Abstract
The solid-state fermentation (SSF) of agro-industrial by-products such as okara, pomegranate peel, and cranberry pomace presents a sustainable approach to enhance the release of bioactive compounds. This study investigated the effects of different microbial cultures—Rhizopus oligosporus, Aspergillus oryzae, Streptococcus thermophilus, and a co-culture of R. oligosporus and S. thermophilus—on the bioconversion of bioactive compounds in 100% okara, okara with 2% pomegranate peel, and okara with 1% cranberry pomace. The objective was to assess whether co-culture fermentation with molds and S. thermophilus augments the release of bioactive compounds in okara-based fermentations through synergistic enzymatic activity. Over a period of 72 h, isoflavone transformation (daidzin, daidzein, genistin, and genistein), pH evolution, and water activity were assessed. The co-culture system exhibited improved bioconversion, leading to significant (p < 0.01) increases in daidzein and genistein in pure okara compared to the starting material. The highest polyphenol content (0.908 mg/g) and antioxidant capacity (24.9 mg Trolox eq/g) were recorded in 100% okara. However, pomegranate peel inhibited β-glucosidase activity, delaying the release of isoflavone aglycones. These findings confirm that co-culture fermentation is an effective strategy for enhancing the bioactive properties of okara-based fermentations. This facilitates the release of bioactive aglycones and supports the upcycling of agro-industrial by-products into functional food ingredients. Future research should focus on optimizing fermentation parameters to further enhance the release of bioactive compounds.
1. Introduction
The fruit production and processing industries generate substantial by-products and waste, leading to significant economic losses and environmental concerns. An estimated one-third of all food produced annually is lost or discarded, amounting to approximately 1.6 billion tons of waste, with a carbon footprint of 3.3 billion tons of carbon dioxide [1]. However, fruit-derived waste materials—including peels, seeds, skins, shells, pods, cores, pulp, and pomace—offer opportunities for sustainable reuse. These by-products contain bioactive compounds such as dietary fiber, flavonoids, phenolic compounds, other antioxidants, polysaccharides, and essential minerals. By extracting and repurposing these compounds, they can be transformed into nutraceuticals, functional foods, or food additives, thereby converting waste into valuable resources. Despite this potential, large-scale biorefinery applications for sustainable conversion remain limited [2]. One key challenge is optimizing extraction techniques for bioactive compounds. Factors such as pressure, temperature, light exposure, and pH significantly influence both the yield and quality of these compounds, making the optimization of these parameters crucial for efficiency. Recently, modern extraction methods—including microwave-assisted, ultrasound-assisted, pressurized solvent, enzymatic, negative-pressure, and high-pressure homogenization techniques—have emerged as sustainable alternatives to conventional methods such as grinding, maceration, Soxhlet extraction, and supercritical fluid extraction. These advancements have improved both the efficiency and environmental sustainability of bioactive compound recovery from agricultural waste [2,3].
1.1. Okara: A Fiber-Rich By-Product
Okara, a by-product of tofu and soymilk production, is generated at a ratio of 1.2 kg per kg of processed soybeans [4]. It is a fiber-rich raw material with prebiotic potential and bioactive components such as isoflavones, lignans, phytosterols, saponins, and phytates, which offer antioxidant, cardioprotective, and chemopreventive benefits. Fermentation of okara enhances its nutritional profile by increasing soluble fiber, protein, amino acids, and bioavailable isoflavones while reducing the phytic acid content [4]. Solid-state fermentation (SSF) with lactic acid bacteria, including Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, and Pediococcus acidilactici, has been shown to improve the sensory properties of okara by reducing its undesirable aroma while enhancing fruity and buttery notes [5]. Furthermore, fermented okara has been explored for its potential in meat substitutes, benefiting from improved water-holding capacity and reduced protein oxidation [6].
1.2. Cranberry Pomace as a Bioactive Source
Cranberry pomace, a by-product of the juice industry, is an excellent source of bioactive compounds with antioxidant properties [7]. Extracts from the pomace of fruit such as grapes, cranberries, and apples have demonstrated antibacterial activity against Listeria, Salmonella, Staphylococcus, and Escherichia species [8]. Additionally, berry pomace possesses techno-functional properties that influence the rheological, textural, sensory, and nutritional attributes of food products [9]. Both the American (Vaccinium macrocarpon) and European (Vaccinium oxycoccus) cranberry species contain high levels of polyphenols, including phenolic acids, anthocyanins, and flavonoids, particularly proanthocyanidins, which are associated with various health benefits. Cranberries have been linked to reduced risk of urinary tract infections, improved oral health, reduced inflammation, and lower cholesterol levels. The phenolic contents of cranberries are influenced by multiple factors, including cultivar type, agricultural practices, geographical region, climate, ripening stage, harvest timing, and storage conditions [10,11]. Studies have identified p-coumaric acid, p-hydroxybenzoic acid, kaempferol-3-O-glucoside, caffeic acid, and ellagic acid as key phenolic compounds in Vaccinium macrocarpon extracts [10].
1.3. Pomegranate Peel as a Functional Ingredient
Pomegranate peel constitutes 30–40% of the fruit and is rich in polyphenols, tannins, flavonoids, polysaccharides, and essential minerals such as calcium, potassium, and magnesium [12,13]. Like cranberry pomace, pomegranate peel exhibits antioxidant, antitumor, anti-inflammatory, neuroprotective, antiviral, and antibacterial properties [14]. SSF using Aspergillus brasiliensis has been shown to yield valuable bioactive molecules such as citric acid, punicalins, punicalagins, and ellagic acid from pomegranate peel [15]. Moreover, pomegranate peel extracts have been explored as natural preservatives in meat products, improving texture, extending shelf life, enhancing color stability, and boosting antioxidant efficacy [16]. These by-products can also be incorporated into functional foods, dietary supplements, herbal teas, baked goods, and nutritional bars [16].
1.4. Objectives and Approach
Optimizing fermentation methods for agro-industrial by-products remains challenging, particularly in balancing bioactive compound release with potential inhibitory effects from polyphenol-rich substrates. To address this gap, this study will evaluate the release of bioactive compounds from a product primarily composed of recycled waste products—notably, cranberry pomace, pomegranate peel, and okara—using co-culture SSF with GRAS (Generally Recognized as Safe) microbial strains: Aspergillus oryzae, Rhizopus oligosporus, and Streptococcus thermophilus. SSF, which utilizes solid substrates with minimal liquid water, provides an environment that is conducive to microbial growth. This technique is particularly effective for extracting valuable biomolecules from lignocellulosic materials, making it a promising approach for food waste valorization. SSF aligns with the 3-R approach (reduce, reuse, recycle) by converting agro-industrial residues into valuable bioproducts, thereby supporting the circular bioeconomy. The primary goal of this project was to assess the potential release of bioactive compounds, using cranberry pomace and okara as the main substrates. These substrates were fermented using Streptococcus thermophilus, Aspergillus oryzae, and Rhizopus oligosporus in SSF. Streptococcus thermophilus, a Gram-positive lactic acid bacterium, is widely used in dairy fermentation. Aspergillus oryzae and Rhizopus oligosporus are filamentous fungi that are used in traditional fermented foods such as tempeh. Co-culture fermentation using these organisms has been shown to be more effective than fermentation by single organisms, due to their complementary enzymatic activities, which enhance bioactive compounds’ release. R. oligosporus produces β-glucosidase, facilitating the conversion of isoflavone glycosides into more bioavailable aglycones, while S. thermophilus helps regulate pH and contributes additional enzymatic activity that optimizes the fermentation conditions. This synergy should lead to an efficient release of the less bioactive and less bioavailable isoflavone glucosides daidzin and genistin into the more active and more bioavailable aglycons daidzein and genistein during SSF [17,18,19]. SSF presents numerous advantages over traditional submerged fermentation, including lower water and energy consumption and minimal residual waste generation. However, challenges such as process scalability, mass and energy transfer efficiency, and substrate homogeneity remain key obstacles. Several parameters—including substrate pretreatment, particle size, medium composition, moisture content, inoculum density, temperature, pH, aeration, and agitation—significantly impact SSF’s efficiency. Thus, optimizing the fermentation conditions is essential for producing a functional food with enhanced bioactive properties. This study aims to determine whether co-culture fermentation with molds and the lactic acid bacterium Streptococcus thermophilus enhances bioactive compounds’ release in okara-based fermentations through synergistic enzymatic activity.
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
Three Generally Recognized as Safe (GRAS) strains were utilized in this study: Aspergillus oryzae (DSM 1862, Leibniz Institute DSMZ, Braunschweig, Germany), Rhizopus microsporus var. oligosporus (DSM 1964), and Streptococcus thermophilus (DSM 20617). The strains were initially inoculated onto 92 × 16 mm standard Petri dishes containing appropriate agar media to confirm their purity and viability. The selected agar media were potato dextrose agar (Biolife, Milan, Italy) for A. oryzae, Sabouraud dextrose agar (Biokar Diagnostics, Allonne, France) for R. oligosporus, and M17 agar (Biolife, Milan, Italy) for S. thermophilus. The incubation conditions were 25 °C for A. oryzae and R. oligosporus, and 37 °C for S. thermophilus.
For S. thermophilus, an isolated colony was suspended in M17 broth (Sigma-Aldrich, Buchs, Switzerland) and incubated at 37 °C with agitation at 150 RPM for 24 h. Anaerobic conditions were achieved by placing the flask in a sealed jar with a GENBox anaer (bioMérieux SA, Petit Lancy, Switzerland) and an anaerobic indicator stick (bioMérieux SA, Petit Lancy, Switzerland).
2.2. Preparation of Solid Substrates
Three different solid substrates were formulated to maintain an initial pH of ~5, suitable for microbial growth:
- 100% okara (Engel-Tofu, Widen, Switzerland);
- 98% okara + 2% pomegranate peel (Oasi delle Spezie, Gudo, Switzerland);
- 99% okara + 1% cranberry pomace (Oasi delle Spezie, Gudo, Switzerland).
The choice of 2% pomegranate peel and 1% cranberry pomace was based on their ability to enhance bioactive compounds’ release while sustaining microbial growth. Preliminary studies showed that higher concentrations inhibited R. oligosporus, A. oryzae, and S. thermophilus due to excessive polyphenols and antimicrobial compounds. Hence, these levels were the highest that still allowed for effective fermentation and enzymatic bioconversion.
An optimized hydration solution (HS), as previously described by Brück et al. [20], was prepared by dissolving 2.5 g/L NaNO3 (Sigma-Aldrich, Buchs, Switzerland), 1 g/L KH2PO4 (Acros Organics, Reinach, Switzerland), 0.5 g/L KCl (Sigma-Aldrich, Buchs, Switzerland), and 0.5 g/L MgSO4 (Sigma-Aldrich, Buchs, Switzerland) in water, followed by autoclaving. Each 100 g of solid substrate was hydrated with 80 mL of HS and kneaded in a sterile plastic bag.
Acidification was performed using an aqueous acetic acid solution (Sigma-Aldrich, Buchs, Switzerland) at a concentration of 45 g/L (pH 2.30). A total of 5 mL of acid solution (AS) was added per 100 g of solid support, followed by kneading. Additionally, 1% (w/w) NaCl (Carl Roth, Arlesheim, Switzerland) was incorporated and mixed as described above.
2.3. Inoculation of Microorganisms
The fungal starters were inoculated at 1 g/kg of solid support, which gave an approximate fungal spore count of 109 spores/100 g. Due to the small mass required for 100 g portions, 100 mg of fungal starter was first suspended in 1 mL of maximum recovery diluent (Biolife, Milan, Italy) containing 1.0 g/L peptone, 8.5 g/L NaCl, and 5.0 g/L Tween® 80 (Sigma-Aldrich, Buchs, Switzerland), before being mixed into the solid support.
