAK-STAT Signaling within the CNS. JAK-STAT 2013, 2, e22925. [Google Scholar] [CrossRef] [Green Version]
- Feriani, A.; Tir, M.; Hachani, R.; Gómez-Caravaca, A.M.; Contreras, M.D.M.; Taamalli, A.; Talhaoui, N.; Segura-Carretero, A.; Ghazouani, L.; Mufti, A.; et al. Zygophyllum album Saponins Prevent Atherogenic Effect Induced by Deltamethrin via Attenuating Arterial Accumulation of Native and Oxidized LDL in Rats. Ecotoxicol. Environ. Saf. 2020, 193, 110318. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.I.; Parmenter, T.R.; Mok, M. The Relationship between Neurobehavioural Problems of Severe Traumatic Brain Injury (TBI), Family Functioning and the Psychological Well-Being of the Spouse/Caregiver: Path Model Analysis. Brain Inj. 2002, 16, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhang, Z.; Zou, Y.; Tian, Q.; Han, S.; Xu, Z.; Liao, J.; Gao, L.; Chen, Q.; Li, M. Tetramethylpyrazine Attenuates Blood-Brain Barrier Disruption in Ischemia/Reperfusion Injury through the JAK/STAT Signaling Pathway. Eur. J. Pharmacol. 2019, 854, 289–297. [Google Scholar] [CrossRef]
- Soendergaard, C.; Bergenheim, F.H.; Bjerrum, J.T.; Nielsen, O.H. Targeting JAK-STAT Signal Transduction in IBD. Pharmacol. Ther. 2018, 192, 100–111. [Google Scholar] [CrossRef]
- Gadina, M.; Johnson, C.; Schwartz, D.; Bonelli, M.; Hasni, S.; Kanno, Y.; Changelian, P.; Laurence, A.; O’Shea, J.J. Translational and Clinical Advances in JAK-STAT Biology: The Present and Future of Jakinibs. J. Leukoc. Biol. 2018, 104, 499–514. [Google Scholar] [CrossRef]
- Reich, N.C. STATs Get Their Move On. JAK-STAT 2013, 2, e27080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konjević, G. STAT Proteins in Cancerogenesis and Therapy of Malignancies. Srp. Arh. Celok. Lek. 2009, 137, 98–105. [Google Scholar] [CrossRef]
- Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. Active Oxygen Species Generated from Photoexcited Fullerene (C 60) as Potential Medicines: O2−. versus 1O2. J. Am. Chem. Soc. 2003, 125, 12803–12809. [Google Scholar] [CrossRef]
- Yu, C.I.; Cheng, C.I.; Kang, Y.F.; Chang, P.C.; Lin, I.P.; Kuo, Y.H.; Jhou, A.J.; Lin, M.Y.; Chen, C.Y.; Lee, C.H. Hispidulin Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia and Attenuates the Activation of Akt, NF-ΚB, and STAT3 Pathway. Neurotox. Res. 2020, 38, 163–174. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, J.; Wu, J.; Wu, Q.; Jia, C.; Xu, Y.X.Z.; Chen, L.; Tu, W.; Yang, G.; Kong, J.; et al. Atractylenolide III Ameliorates Cerebral Ischemic Injury and Neuroinflammation Associated with Inhibiting JAK2/STAT3/Drp1-Dependent Mitochondrial Fission in Microglia. Phytomedicine 2019, 59, 152922. [Google Scholar] [CrossRef] [PubMed]
- Akaike, A.; Takada-Takatori, Y.; Kume, T.; Izumi, Y. Mechanisms of Neuroprotective Effects of Nicotine and Acetylcholinesterase Inhibitors: Role of A4 and A7 Receptors in Neuroprotection. J. Mol. Neurosci. 2010, 40, 211–216. [Google Scholar] [CrossRef]
