Malnutrition and Gastrointestinal and Respiratory Infections in Children: A Public Health Problem
Abstract
:1. Introduction
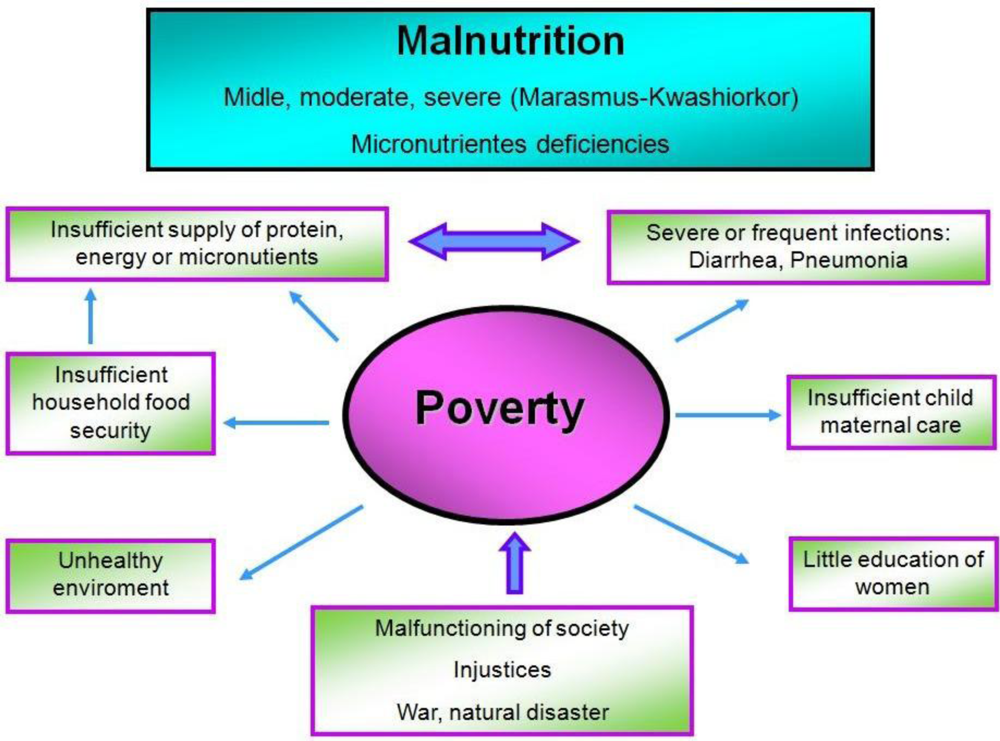
2. Malnutrition
3. Immune System
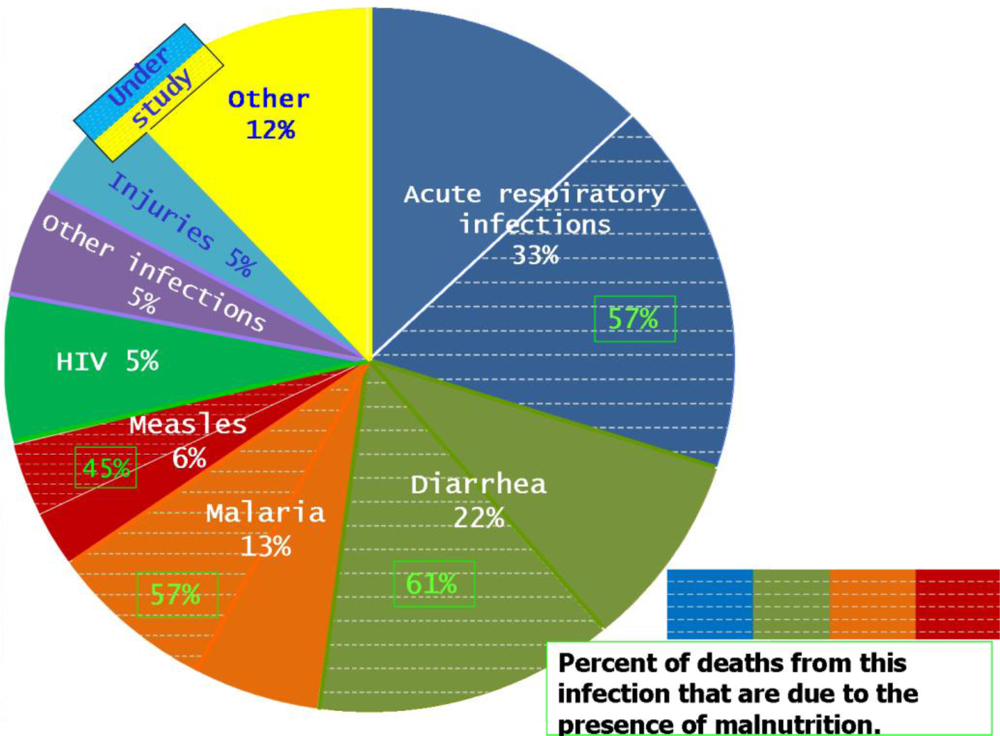
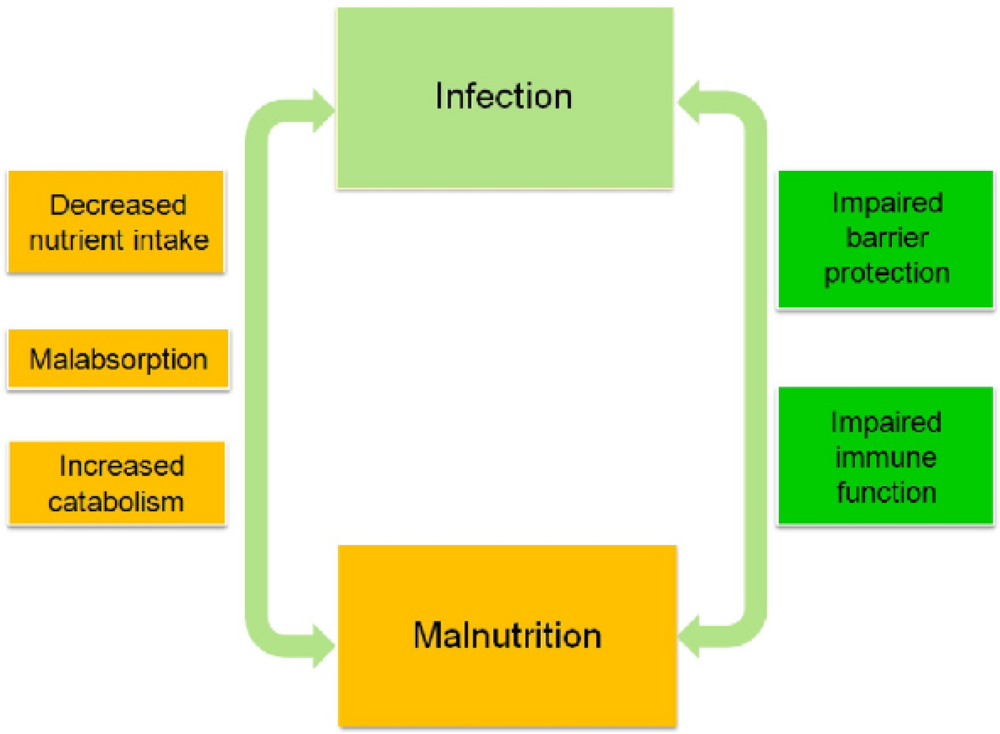
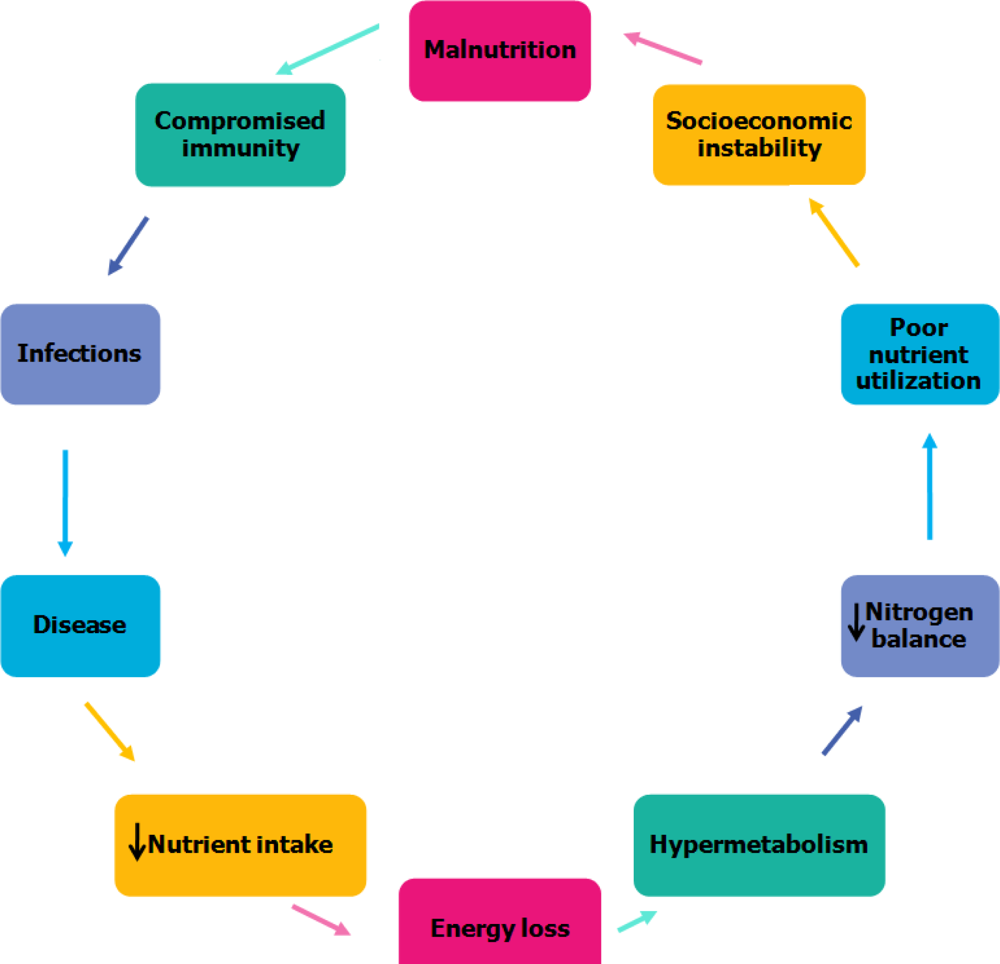
4. Relationship between Malnutrition and Infection
5. Gastrointestinal Infections Associated with Malnutrition
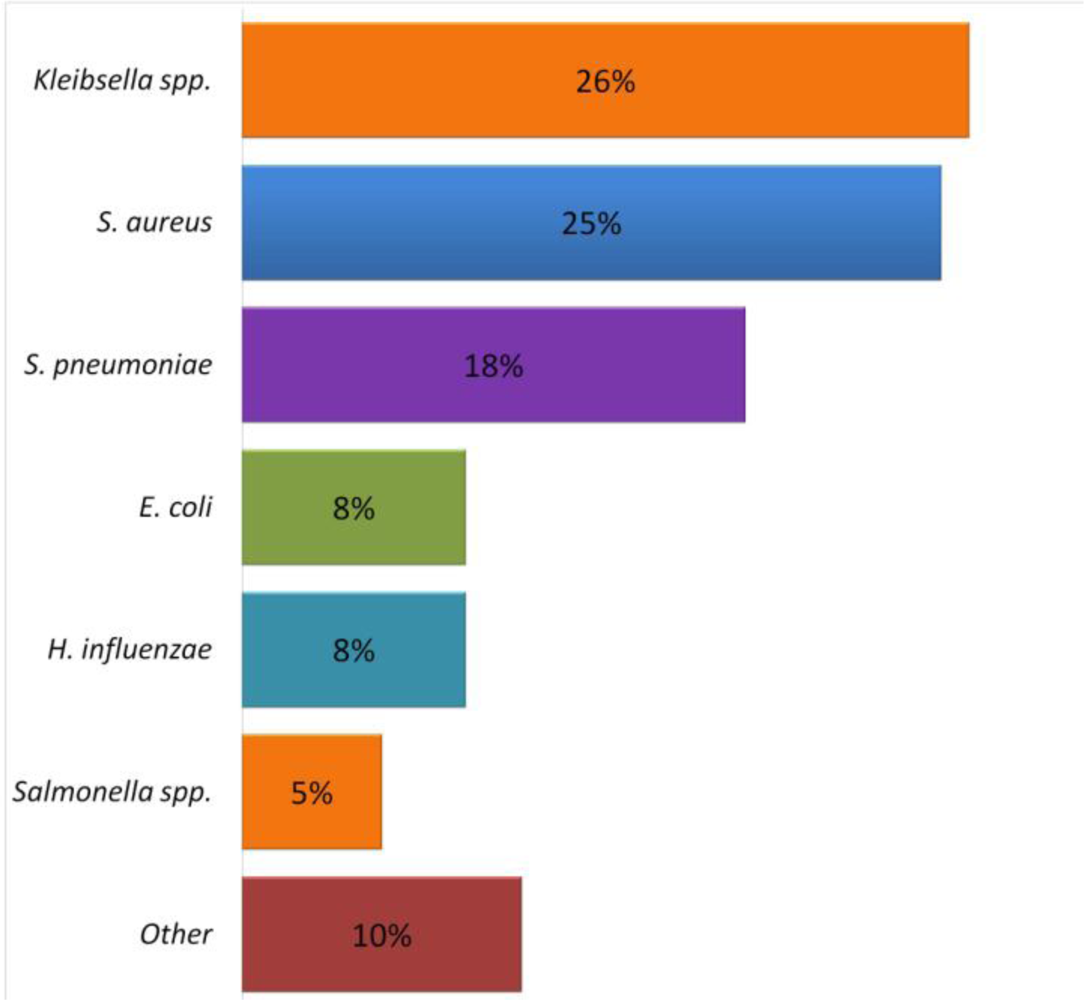
6. Respiratory Infections Associated with Malnutrition
7. Malnutrition, Leptin and Bacterial Infections
8. Conclusions
Acknowledgments
References
- Pelletier, DL; Frongillo, EA, Jr; Schroeder, DG; Habicht, JP. The effects of malnutrition on child mortality in developing countries. Bull. World Health Organ 1995, 73, 443–448. [Google Scholar]
- Kwena, AM; Terlouw, DJ; de Vlas, SJ; Phillips-Howard, PA; Hawley, WA; Friedman, JF; Vulule, JM; Nahlen, BL; Sauerwein, RW; Terkuile, FO. Prevalence and severity of malnutrition in pre-school children in a rural area of western Kenya. Am. J. Trop. Med. Hyg 2003, 68, 94–99. [Google Scholar]
- Ahmed, T; Haque, R; Mansur, A; Ahmed, S; Petri, WA, Jr; Cravioto, A. Use of metagenomics to understand the genetic basis of malnutrition. Nutr. Rev 2009, 67, S201–S206. [Google Scholar]
- Pelletier, D. The potentiating effects of malnutrition on child mortality: Epidemiologic evidence and policy implications. Nutr. Rev 1994, 52, 409–415. [Google Scholar]
- Pelletier, D; Frongillo, EA. Changes in child survival are strongly associated with changes in malnutrition in developing countries. J. Nutr 2003, 133, 107–119. [Google Scholar]