For S. thermophilus, an initial concentration of 109 cells/100 g of solid support was targeted to ensure a rapid fermentation process and maintain cell viability. Cell counts were performed using a Neubauer counting chamber (depth: 0.02 mm, surface area: 0.0025 mm2), with cultures suspended in M17 broth.
In co-culture fermentations, R. oligosporus (50 mg tempeh starter) and S. thermophilus (0.5 × 109 cells/100 g) were co-inoculated per 100 g of hydrated, acidified, and salted solid support.
2.4. Fermentation Conditions
Homogenized paste was molded into ~30 g disks using 60 × 15 mm sterile Petri dishes. The disks were placed on perforated baking trays (GN-Profi, Isernhagen, Germany) and incubated at strain-specific temperatures: 25 °C for A. oryzae and R. oligosporus, and 30 °C for the co-culture of S. thermophilus and R. oligosporus.
2.5. Monitoring and Sampling
Fermentation was monitored through CO2 concentration [ppm] and relative humidity [%] measurements using a climate analyzer (Testo, Egg, Switzerland) and a Bluetooth® probe (Testo, Egg, Switzerland). The disks were photographed every 24 h.
At 0, 24, 48, and 72 h, 2 g of fermented solid was collected from each disk and stored at −18 °C. The pH measurements were conducted using a 691 Metrohm pH meter (Zofingen, Switzerland), with 2 g of fermented material suspended in 20 mL of demineralized water.
Water activity (aw) was measured using an AW SPRINT TH500 (Novasina, Lachen, Switzerland) at 0 h and 72 h.
2.6. Extraction Procedures of Fermented Substrates
Methanol extraction of polyphenols was carried out using 5 mL of anhydrous methanol (Thermo Fischer Scientific, Reinach, Switzerland) on 500 mg samples. The extraction process included ultrasonication for 15 min, followed by centrifugation at 3000 RPM for 10 min. The supernatant was collected, and the remaining pellet was re-extracted using the same procedure. The combined supernatants were filtered through CHROMAFIL® Xtra PTFE-45/13 filters before photometric and HPLC analysis.
2.7. Polyphenol and Antioxidant Assays
The Folin–Ciocâlteu total polyphenol assay was conducted using a modular multimode microplate reader (Synergy H1, BioTek Instruments, Sursee, Switzerland) and a 96-well microplate (Paul Boettger GmbH & Co. KG, Bodenmais, Germany) [21,22]. The dispenser was programmed to deliver 250 µL at a speed of 300 µL/s, with a refill mode set to 900 µL at the same speed. The plate was shaken in orbital mode for 10 s, with an amplitude of 4 mm. Absorbance was measured at 755 nm, using ten flashes per well and a shuttle time of 10 × 10−3 s.
The reagents for the assay included ultrapure water (Merck Millipore, Zug, Switzerland), Folin reagent (Sigma-Aldrich, Buchs, Switzerland) diluted 1:15, a 5% Na2CO3 solution (Thermo Fischer Scientific, Reinach, Switzerland), and gallic acid (Sigma-Aldrich, Buchs, Switzerland).
To prepare the standards, a 1000 mg/L stock solution was made by dissolving 10 mg of gallic acid in 10 mL of Milli-Q water. Serial dilutions of this stock solution were performed to obtain standard concentrations of 100 mg/L, 50 mg/L, 10 mg/L, 5 mg/L, and 1 mg/L, using Milli-Q water as the diluent.
For plate preparation, 25 µL of each sample, blank, or standard was added to each well in triplicate. An automated injector was used to add 250 µL of diluted Folin reagent (1:15) to each well, followed by a 10 min reaction time. Then, 25 µL of 5% Na2CO3 solution was added using the automated injector, and the plate was incubated for 20 min.
The ABTS assay for total antioxidant capacity was also performed using the Synergy H1 microplate reader and a 96-well microplate. The dispenser was set to deliver 280 µL at a speed of 300 µL/s. The plate was shaken in orbital mode for 10 s, with an amplitude of 4 mm, and the absorbance was measured at 734 nm.
The reagents for the ABTS assay included ultrapure water (Merck Millipore, Zug, Switzerland), Trolox (Acros Organics, Reinach, Switzerland), absolute ethanol (Sigma-Aldrich, Buchs, Switzerland), ABTS (Sigma-Aldrich, Buchs, Switzerland), and potassium persulfate (Acros Organics, Reinach, Switzerland). A 24.5 mmol/L potassium persulfate solution was prepared, and the ABTS stock solution was made by dissolving 96 mg of ABTS in approximately 20 mL of ultrapure water, adding 2.5 mL of the potassium persulfate solution, and filling to 25 mL. This solution was left to stand for 12–16 h. The working ABTS reagent was prepared by diluting 1 mL of the ABTS stock solution in 50 mL of absolute ethanol. The reagent should appear slightly blue; if the color fades, a fresh dilution must be prepared using a new batch of ethanol.
Trolox standards were prepared by dissolving 10.3 mg of Trolox (97% purity) in 10 mL of ethanol to create a 1000 mg/L stock solution. Serial dilutions were then performed to obtain standards of 100 mg/L, 50 mg/L, 10 mg/L, 5 mg/L, and 1 mg/L, using ethanol as the diluent.
For plate preparation, 20 µL of each sample or standard was added to each well in triplicate. An automated injector was used to add 280 µL of the ABTS reagent, and the reaction was allowed to proceed for 6 min.
2.8. HPLC Analysis of Bioactive Compounds
To prepare the stock solutions at a concentration of 1 g/L, 1.0 mg of each standard was weighed and transferred into separate 1.5 mL microcentrifuge tubes (Sigma-Aldrich, Buchs, Switzerland). For ellagic acid (Sigma-Aldrich, Buchs, Switzerland), 1.0 mL of DMSO (Thermo Fischer Scientific, Reinach, Switzerland) was added directly. In the case of genistin (Sigma-Aldrich, Buchs, Switzerland), genistein (Sigma-Aldrich, Buchs, Switzerland), daidzin (Sigma-Aldrich, Buchs, Switzerland), and daidzein (Sigma-Aldrich, Buchs, Switzerland), a mixture of 100 μL of DMSO and 900 μL of methanol (anhydrous ≥ 99.8%, ChromAR® for HPLC, Thermo Fischer Scientific, Reinach, Switzerland) was used. All solutions were thoroughly mixed using a vortex to ensure complete dissolution of the compounds. Once prepared, the stock solutions were stored at 4 °C to maintain stability.
The standards were further diluted to concentrations of up to 1 mg/L by pipetting the calculated volume of each stock solution into a 1.5 mL microcentrifuge tube and adding the required amount of methanol to reach a final volume of 1 mL.
Quantification of these compounds was performed using an LC system (1220 Infinity, Agilent Technologies, Basel, Switzerland) equipped with a Kinetex® 2.6 μm C18 100 Å column (100 × 2.1 mm, 00D-4725-AN, Phenomenex Inc., Basel, Switzerland). The instrument was controlled by OpenLab ChemStation. The column temperature was maintained at 35 °C, with a flow rate of 0.4 mL/min. The mobile phase consisted of two eluents: eluent A, composed of water with 0.1% formic acid (Acros Organics, Reinach, Switzerland), and eluent B, consisting of acetonitrile (Thermo Fischer Scientific, Reinach, Switzerland) with 0.1% formic acid. The gradient details are provided in Table 1. The limit of detection (LOD) values were determined as 0.03 mg/L for daidzin and daidzein, 0.07 mg/L for genistin, and 0.02 mg/L for genistein. The limit of quantification (LOQ) values were three times the LOD values.

Table 1.
Gradient program of HPLC-DAD analysis.
2.9. Statistical Analysis
The Interquartile Range (IQR) was used as a robust statistical measure to detect outliers in the blank and calibration curve data for the polyphenol and antioxidant assays. For bioactive compound analysis, outlier detection in triplicate calibration points and samples was performed using the Hampel test.
To evaluate differences between the two timepoints (T0 and T72), a two-sample t-test assuming unequal variances (Welch’s t-test) was performed. This approach was selected to account for potential heteroscedasticity arising from variability in substrates and strains. The null hypothesis stated that there was no significant difference in the means between T0 and T72.
Since the analysis focused exclusively on comparing these two timepoints, without examining interactions between different factors (substrates and strains), a t-test was selected for assessing the effect of time between two specific conditions.
A p-value threshold of 0.05 was used to determine statistical significance, with values below this threshold indicating a significant temporal effect.
3. Results and Discussion
3.1. Solid-State Fermentation with R. oligosporus
SSF with R. oligosporus demonstrated distinct growth patterns depending on the substrate composition. A 100% okara substrate supported the most rapid and uniform fungal growth, with extensive colonization and sporulation observed within 48 to 72 h. The addition of 2% pomegranate peel slowed fungal growth, with only slight mycelial formation visible under the disk at 72 h. In comparison, incorporating 1% cranberry pomace also delayed growth slightly but allowed for better fungal colonization than the pomegranate peel formulation, with dense mycelium formation nearly matching that of pure okara by 72 h (Figure 1). Higher concentrations of pomegranate peel or cranberry pomace completely inhibited the fermentation process.

Figure 1.
SSF with R. oligosporus: Growth progression.
The composition of the substrate had a profound impact on fungal growth. Pomegranate peel exhibited the strongest inhibitory effect on fungal growth, likely due to its antimicrobial properties. Gallic acid, a key compound in pomegranate peel, is recognized for its antifungal activity, disrupting fungal cell membranes and inducing oxidative stress, thereby compromising fungal cell integrity [23]. This antifungal mechanism makes it a promising natural alternative to conventional antifungal agents. Similarly, cranberry-derived compounds, such as proanthocyanidins and other phenolics, have been extensively studied for their antifungal properties, particularly against yeast-like fungi such as Candida albicans. These compounds interfere with biofilm formation, cell adhesion, and membrane integrity [24]. However, their effects on filamentous fungi like R. oligosporus and A. oryzae are less pronounced and not as well documented.