- Knudsen, L.E.; Hansen, Å.M. Biomarkers of Intermediate Endpoints in Environmental and Occupational Health. Int. J. Hyg. Environ. Health 2007, 210, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S. Neurotransmission Dysfunction by Mixture of Pesticides and Preventive Effects of Quercetin on Brain, Hippocampus and Striatum in Rats. Toxicol. Environ. Health Sci. 2020, 12, 203–212. [Google Scholar] [CrossRef]
- Maslova, M.N.; Reznik, L.V. Functional and biochemical changes in rat brain in the initial stages of hyperbaric oxygenation. Dokl. Akad. Nauk SSSR 1971, 197, 494–496. [Google Scholar]
- Nasuti, C.; Gabbianelli, R.; Falcioni, M.L.; Di Stefano, A.; Sozio, P.; Cantalamessa, F. Dopaminergic System Modulation, Behavioral Changes, and Oxidative Stress after Neonatal Administration of Pyrethroids. Toxicology 2007, 229, 194–205. [Google Scholar] [CrossRef]
- Preud’homme, V.; Milla, S.; Gillardin, V.; De Pauw, E.; Denoël, M.; Kestemont, P. Effects of Low Dose Endosulfan Exposure on Brain Neurotransmitter Levels in the African Clawed Frog Xenopus Laevis. Chemosphere 2015, 120, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.F.; Medeiros, K.A.A.L.; Lins, L.C.R.F.; Bispo, J.M.M.; Gois, A.M.; Freire, M.A.M.; Marchioro, M.; Santos, J.R. Intracerebroventricular Injection of Deltamethrin Increases Locomotion Activity and Causes Spatial Working Memory and Dopaminergic Pathway Impairment in Rats. Brain Res. Bull. 2020, 154, 1–8. [Google Scholar] [CrossRef]
- Hossain, M.M.; Belkadi, A.; Al-Haddad, S.; Richardson, J.R. Deltamethrin Exposure Inhibits Adult Hippocampal Neurogenesis and Causes Deficits in Learning and Memory in Mice. Toxicol. Sci. 2020, 178, 347–357. [Google Scholar] [CrossRef]
- Khakpai, F.; Zarrindast, M.R.; Nasehi, M.; Haeri-Rohani, A.; Eidi, A. The Role of Glutamatergic Pathway between Septum and Hippocampus in the Memory Formation. Excli J. 2013, 12, 41–51. [Google Scholar]
- Yeung, A.W.K.; Georgieva, M.G.; Atanasov, A.G.; Tzvetkov, N.T. Monoamine Oxidases (MAOs) as Privileged Molecular Targets in Neuroscience: Research Literature Analysis. Front. Mol. Neurosci. 2019, 12, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | GenBank Accession Number | Gene Sequence (5′-3′) |
|---|---|---|
| JAK2 | NM_031514.1 | F: GTGTGGAGATGTGCCGCTAT R: GCACTGTAGCACACTCCCTT |
| STAT3 | NM-012747.2 | F: GCAGTTTAGACAGGGAGGGG R: CACTGTCTCTGGGGCTGAAG |
| β-actin | NM_031144.3 | F: AGGAGTACGATGAGTCCGGC R: CGCAGCTCAGTAACAGTCCG |
| Locomotor Behavior | Anxiety Like Behavior | Exploratory Behavior | Spontaneous Alternative Behavior Percent (SAP) | ||||
|---|---|---|---|---|---|---|---|
| Number of Crossed Peripheral Squares | Rearing Frequency | Freezing Time (Second) | Streched Attend Posture (Frequency) | Number of Crossed Central Squares | Time Rats Spent in Central Squares (Second) | ||
| control | 6.33 ± 6.94 b | 9.16 ± 5.60 ab | 69.33 ± 51.63 a | 6.00 ± 2.75 a | 6.00 ± 6.60 a | 8.33 ± 7.20 a | 25.83 ± 4.62 b |