- Pelletier, D; Frongillo, E; Habicht, JP. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am. J. Public Health 1993, 83, 1130–1133. [Google Scholar]
- Black, RE; Cousens, S; Johnson, HL; Lawn, JE; Rudan, I; Bassani, DG; Jha, P; Campbell, H; Fischer, WC; Cibulskis, R; Eisele, T; Liu, L; Mathers, C. Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet 2010, 375, 1969–1987. [Google Scholar]
- Yoon, PW; Black, RE; Moulton, LH; Becker, S. The effect of malnutrition on the risk of diarrheal and respiratory mortality in children <2 y of age in Cebu, Philippines. Am. J. Clin. Nutr 1997, 65, 1070–1077. [Google Scholar]
- Scrimshaw, NS; SanGiovanni, JP. Synergism of nutrition, infection, and immunity: An overview. Am. J. Clin. Nutr 1997, 66, 464S–477S. [Google Scholar]
- Reddy, V; Raghutamulu, N; Bhaskaram, P. Secretory IgA in protein calorie malnutrition. Arch. Dis. Child 1976, 51, 871–874. [Google Scholar]
- Ha, CL; Woodward, B. Reduction in the quantity of the polymeric immunoglobulin receptor is sufficient to account for the low concentration of intestinal secretory immunoglobulin a in a weanling mouse model of wasting protein-energy malnutrition. J. Nutr 1997, 127, 427–435. [Google Scholar]
- Gómez, F; Ramos-Galván, R; Frenk, S; Cravioto, J; Chávez, R; Vázquez, J. Mortality in second and third degree malnutrition. J. Trop. Pediatr 1956, 2, 77–83. [Google Scholar]
- Arroyo, P; Mandujano, M. Joaquin Cravioto (1922–1998). J. Nutr 2000, 130, 2867–2869. [Google Scholar]