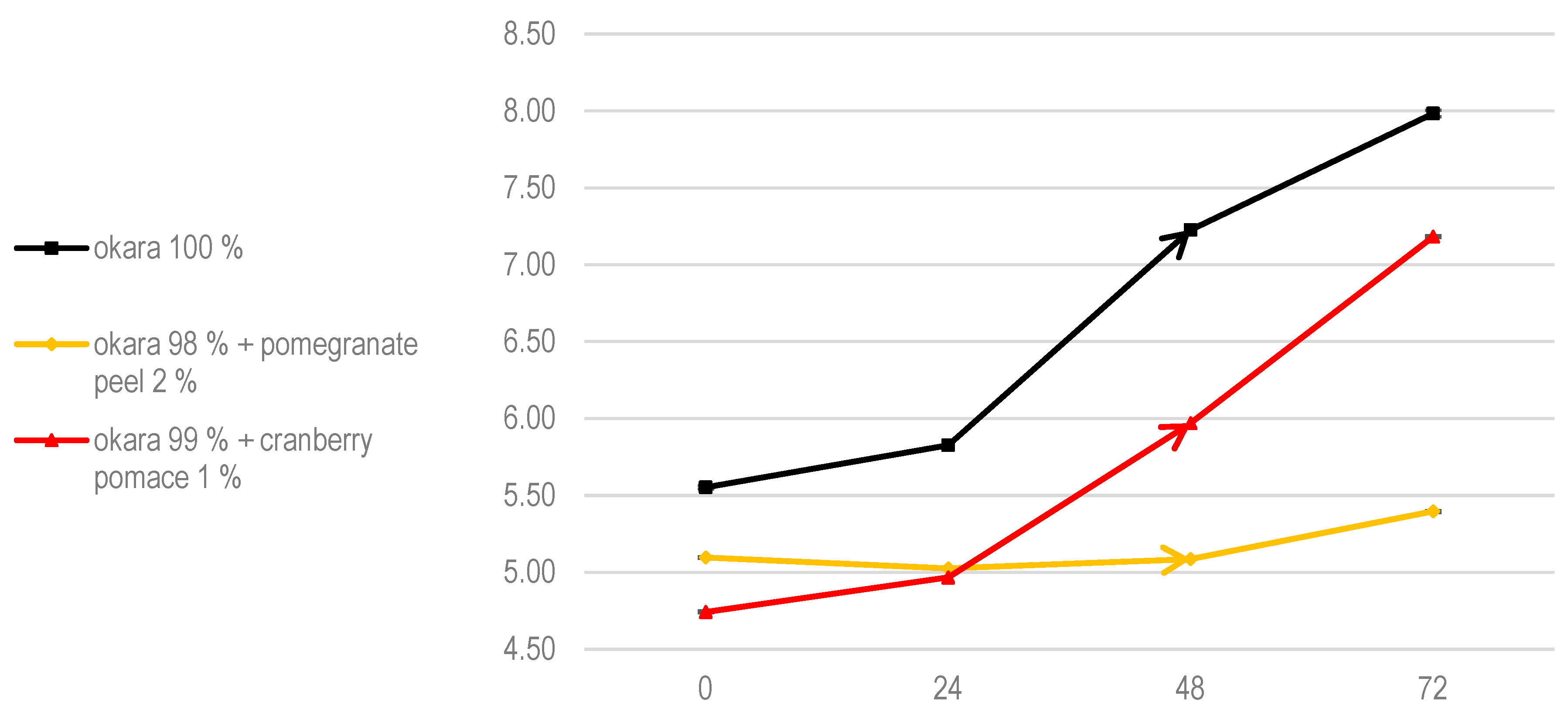
Fungal growth is also influenced by pH (Figure 2). The addition of cranberry pomace significantly acidified the substrate, leading to a marked slowdown in fungal colonization. Studies indicate that R. oligosporus exhibits optimal hyphal extension at pH levels between 5.5 and 5.8 [25]. As shown in Figure 2, the initial pH of the 100% okara substrate fell within this optimal range, facilitating mycelial expansion. The observed changes in pH were correlated with growth dynamics: faster pH fluctuations aligned with rapid and extensive fungal growth in pure okara, whereas slower pH shifts reflected the delayed colonization seen in pomegranate peel and cranberry pomace fermentations.
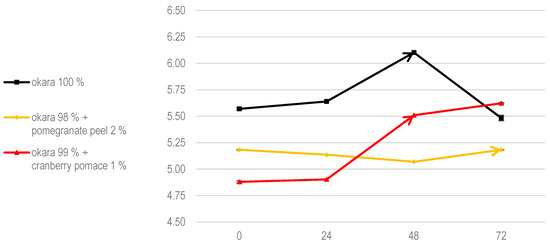
Figure 2.
The pH evolution of SSF with R. oligosporus. Data are expressed as means ± SD (n = 3).
Despite these differences, aw remained optimal across all fermentations (Table 2). Slight variations in aw values may be attributed to the intrinsic properties of the added pomegranate peel or cranberry pomace, which may have slightly altered moisture retention. However, the stability of aw ensured a conducive environment for fermentation. The literature suggests that R. oligosporus exhibits maximum hyphal extension at aw ≈ 1.0, a value closely matching the conditions in these experiments [25].

Table 2.
Water activity (aw) values during SSF with R. oligosporus. Single measurements are reported (n = 1).
The substrate composition also influenced the evolution of total polyphenol contents during SSF with R. oligosporus. The pomegranate peel fermentation consistently exhibited the highest polyphenol levels due to its naturally rich composition and the enzymatic release of additional polyphenols (Figure 3). From 24 to 72 h, the polyphenol levels increased, reflecting the fungal enzymatic breakdown of pomegranate peel’s components. Cranberry pomace fermentations maintained moderate polyphenol levels, with a steady increase over time. Comparing the polyphenol levels at 0 h to those at 72 h, significant increases were observed for fermentations with okara with 1% cranberry pomace (p < 0.05). In contrast, solid-state fermentation (SSF) with the 100% okara substrate showed the lowest polyphenol content. The reported phenolic content of soy-derived okara varies significantly between studies, largely due to differences in extraction methods and experimental conditions. One study estimated the total phenolic content of okara at 0.65 mg/g GAE based on dry matter content [26].
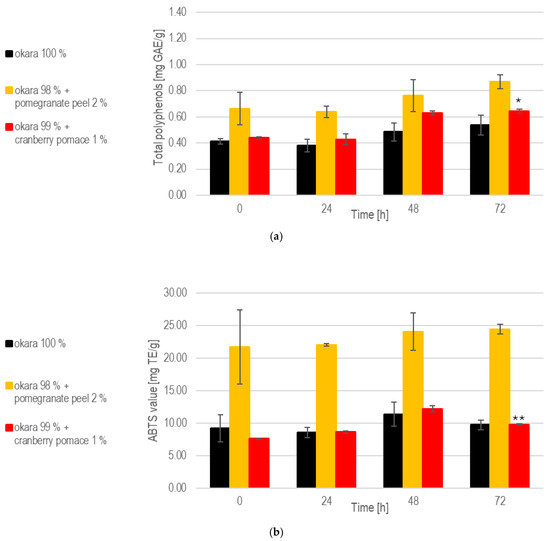
Figure 3.
SSF with R. oligosporus: (a) Total polyphenols. (b) Antioxidant capacity. Data are expressed as means ± SD (n ≥ 2); * = p < 0.05, ** = p < 0.01.
These values indicate that substrates enriched with bioactive compounds, such as pomegranate peel and cranberry pomace, enhance antioxidant capacity during SSF. The pomegranate peel formulation exhibited the highest antioxidant potential due to its polyphenol-rich composition and the fungal breakdown of phenolic compounds. This aligns with previous observations of increasing polyphenol levels throughout fermentation. As reported in a prior study, unfermented okara demonstrated an ABTS+· scavenging activity of 13.5 mg TE/g, and fermentation is expected to further enhance its antioxidant activity [27]. However, only SSF with 1% cranberry pomace led to a significant increase in antioxidant levels by the end of fermentation (p < 0.01).
3.2. Solid-State Fermentation with Aspergillus oryzae
The 100% okara and 1% cranberry pomace fermentations supported faster fungal growth, with the cranberry pomace formulation exhibiting distinct color variations, reflecting differences in fungal metabolism (Figure 4). In contrast, the pomegranate peel formulation slowed fungal growth and resulted in less uniform colonization. This may be attributed to its bioactive compounds, which, as previously discussed, can influence fungal proliferation and metabolism. Interestingly, A. oryzae appeared to be less affected by pomegranate peel than R. oligosporus, with a more pronounced inhibitory effect observed on R. oligosporus.

Figure 4.
SSF with A. oryzae: Growth progression.
The pH evolution trends aligned with these growth patterns (Figure 5). Both the 100% okara and cranberry pomace fermentations supported faster fungal colonization and metabolic activity, whereas the pomegranate peel formulation exhibited delayed growth and a reduced metabolic impact. The literature suggests that A. oryzae mycelium expands optimally within a pH range of 5.0 to 6.0, a slightly acidic environment that promotes mycelial growth and enzyme production [28]. As shown in Figure 5, this pH range was maintained across all tested substrates. Additionally, okara and cranberry pomace enhanced moisture retention, creating favorable conditions for fungal growth. In contrast, pomegranate peel appeared to slightly reduce water availability, which may have contributed to the slower fungal growth observed in this formulation.
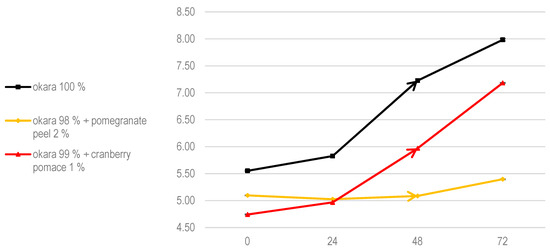
Figure 5.
The pH evolution of SSF with A. oryzae. Data are expressed as means ± SD (n = 3).
Despite this, previous studies indicate that fungal enzymes are well adapted to low aw, typically in the range of 0.5–0.6, a condition maintained throughout these experiments (Table 3). This suggests that gallic acid from pomegranate peel is the primary factor inhibiting A. oryzae growth, as neither pH nor water activity is limiting.

Table 3.
The aw values during SSF with A. oryzae. Single measurements are reported (n = 1).
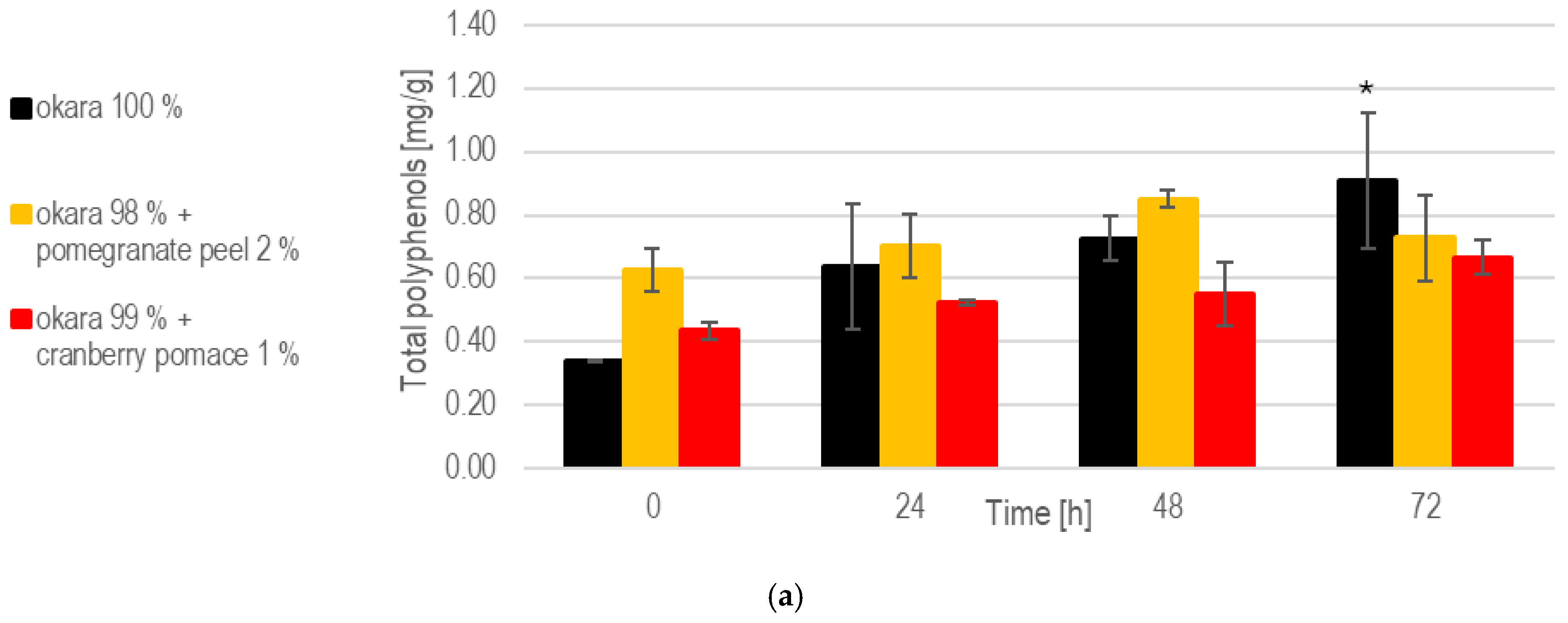
Regarding polyphenol contents, the pomegranate peel formulation consistently exhibited the highest levels throughout fermentation (Figure 6). The 1% cranberry pomace formulation also showed a significant (p < 0.05) enrichment. Similarly, the 100% okara substrate showed a moderate but statistically insignificant increase in total polyphenol content. The pomegranate peel formulation achieved the highest antioxidant potential, driven by its rich composition and the enzymatic activity of A. oryzae. However, no significant increase in antioxidant capacity was observed. The fermentations with 100% okara (p < 0.05) and 1% cranberry pomace (p < 0.01) showed significant improvements in antioxidant capacity.