| DM | 1.66 ± 1.16 a | 7.00 ± 3.79 a | 185.00 ± 47.95 b | 10.50 ± 3.08 b | 1.16 ± 1.16 a | 3.16 ± 5.91 a | 14.66 ± 5.50 a |
| S/DM Nps | 3.00 ± 2.44 b | 7.33 ± 1.96 a | 65.83 ± 19.34 a | 6.30 ± 4.36 a | 3.00 ± 2.44 a | 8.66 ± 8.35 a | 17.66 ± 3.26 a |
| CS/DM Nps | 3.83 ± 3.48 b | 12.16 ± 2.85 b | 73.50 ± 32.74 a | 6.00 ± 1.78 a | 3.83 ± 3.48 a | 6.00 ± 5.09 a | 18.33 ± 4.96 a |
| Groups | Glutathione Content (nmol/ 100 mg Tissue) | Glutathione Peroxidase (mU/100 mg Tissue) | Glutathione-S-Transferase (U/100 mg Tissue) | Lipid Peroxidation (nmol MDA/100 mg Tissue/Hour) | Superoxide Dismutase (mU/100 mg Tissue) |
|---|---|---|---|---|---|
| Control | 11.63 ± 1.98 a | 169.87 ± 6.33 a | 68.56 ± 11.68 a | 4.16 ± 0.49 a | 82.03 ± 2.33 a |
| DM | 4.55 ± 1.55 b | 54.91 ± 7.30 b | 24.64 ± 3.03 b | 7.32 ± 0.55 bc | 63.93 ± 3.87 b |
| S/DM Nps | 7.17 ± 1.71 c | 149.35 ± 4.53 c | 59.74 ± 6.36 a | 6.67 ± 1.28 b | 74.73 ± 2.72 c |
| CS/DMNps | 3.73 ± 2.03 b | 153.92 ± 12.20 c | 40.60 ± 4.30 c | 8.38 ± 1.72 c | 75.76 ± 1.99 c |
| Groups | Acetylcholinesterase (pg/mL) | Monoamine Oxidase (mU/mL) | Vascular Endothelial Growth Factor (pg/mL) |
|---|---|---|---|
| Control | 18.43 ± 2.70 a | 155.23 ± 3.71 a | 137.66 ± 4.43 a |
| DM | 42.00 ± 8.66 b | 215.37 ± 5.75 b | 324.33 ± 3.95 b |
| S/DM Nps | 27.63 ± 2.99 a | 177.36 ± 8.85 a | 94.33 ± 5.59 a |
| CS/DM Nps | 24.70 ± 7.35 a | 162.00 ± 7.75 a | 149.33 ± 6.88 a |
| Groups | JAK2 | STAT3 |
|---|---|---|
| Control | 1.01 ± 0.015 a | 1.00 ± 0.011 a |
| DM | 6.43 ± 1.55 b | 5.7 ± 0.60 b |
| S/DM Nps | 3.33 ± 0.288 c | 2.79 ± 0.28 c |
| CS/DM Nps | 3.46 ± 0.408 c | 2.23 ± 0.40 c |
| Groups | JAK2 | STAT3 |
|---|---|---|
| Control | 1.01 ± 0.01 a | 1.01 ± 0.015 a |
| DM | 6.66 ± 2.17 b | 6.57 ± 0.59 b |
| S/DM Nps | 3.37 ± 0.46 c | 2.96 ± 0.60 c |
| CS/DM Nps | 3.31 ± 1.22 c | 2.37 ± 0.46 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, A.G.; Moselhy, W.A.; Mohammed, H.M.; Khalil, F.; Shaban, M.; El-Nahass, E.-S.; Al-Muzafar, H.M.; Adel Amin, K.; Abdou, K.A. Deltamethrin and Its Nanoformulations Induce Behavioral Alteration and Toxicity in Rat Brain through Oxidative Stress and JAK2/STAT3 Signaling Pathway. Toxics 2022, 10, 303. https://doi.org/10.3390/toxics10060303
Khalifa AG, Moselhy WA, Mohammed HM, Khalil F, Shaban M, El-Nahass E-S, Al-Muzafar HM, Adel Amin K, Abdou KA. Deltamethrin and Its Nanoformulations Induce Behavioral Alteration and Toxicity in Rat Brain through Oxidative Stress and JAK2/STAT3 Signaling Pathway. Toxics. 2022; 10(6):303. https://doi.org/10.3390/toxics10060303
Chicago/Turabian StyleKhalifa, Ahlam G., Walaa A. Moselhy, Hanaa M. Mohammed, Fatma Khalil, Mohamed Shaban, El-Shaymaa El-Nahass, Hessah Mohammed Al-Muzafar, Kamal Adel Amin, and Khaled A. Abdou. 2022. "Deltamethrin and Its Nanoformulations Induce Behavioral Alteration and Toxicity in Rat Brain through Oxidative Stress and JAK2/STAT3 Signaling Pathway" Toxics 10, no. 6: 303. https://doi.org/10.3390/toxics10060303