- Müller, O; Krawinkel, M. Malnutrition and health in developing countries. CMAJ 2005, 173, 279–286. [Google Scholar]
- Meléndez, G. Fundación Mexicana para la Salud-FUNSALUD. Available online: http://www.informador.com.mx/mexico/2010/198618/6/afecta-desnutricion-a-18-millones-de-mexicanos-menores-de-cinco-anos.htm (accessed on 31 January 2011).
- Schaible, UE; Kaufmann, SHE. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med 2007, 4, e115. [Google Scholar]
- Pinstrup-Andersen, P; Burger, S; Habicht, JP; Peterson, K. Protein-energy malnutrition. In Disease Control Priorities in Developing Countries, 2nd ed; Jamison, DT, Mosley, WH, Measham, AR, Bobadilla, JL, Eds.; Oxford University Press: New York, NY, USA, 1993; pp. 391–420. [Google Scholar]
- Bloss, E; Wainaina, F; Bailey, RC. Prevalence and predictors of underweight, stunting, and wasting among children aged 5 and under in Western Kenya, Bailey. J. Trop. Pediatr 2004, 50, 260–270. [Google Scholar]
- Gomez, F; Galvan, R; Cravioto, J; Frenk, S. Malnutrition in infancy and childhood, with special reference to Kwashiorkor. Adv. Pediatr 1955, 7, 131–169. [Google Scholar]
- Waterlow, J; Buzina, R; Keller, W. The presentation and use of height and weight data for comparing the nutritional status of groups of children under the age of 10 years. Bull. World Health Organ 1977, 55, 489–498. [Google Scholar]
- de Onis, M; Frongillo, EA; Blössner, M. Is malnutrition declining? An analysis of changes in levels of child malnutrition since 1980. Bull. World Health Organ 2000, 78, 1222–1233. [Google Scholar]