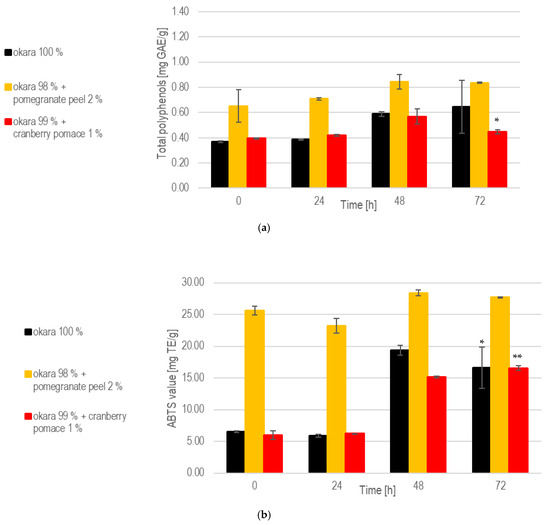
Figure 6.
SSF with A. oryzae: (a) Total polyphenols during SSF with A. oryzae. (b) Antioxidant capacity. Data are expressed as means ± SD (n ≥ 2), * = p < 0.05, ** = p < 0.01.
3.3. SSF with Co-Culture of R. oligosporus and S. thermophilus
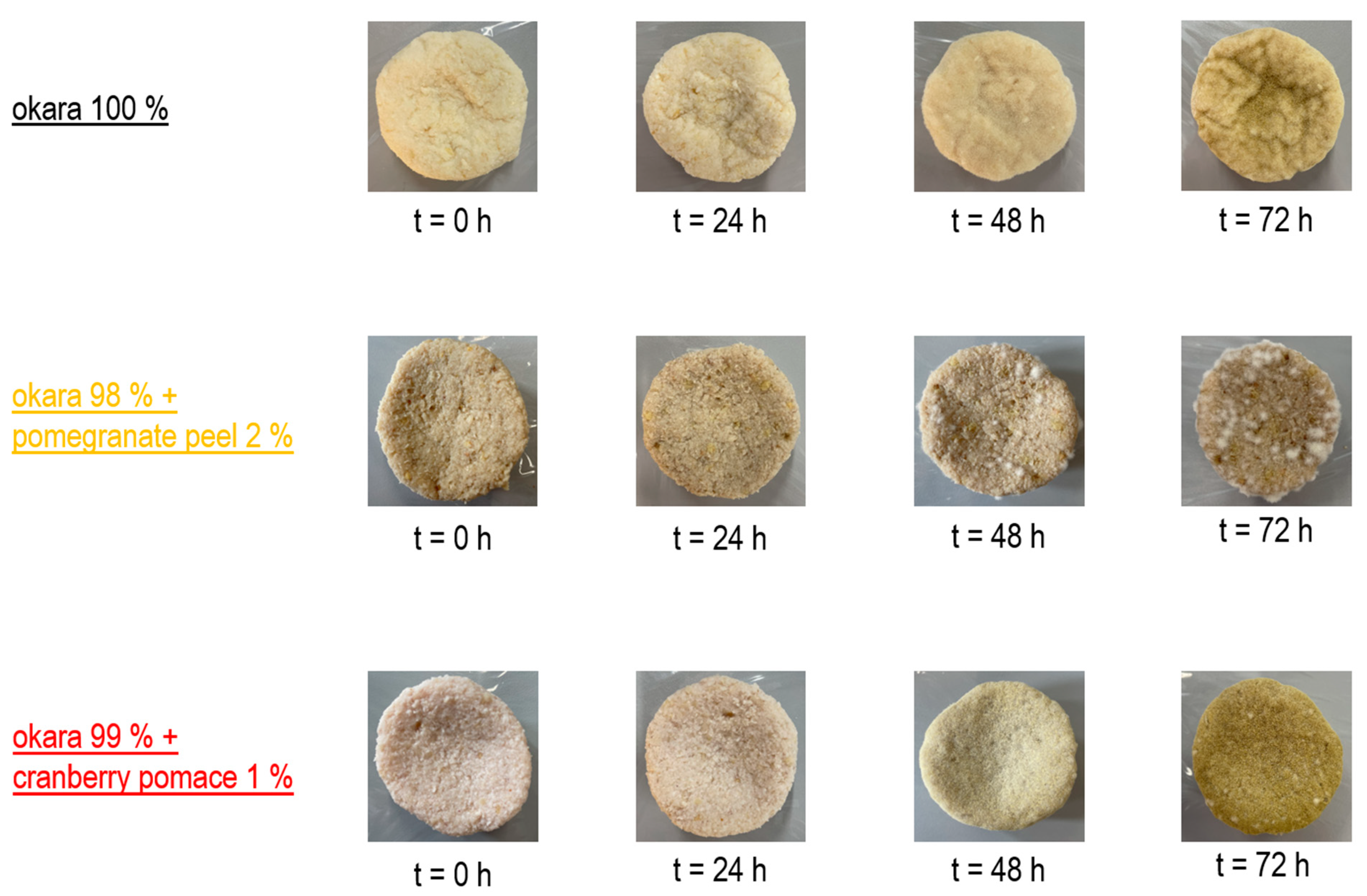
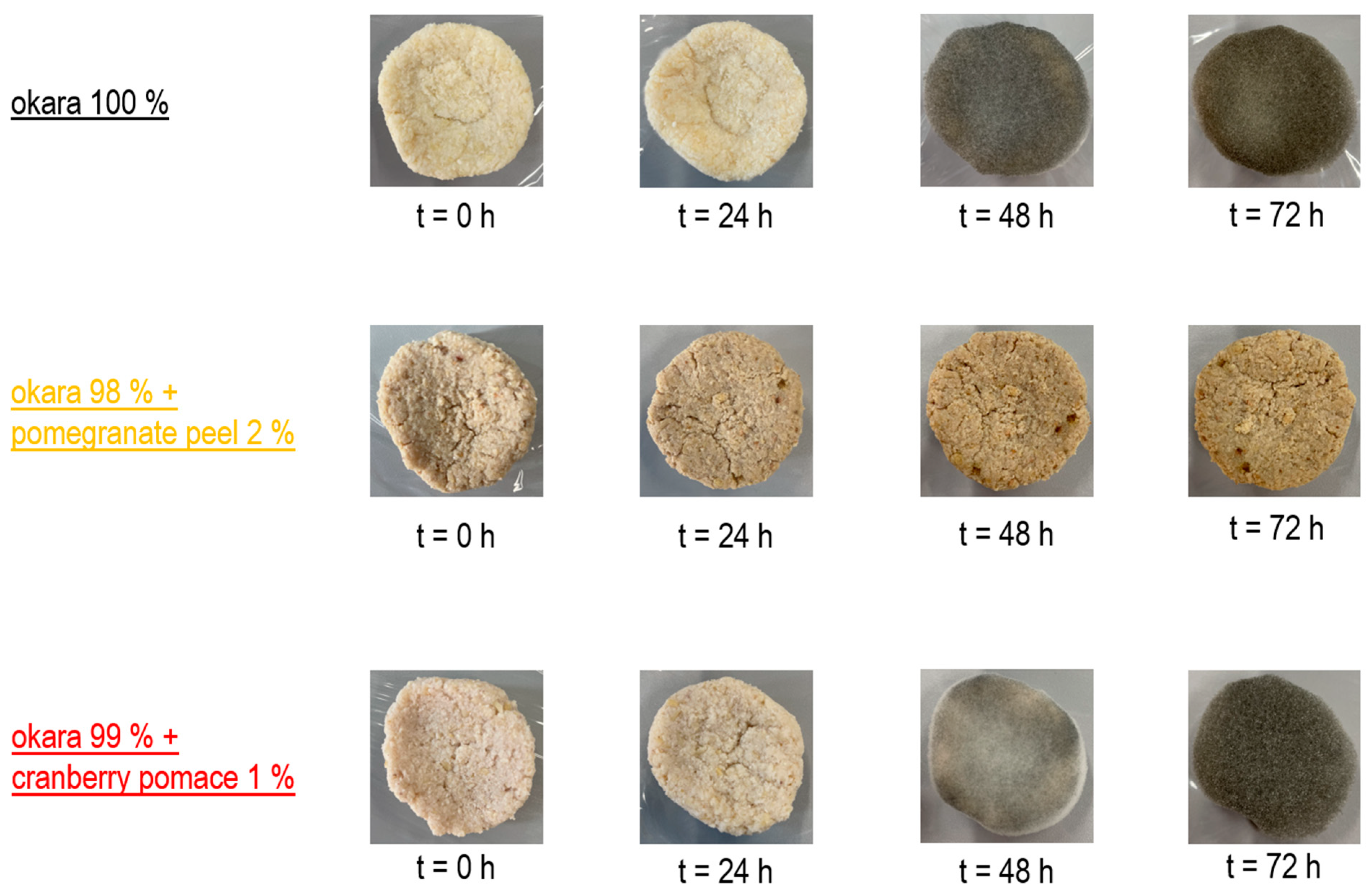
The co-culture grew robustly on both the 100% okara substrate and the cranberry pomace formulation. However, the pure okara substrate supported the most extensive fungal colonization, while cranberry pomace slightly inhibited growth, and pomegranate peel exhibited a stronger inhibitory effect (Figure 7). These results align with those of previous single-culture fermentation experiments.

Figure 7.
SSF with co-culture of R. oligosporus and S. thermophilus: Growth progression.
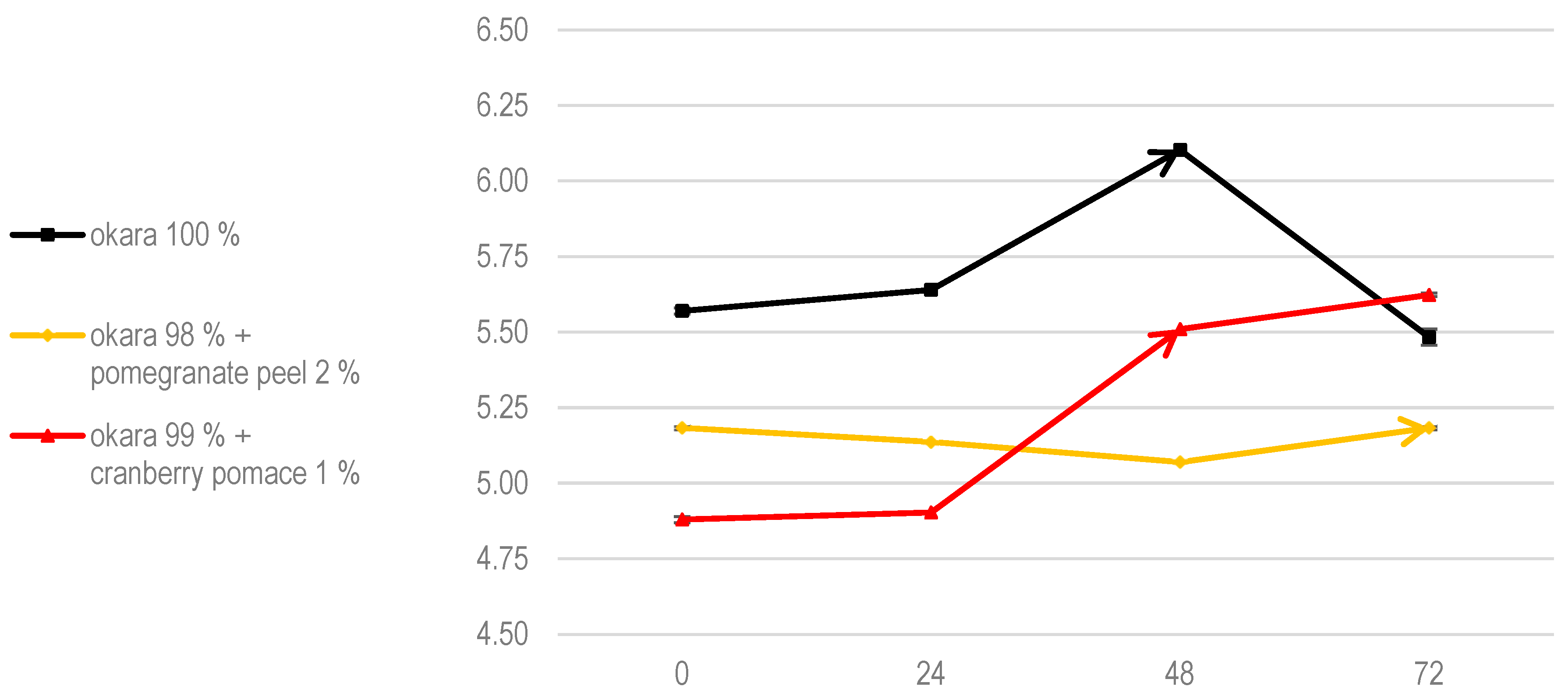
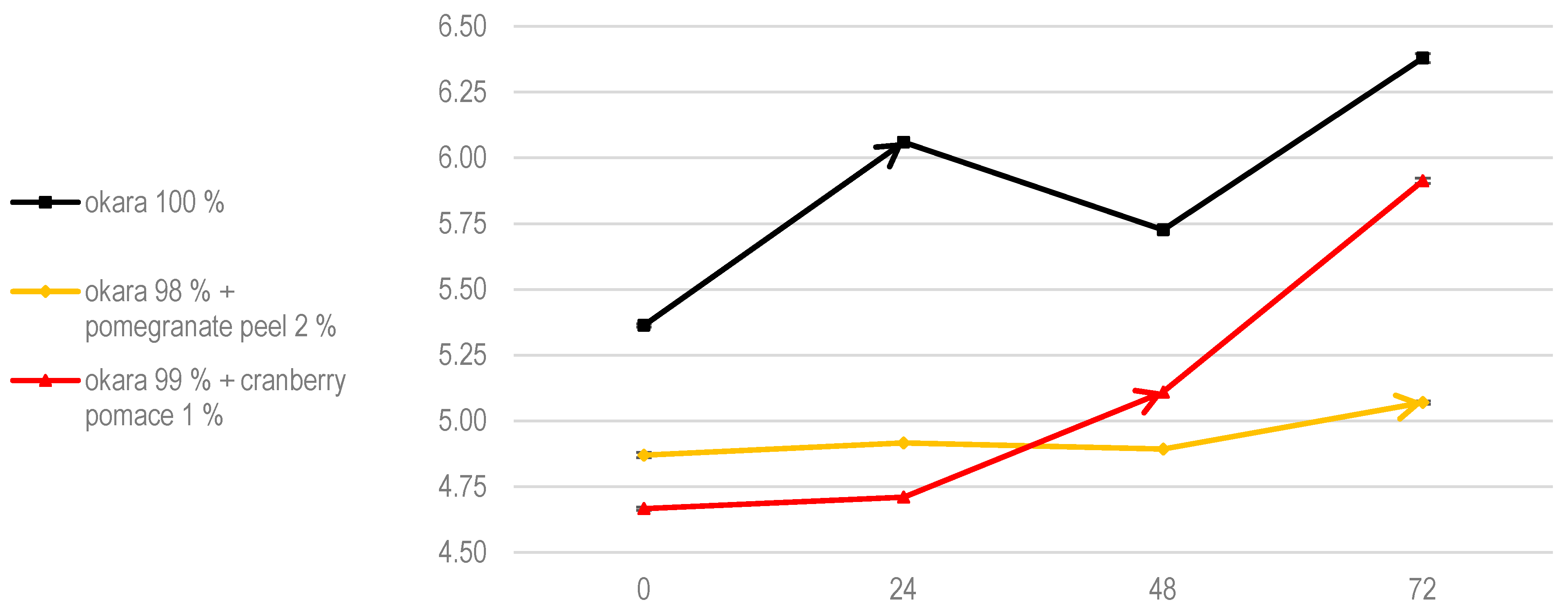
Regarding pH evolution, pure okara fermentations showed the most significant changes, indicating a high level of metabolic activity within the co-culture (Figure 8). Cranberry pomace promoted more gradual pH changes, while pomegranate peel significantly slowed the pH evolution, likely due to its inhibitory effects. When compared to SSF with R. oligosporus alone, a pH drop was observed at 48 h in the 100% okara substrate.
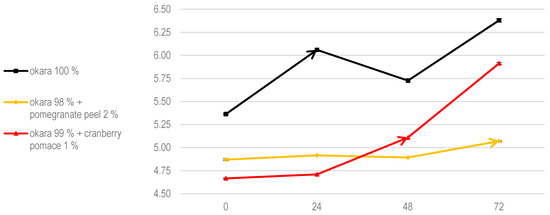
Figure 8.
The pH of SSF with co-culture of R. oligosporus and S. thermophilus. Data are expressed as means ± SD (n = 3).
This was likely due to the synergy between R. oligosporus and S. thermophilus, as R. oligosporus produces amylase during SSF, breaking down the complex carbohydrates in okara into simpler sugars [29]. These sugars are then fermented by S. thermophilus into lactic acid through the glycolytic pathway [30]. According to Food Microbiology by Adams, Moss, and McClure, during tempeh fermentation with R. oligosporus, the pH increases to around 7 due to the action of fungal proteases and lipases, which enhance the free amino acid contents and hydrolyze triglycerides into free fatty acids [31].
The enriched fermentations containing pomegranate peel and cranberry pomace exhibited improved water retention compared to the pure okara substrate, with cranberry pomace retaining the most moisture. Despite the differences in moisture retention, all SSFs provided a sufficient aw to support optimal fungal growth (Table 4).

Table 4.
The aw values during SSF with co-culture of R. oligosporus and S. thermophilus. Single measurements are reported (n = 1).
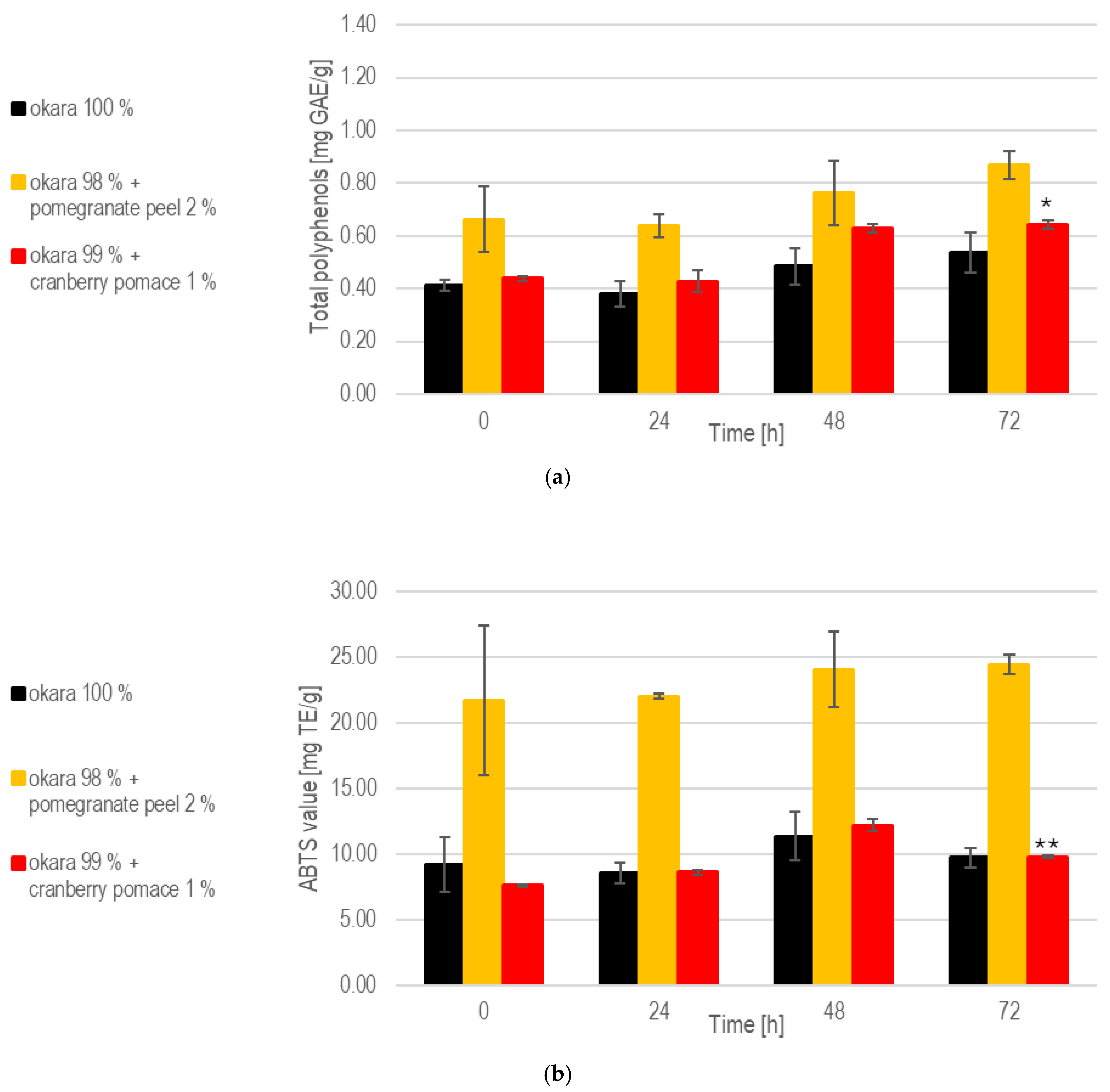
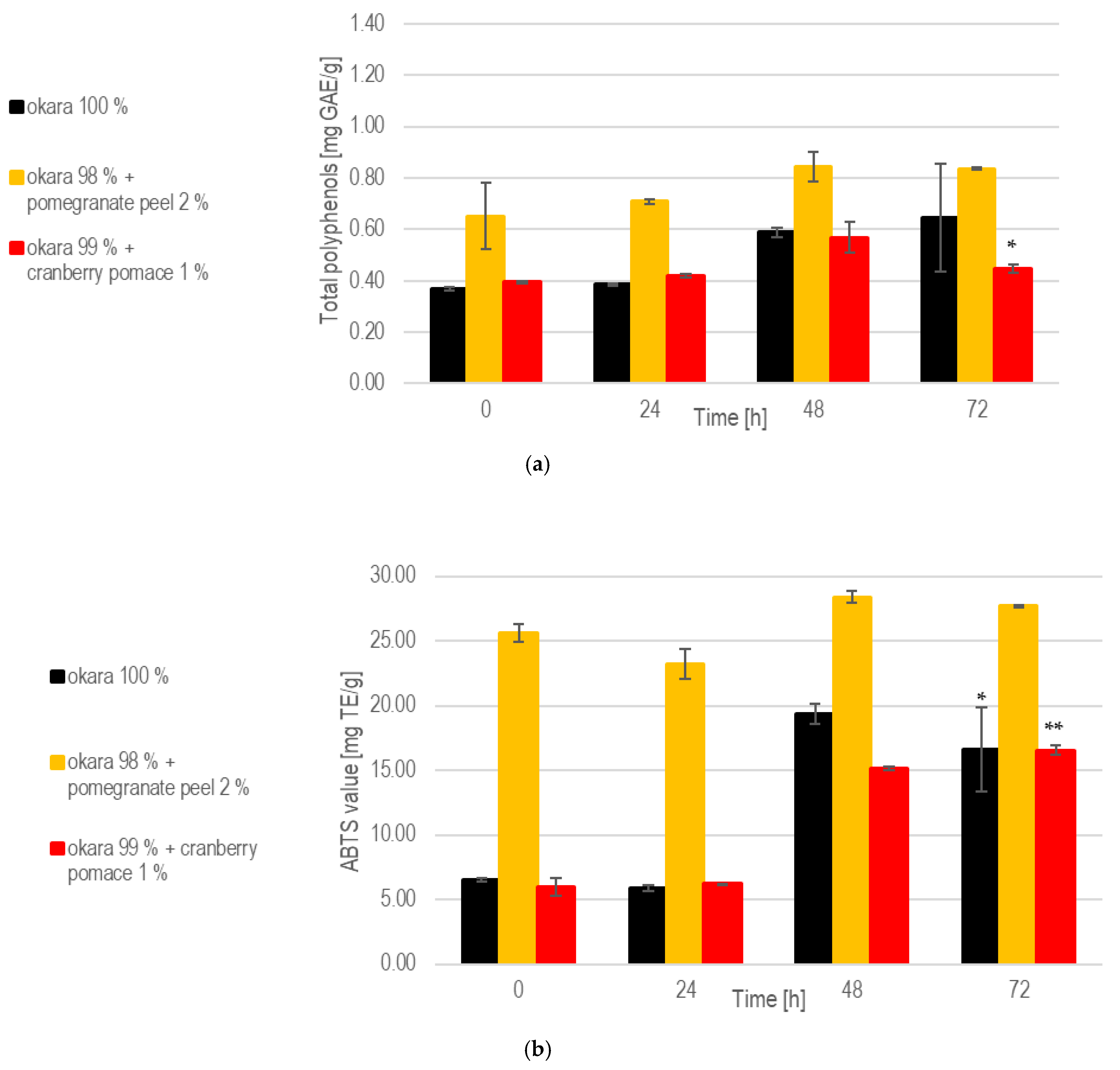
While the enriched fermentations initially provided higher polyphenol contents, the 100% okara formulation showed a more consistent release of polyphenols over time (Figure 9). Notably, at 72 h, the 100% okara fermentations showed a significant (p < 0.05) increase compared to the initial concentrations at 0 h, reaching 0.908 mg/g. This was the highest value among all fermentations. The total polyphenol content in the 1% cranberry substrate attained 0.667 mg/g at 72 h, indicating that this SSF setup effectively enhances polyphenols’ release.