- Van den Broeck, J; Eeckels, R; Vuylsteke, J. Influence of nutritional status on child mortality in rural Zaire. Lancet 1993, 341, 1491–1495. [Google Scholar]
- Waterlow, JC. Malnutrición proteico-energética; Organización Panamericana de la Salud: Washington, DC, USA, 1996; pp. 261–262. [Google Scholar]
- Woodward, B. Protein, calories, and immune defenses. Nutr. Rev 1998, 56, S84–S92. [Google Scholar]
- Bhaskaram, P. Nutritional modulation of immunity to infection. Indian J. Pathol. Microbiol 1992, 35, 392–400. [Google Scholar]
- Keusch, GT. The history of nutrition: Malnutrition, infection and immunity. J. Nutr 2003, 133, 336S–340S. [Google Scholar]
- Rodríguez, L; González, C; Flores, L; Jiménez-Zamudio, L; Graniel, J; Ortiz, R. Assessment by flow cytometry of cytokine production in malnourished children. Clin. Diag. Lab. Immunol 2005, 12, 502–507. [Google Scholar]
- Benguigui, Y; Stein, F. Integrated management of childhood illness: An emphasis on the management of infectious diseases. Sem. Pediatr. Infect. Dis 2006, 17, 80–98. [Google Scholar]
- Borelli, P; Blatt, SL; Rogero, MM; Fock, RA. Haematological alterations in protein malnutrition. Rev. Bras. Hematol. Hemoter 2004, 26, 49–56. [Google Scholar]
- Field, C. Use of T cell function to determine the effect of physiologically active food components. Am. J. Clin. Nutr 2000, 71, 1720S–1725S. [Google Scholar]
- Fearon, DT; Locksley, RM. The instructive role of innate immunity in the acquired immune response. Science 1996, 272, 50–54. [Google Scholar]
- Medzhitov, R; Janeway, CA, Jr. An ancient system of host defense. Curr. Opin. Immunol 1998, 10, 12–15. [Google Scholar]