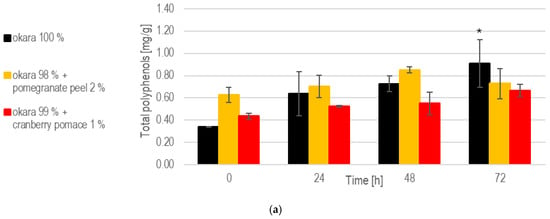
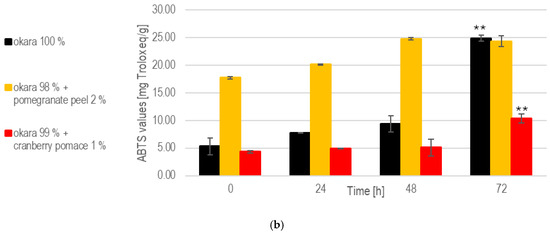
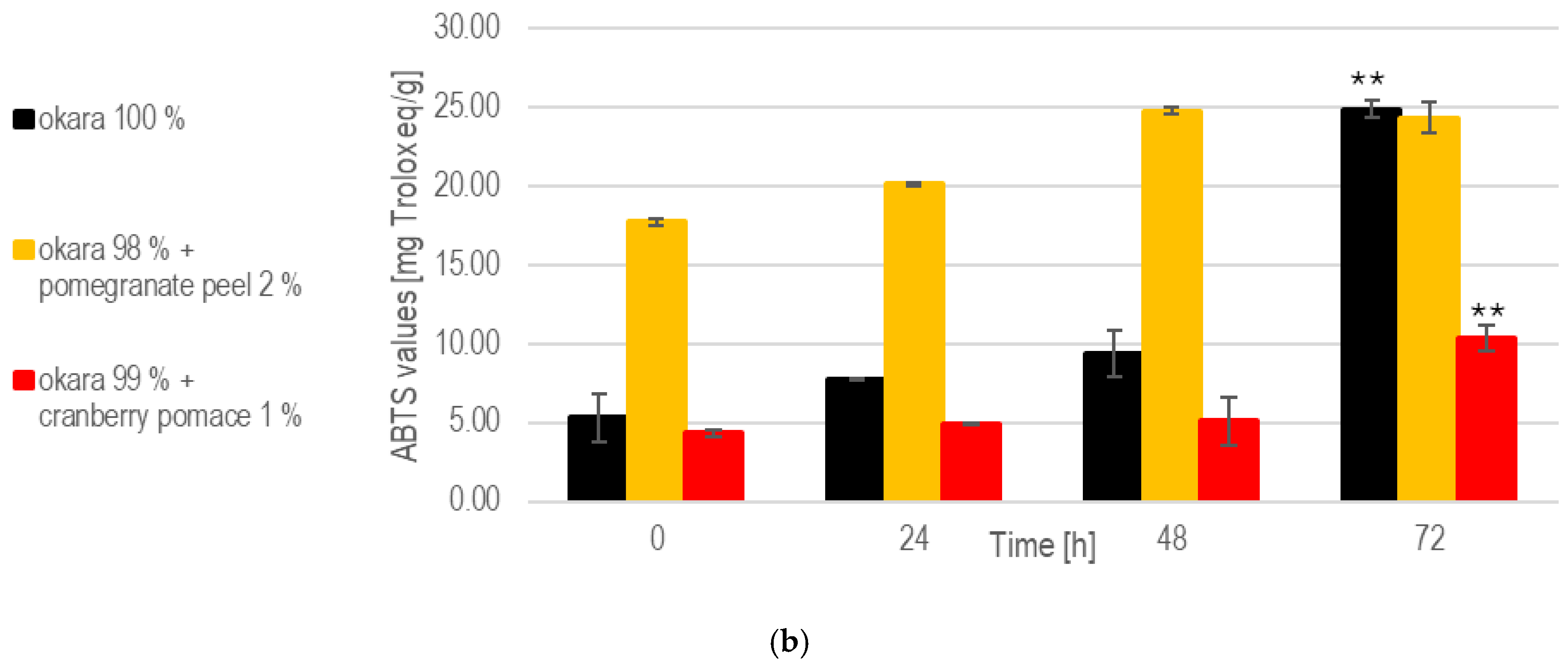
Figure 9.
SSF with co-culture of R. oligosporus and S. thermophilus: (a). Total polyphenols. (b). Antioxidant capacity. Data are expressed as means ± SD (n ≥ 2); * = p < 0.05, ** = p < 0.01. The co-culture of R. oligosporus and S. thermophilus appears to be the most optimal choice for achieving a versatile and well-balanced SSF. This combination maximizes polyphenol release and antioxidant capacity while maintaining robust metabolic activity across all substrates. Unlike individual strains, the co-culture demonstrates complementary metabolic interactions, ensuring efficient substrate transformation and bioactive enhancement. It performs exceptionally well with pure okara and maintains a high antioxidant capacity even when using pomegranate-peel-enriched substrates. However, individual strains may still be preferred for optimizing specific parameters in certain contexts.
At 72 h, the 100% okara formulation demonstrated a significant (p < 0.01) increase in antioxidant capacity (24.9 mg Trolox eq/g), surpassing that of all other SSFs. The pomegranate peel formulation exhibited the strongest antioxidant potential due to its initial composition. However, a significant increase in antioxidant activity during fermentation was not observed. While the 100% okara formulation showed a slower increase in antioxidant capacity, it eventually reached levels comparable to those of the pomegranate peel formulation, demonstrating an almost fivefold increase in antioxidant capacity. This places the 100% okara substrate among the top performers across all of the SSF experiments. In contrast, the cranberry pomace formulation exhibited more modest, but significant (p < 0.01), increases in antioxidant capacity.
3.4. Isoflavone Transformation
Given the promising results observed with the co-culture, further investigation was conducted on isoflavones, specifically the glucosides daidzin and genistin, as well as the aglycons daidzein and genistein.
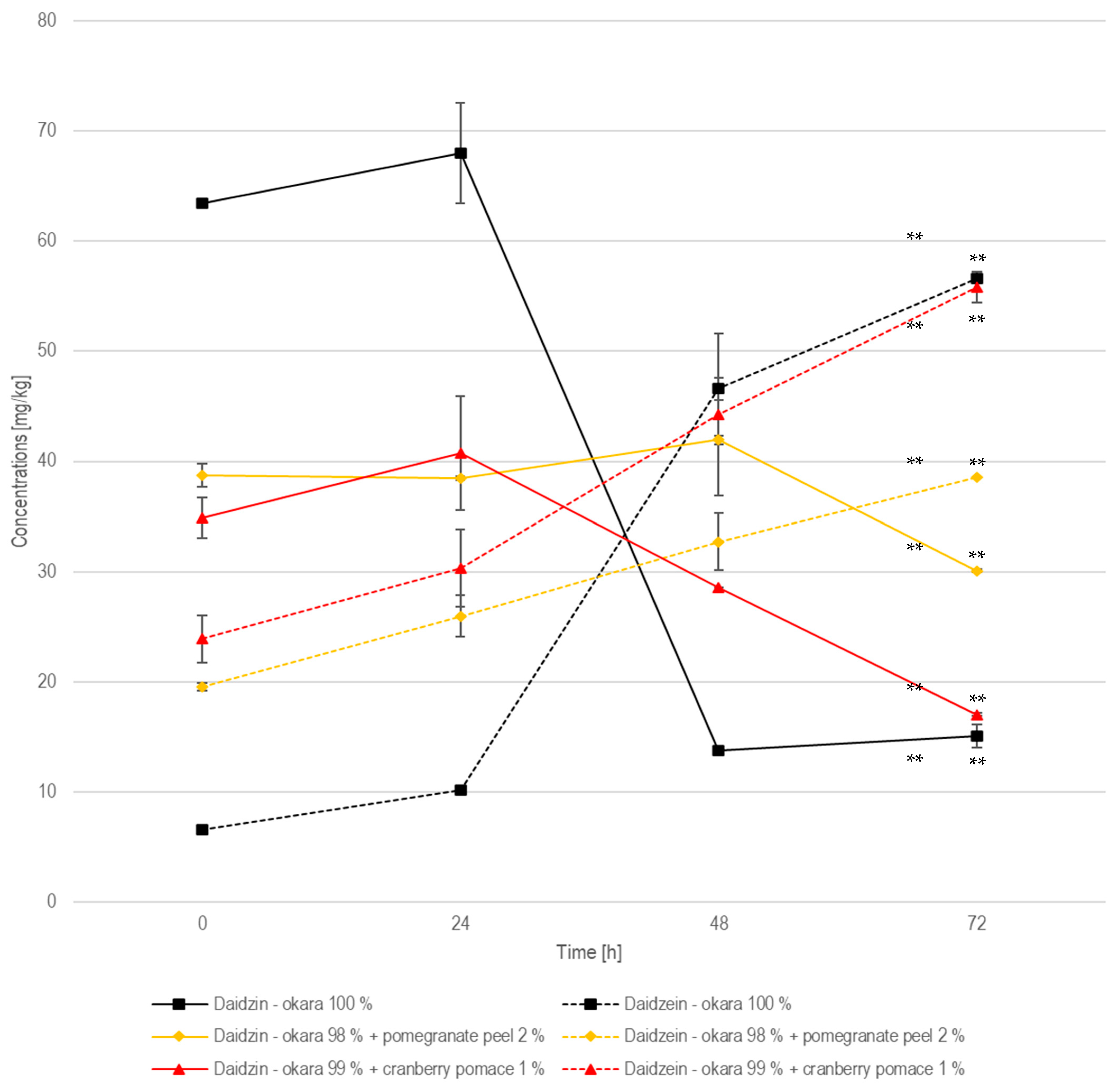
In the 100% okara formulation, the daidzin concentration started at 63.5 mg/kg, increased slightly to 68.0 mg/kg at 24 h, and then declined sharply to 13.8 mg/kg at 48 h (Figure 10). Meanwhile, daidzein showed a marked increase, from 6.64 mg/kg at 0 h to 10.2 mg/kg at 24 h, followed by a more pronounced rise to 46.6 mg/kg at 48 h, reaching 56.6 mg/kg at 72 h. This suggests that enzymatic hydrolysis is most effective between 24 and 72 h, resulting in a significant conversion of daidzin to daidzein (p < 0.01). The existing literature reports that the glucoside form of daidzin is more abundant in raw okara, whereas the aglycone form, daidzein, dominates after fermentation due to a hydrolysis enabled by β-glucosidase, an enzyme expressed by R. oligosporus. A study showed a 75.9% increase in daidzein concentration when comparing fermented to non-fermented okara, which aligns with the daidzein increase observed in the present study [32]. In the pomegranate-peel-enriched formulation, daidzin remained relatively stable, fluctuating between 38.8 mg/kg and 41.9 mg/kg in the first 48 h, before declining to 30.1 mg/kg by 72 h. The concentration of daidzein increased from 19.6 mg/kg to 38.6 mg/kg at 72 h. Compared to the 100% okara formulation, the transformation from daidzin to daidzein was significant (p < 0.01) but less pronounced, with higher residual daidzin and lower final daidzein levels. This suggests that pomegranate peel moderately inhibits β-glucosidase activity, likely due to its polyphenolic content, which may interact with the enzyme’s functions.
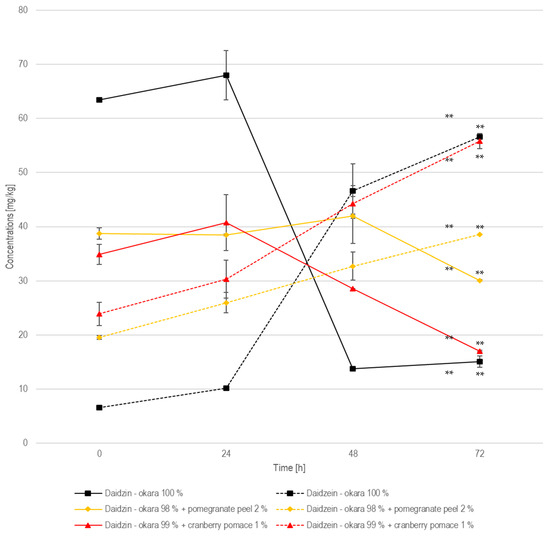
Figure 10.
Daidzin and daidzein during SSF with co-culture of R. oligosporus and S. thermophilus for the samples containing 100% okara, 98% okara + 2% pomegranate peel, and 99% okara + 1% cranberry pomace. Data are expressed as means ± SD (n ≥ 2), ** = p < 0.01.
In the cranberry-pomace-enriched formulation, the daidzin concentration started at 34.9 mg/kg, increased slightly to 40.8 mg/kg at 24 h, and then decreased progressively to 28.6 mg/kg at 48 h and dropped to 17.0 mg/kg at 72 h. Daidzein started at 23.9 mg/kg, increased to 30.3 mg/kg at 24 h, and then rose to 44.2 mg/kg at 48 h and reached 55.8 mg/kg at 72 h. The significant (p < 0.01) transformation of daidzin to daidzein in this formulation is comparable to that in the 100% okara formulation, indicating that cranberry pomace does not hinder enzymatic activity as pomegranate peel does.
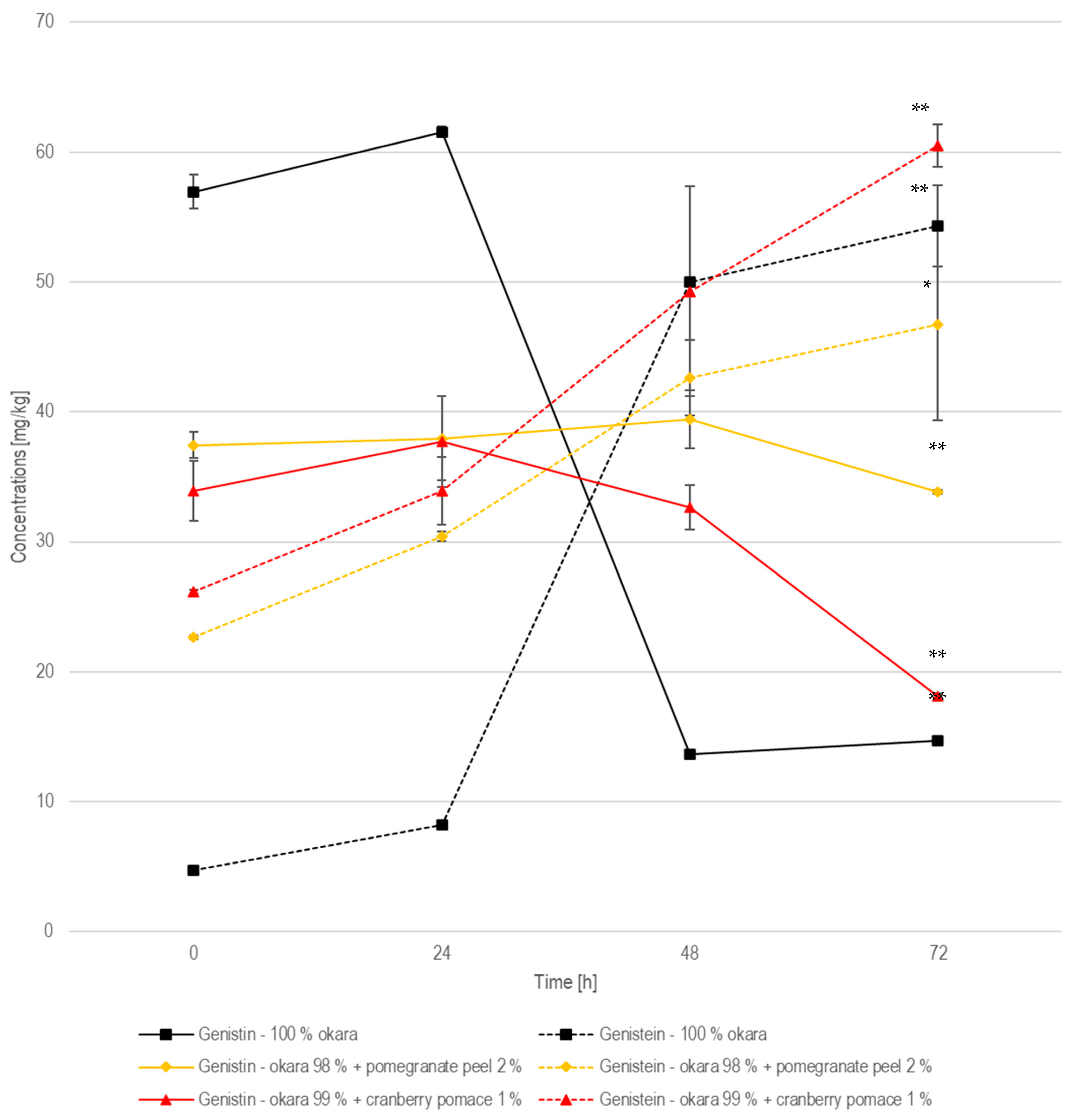
In the 100% okara formulation, the genistin concentration followed a similar pattern, starting at 57.0 mg/kg, increasing to 61.6 mg/kg at 24 h, and then declining sharply to 13.7 mg/kg at 48 h and remaining low at 14.7 mg/kg at 72 h (Figure 11). Genistein, on the other hand, started at 4.74 mg/kg, rose to 8.19 mg/kg at 24 h, and then increased significantly (p < 0.01) to 50.0 mg/kg at 48 h, before finally reaching 54.3 mg/kg at 72 h. This trend indicates that genistin was rapidly converted into genistein, like the conversion of daidzin into daidzein. According to the same previously cited study, a 122.5% increase in genistein concentration was observed from non-fermented to fermented okara, while this study recorded a 1050% (±7.90%) increase [32].
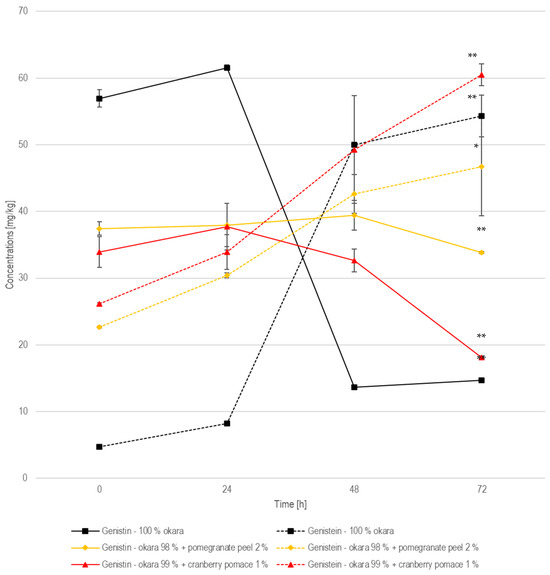
Figure 11.
Genistin and genistein during SSF with co-culture of R. oligosporus and S. thermophilus for the samples containing 10% okara, 98% okara + 2% pomegranate peel, and 99% okara + 1% cranberry pomace. Data are expressed as means ± SD (n ≥ 2), * = p < 0.05, ** = p < 0.01.