- Greenberg, S; Grinstein, S. Phagocytosis and innate immunity. Curr. Opin. Immunol 2002, 14, 136–145. [Google Scholar]
- Janeway, CA, Jr; Medzhitov, R. Innate immune recognition. Ann. Rev. Immunol 2002, 20, 197–216. [Google Scholar]
- Kumar, H; Kawai, T; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol 2011, 30, 16–34. [Google Scholar]
- Brown, KH. Dietary management of acute diarrheal disease: Contemporary scientific issues. J. Nutr 1994, 124, 1455S–1460S. [Google Scholar]
- Brown, KH. Diarrhea and malnutrition. J. Nutr 2003, 133, 328S–332S. [Google Scholar]
- Cunningham-Rundles, S; McNeeley, FD; Moon, A. Mechanisms of nutrient modulation of the immune response. J. Allergy Clin. Immunol 2005, 115, 1119–1128. [Google Scholar]
- Wilmore, DW. Infection and Injury: Effects on Whole Body Protein Metabolism. In Protein and Amino Acids; Committee on Military Nutrition Research, Institute of Medicine, Ed.; National Academic Press: Washington, DC, USA, 1999; pp. 155–167. [Google Scholar]
- Powanda, MC; Beisel, WR. Metabolic effects of infection on protein and energy status. J. Nutr 2003, 133, 322S–327S. [Google Scholar]
- Phillips, RS; Enwonwu, CO; Okolo, S; Hassan, A. Metabolic effects of acute measles in chronically malnourished Nigerian children. J. Nutr. Biochem 2004, 15, 281–288. [Google Scholar]
- De Onis, M; Monteiro, C; Akré, J; Clugston, G. The worldwide magnitude of protein—energy malnutrition: An overview from the WHO global database on child growth. Bull. World Health Organ 1993, 71, 703–712. [Google Scholar]