In relation to the other isoflavones in the pomegranate-peel-enriched formulation, the genistin concentration started at 37.4 mg/kg, remained relatively stable at 38.0 mg/kg at 24 h, and then slightly increased to 39.4 mg/kg at 48 h, before declining to 33.9 mg/kg at 72 h. Meanwhile, genistein started at 22.7 mg/kg, rose to 30.4 mg/kg at 24 h, and then increased further to 42.6 mg/kg at 48 h, before finally reaching 46.7 mg/kg at 72 h. Compared to the pure okara formulation, where genistin underwent a rapid and complete hydrolysis, the pomegranate-peel-enriched samples showed a slower and less efficient conversion, similar to what was observed for the daidzin-to-daidzein transformation. The slower genistin degradation rate and the more gradual genistein accumulation indicate that the enzyme remained active, but at a reduced efficiency. However, the conversion of genistin to genistein was statistically (p < 0.05) significant after 72 h of fermentation.
When examining the other isoflavones, genistin in the cranberry-pomace-enriched formulation started at 33.9 mg/kg, slightly increased to 37.7 mg/kg at 24 h, and then decreased to 32.7 mg/kg at 48 h, before ultimately declining to 18.1 mg/kg at 72 h. Genistein showed a significant (p < 0.01) increase, starting at 26.1 mg/kg, rising to 33.9 mg/kg at 24 h, and then increasing to 49.3 mg/kg at 48 h, before reaching the highest concentration of 60.5 mg/kg at 72 h. This formulation supported a high and extensive conversion of genistin to genistein, with the highest final concentration of genistein. However, the 100% okara formulation exhibited the most rapid and complete enzymatic hydrolysis, with the lowest residual genistin.
The 100% okara and cranberry pomace fermentations showed the most efficient isoflavone conversions, with low residual concentrations of the glucosides daidzin and genistin, and high final concentrations of the aglycons daidzein and genistein. This indicates strong β-glucosidase activity. Pomegranate peel slowed down both transformations, likely due to polyphenol-mediated enzyme inhibition, resulting in higher residual daidzin and genistin. While cranberry pomace did not inhibit enzymatic activity, the 100% okara substrate achieved the most rapid and complete enzymatic hydrolysis, making it the most effective formulation for maximizing isoflavone aglycone contents.
4. Conclusions
This study demonstrated that SSF with R. oligosporus and S. thermophilus as a co-culture is an effective approach for enhancing the bioactive potential of okara and its enriched fermentations. Over 72 h of fermentation, daidzein increased by 752% (±3.18%) and genistein by 1050% (±7.90%) in the 100% okara substrate, reflecting the transformation of isoflavone glycosides into their aglycone forms. Additionally, the 100% okara formulation exhibited the most consistent release of polyphenols, achieving the highest total polyphenol content (TPC) among all fermentations and substrates. The cranberry-pomace-enriched formulation, on the other hand, reached the highest TPC among all tested fermentations within the 1% cranberry substrate.
Regarding antioxidant capacity, the 100% okara substrate fermented with the co-culture demonstrated the highest final antioxidant activity at 72 h, outperforming all other SSF conditions within its formulation. The addition of pomegranate peel appeared to inhibit β-glucosidase activity, leading to slower isoflavone bioconversion, as evidenced by higher residual genistin and daidzein concentrations.
While this study confirms the efficiency of co-culture fermentation in enhancing bioactive compounds, further research is needed to optimize the fermentation parameters to maximize bioactives’ release while minimizing potential inhibitory effects from polyphenol-rich substrates like pomegranate peel. Key parameters requiring optimization include fermentation duration, pH regulation, and moisture control to ensure optimal enzymatic activity. Given the inhibitory effects of pomegranate peel on β-glucosidase, strategies such as pre-incubation with β-glucosidase-producing microorganisms, adjusting the substrate composition, or modifying the fermentation conditions (e.g., pH and aeration) could mitigate this effect. Additionally, optimizing the solid-to-liquid ratio and nutrient supplementation may enhance microbial performance and bioactives’ release.
To improve scalability in SSF, follow-up studies should explore specific engineering approaches such as optimizing the bioreactor design (e.g., tray, packed-bed, or rotating-drum bioreactors) to enhance aeration, heat dissipation, and adequate mixing. Process modifications like automated moisture control, adaptive inoculation strategies, or co-fermentation with microbial consortia tailored for large-scale applications should be addressed. Implementing pilot-scale experiments with real-world operational parameters could further strengthen this study’s practical applicability and bridge the gap between laboratory-scale findings and industrial implementation.
Author Contributions
Conceptualization, W.M.B. and W.A.; methodology, Y.E., W.M.B. and W.A.; validation, W.M.B. and W.A.; formal analysis, Y.E., W.M.B. and W.A.; investigation, Y.E.; resources, W.M.B. and W.A.; data curation, Y.E.; writing—original draft preparation, Y.E. and W.M.B.; writing—review and editing, Y.E., W.M.B. and W.A.; visualization, Y.E.; supervision, W.M.B. and W.A.; project administration, W.M.B. and W.A.; funding acquisition, W.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data of this article are available at Zenodo: 10.5281/zenodo.15018180.
Acknowledgments
The authors would like to acknowledge the support of Nancy Nicolet, Martine Baudin, Bruno Lehner, Martine Emery Mabillard, and Pascal Jacquemettaz from the Microbiological and Analytical and Bioanalytical Platforms of the Institute of Life Technologies, University of Applied Sciences and Arts Western Switzerland (HES-SO Valais Wallis).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nirmal, N.; Khanashyam, A.; Mundanat, A.; Shah, K.; Babu, K.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Ahmmed, R.; Ahsan, S.M.; Rana, J.; Ghosh, M.K.; Nandi, R. A comprehensive review of food waste valorization for the sustainable management of global food waste. Sustain. Food Technol. 2024, 2, 48–69. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Komlenić, D.K.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of Okara (Soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Saadoun, J.H.; Calani, L.; Cirlini, M.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G. Effect of fermentation with single and co-culture of lactic acid bacteria on Okara: Evaluation of bioactive compounds and volatile profiles. Food Funct. 2021, 12, 3033–3043. [Google Scholar] [CrossRef]
- Razavizadeh, S.; Alenčikienė, G.; Šalaševičienė, A.; Vaičiulytė-Funk, L.; Ertbjerg, P.; Zabulione, A. Impact of fermentation of Okara on physicochemical, techno-functional, and sensory properties of meat analogues. Eur. Food Res. Technol. 2021, 247, 2379–2389. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- Franco, L.O.; Lopes, S.T.; Nazari, M.T.; Biduski, B.; Pinto, V.Z.; Santos, J.S.D.; Bertolin, T.E.; Santos, L.R.D. Fruit pomace as a promising source to obtain biocompounds with antibacterial activity. Crit. Rev. Food Sci. Nutr. 2022, 63, 12597–12609. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Use of berry pomace to design functional foods. Food Rev. Int. 2021, 39, 3204–3224. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- Howell, A.B.; Botto, H.; Combescure, C.; Blanc-Potard, A.B.; Gausa, L.; Matsumoto, T.; Tenke, P.; Sotto, A.; Lavigne, J.P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: A multicentric randomized double-blind study. BMC Infect. Dis. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Ain, H.B.U.; Tufail, T.; Bashir, S.; Ijaz, N.; Hussain, M.; Ikram, A.; Farooq, M.A.; Saewan, S.A. Nutritional importance and industrial uses of pomegranate peel: A critical review. Food Sci. Nutr. 2023, 11, 2589–2598. [Google Scholar] [CrossRef]
- Fahmy, H.A.; Farag, M.A. Ongoing and potential novel trends of pomegranate fruit peel; a comprehensive review of its health benefits and future perspectives as nutraceutical. J. Food Biochem. 2021, 46, e14024. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, M.; Wen, J.; Ren, F.; Yang, Z.; Jiang, X.; Chen, Y. The bioactivity and applications of pomegranate peel extract: A review. J. Food Biochem. 2022, 46, e14105. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Nevárez-Moorillón, G.V.; Chávez-González, M.L.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Pedroza-Islas, R.; Huerta-Ochoa, S.; Prado-Barragán, L.A. Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Fermentation 2023, 9, 530. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive compounds from pomegranate peels—Biological properties, structure–function relationships, health benefits and food applications—A comprehensive review. J. Funct. Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Stodolak, B.; Starzyńska-Janiszewska, A.; Mika, M.; Wikiera, A. Rhizopus oligosporus and Lactobacillus plantarum Co-Fermentation as a Tool for Increasing the Antioxidant Potential of Grass Pea and Flaxseed Oil-Cake Tempe. Molecules. 2020, 25, 4759. [Google Scholar] [CrossRef]
- Feng, X.M.; Larsen, T.O.; Schnürer, J. Production of volatile compounds by Rhizopus oligosporus during soybean and barley tempeh fermentation. Int. J. Food Microbiol. 2007, 113, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and Health Benefits of Major Isoflavone Aglycones and Their Metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Brück, W.M.; Diaz Escobar, V.D.; Droz-dit-Busset, L.; Baudin, M.; Nicolet, N.; Andlauer, W. Fermentative Liberation of Ellagic Acid from Walnut Press Cake Ellagitannins. Foods 2022, 11, 3102. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of Bioactive Compounds in Berry Juices by Traditional and Optimised Analytical Methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Fan, G.; Xu, Z.; Liu, X.; Yin, W.; Sun, L.; Wu, D.; Wei, M.; Wang, W.; Cai, Y. Antifungal Efficacy of Gallic Acid Extracted From Pomegranate Peel Against Trichophyton rubrum: In Vitro Case Study. Nat. Prod. Commun. 2023, 18, 1934578X221148607. [Google Scholar] [CrossRef]
- Feldman, M.; Tanabe, S.; Howell, A.; Grenier, D. Cranberry Proanthocyanidins Inhibit the Adherence Properties of Candida albicans and Cytokine Secretion by Oral Epithelial Cells. BMC Complement. Altern. Med. 2012, 12, 6. [Google Scholar] [CrossRef]
- Sparringa, R.; Kendall, M.; Westby, A.; Owens, J. Effects of Temperature, pH, Water Activity and CO2 Concentration on Growth of Rhizopus oligosporus NRRL 2710. J. Appl. Microbiol. 2002, 92, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rani, A.; Husain, L. Investigations of Amino Acids Profile, Fatty Acids Composition, Isoflavones Content and Antioxidative Properties in Soy Okara. Asian J. Chem. 2016, 28, 903–906. [Google Scholar] [CrossRef]
- Hang, M.; Zhao, X. Fermentation Time and Extraction Solvents Influenced In Vitro Antioxidant Property of Soluble Extracts of Mao-Tofu Fermented with Mucor sp. Biotechnology 2010, 10, 60–69. [Google Scholar] [CrossRef]
- Daba, G.M.; Mostafa, F.A.; Elkhateeb, W.A. The Ancient Koji Mold (Aspergillus oryzae) as a Modern Biotechnological Tool. Bioresour. Bioprocess. 2021, 8, 52. [Google Scholar] [CrossRef]
- Anigboro, A.A.; Aganbi, E.; Tonukari, N.J. Solid State Fermentation of Maize (Zea mays) Offal by Rhizopus oligosporus under Acidic and Basic Conditions. J. Sci. Res. 2020, 12, 751–756. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, Y.; Zhang, Y.; Leng, C.; Chen, H.; Sun, J.; Fan, X.; Li, A.; Feng, Z. Metabolic Profiles of Carbohydrates in Streptococcus thermophilus During pH-Controlled Batch Fermentation. Front. Microbiol. 2020, 11, 1131. [Google Scholar] [CrossRef]
- Adams, M.R.; Moss, M.O.; McClure, P. Food Microbiology, 4th ed.; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Gupta, S.; Chen, W.N. A Metabolomics Approach to Evaluate Post-Fermentation Enhancement of Daidzein and Genistein in a Green Okara Extract. J. Sci. Food Agric. 2021, 101, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).