- Beisel, WR. Nutrition in pediatric HIV infection: Setting the research agenda. Nutrition and immune function: Overview. J. Nutr 1996, 126, 2611S–2615S. [Google Scholar]
- McGee, DW; McMurray, DN. The effect of protein malnutrition on the IgA immune response in mice. Immunology 1988, 64, 697–702. [Google Scholar]
- Nikawa, T; Odahara, K; Koizumi, H; Kido, Y; Teshima, S; Rokutan, K; Kishi, K. Vitamin A prevents the decline in immunoglobulin a and th2 cytokine levels in small intestinal mucosa of protein-malnourished mice. J. Nutr 1999, 129, 934–941. [Google Scholar]
- Sullivan, PB; Thomas, JE; Wight, DGD; Neale, G; Eastham, TC; Lloyd-Evans, N. Helicobacter pylori in Gambian children with chronic diarrhoea and malnutrition. Arch. Dis. Child 1990, 65, 189–191. [Google Scholar]
- Anstead, GM; Chandrasekar, B; Zhao, W; Yang, J; Perez, LE; Melby, PC. Malnutrition alters the innate immune response and increases early visceralization following Leishmania donovani infection. Infect. Immun 2001, 69, 4709–4718. [Google Scholar]
- Fleck, A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc. Nutr. Soc 1989, 48, 347–354. [Google Scholar]
- Reid, M; Badaloo, A; Forrester, T; Morlese, JF; Heird, WC; Jahoor, F. The acute-phase protein response to infection in edematous and nonedematous protein-energy malnutrition. Am. J. Clin. Nutr 2002, 76, 1409–1415. [Google Scholar]
- Sauerwein, RW; Mulder, JA; Mulder, L; Lowe, B; Peshu, N; Demacker, PN; van der Meer, JW; Marsh, K. Inflammatory mediators in children with protein-energy malnutrition. Am. J. Clin. Nutr 1997, 65, 1534–1539. [Google Scholar]
- Neyestani, TR; Woodward, B. Blood concentrations of Th2-type immunoglobulins are selectively increased in weanling mice subjected to acute malnutrition. Exp. Biol. Med. (Maywood) 2005, 230, 128–134. [Google Scholar]
- Peters-Golden, M; Canetti, C; Mancuso, P; Coffey, MJ. Leukotrienes: Underappreciated mediators of innate immune responses. J Immunol 2005, 174, 589–594. [Google Scholar]
- Ikeda, S; Saito, H; Fukatsu, K; Inoue, T; Han, I; Furukawa, S; Matsuda, T; Hidemura, A. Dietary restriction impairs neutrophil exudation by reducing CD11b/CD18 expression and chemokine production. Arch. Surg 2001, 136, 297–304. [Google Scholar]
- Black, RE; Morris, SS; Bryce, J. Where and why are 10 million children dying every year? Lancet 2003, 361, 2226–2234. [Google Scholar]
- Mondal, D; Haque, R; Sack, B; Kirkpatrick, B; Petri, W. Attribution of malnutrition to cause-specific diarrheal illness: Evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am. J. Trop. Med. Hyg 2009, 80, 824–826. [Google Scholar]
- Wapnir, R. Zinc deficiency, malnutrition and the gastrointestinal Tract. J. Nutr 2000, 130, 1388S–1392S. [Google Scholar]
- Kosek, M; Bern, C; Guerrant, RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ 2003, 81, 197–204. [Google Scholar]
- Bernal, C; Velásquez, C; Alcaraz, G; Botero, J. Treatment of severe malnutrition in children: Experience in implementing the world health organization guidelines in turbo, Colombia. J. Pediatr. Gastroenterol. Nutr 2008, 46, 322–328. [Google Scholar]
- Bjerknes, M; Cheng, H. Gastrointestinal stem cells. II. Intestinal stem cells. Am. J. Physiol. Gastrointest. Liver Physiol 2005, 289, G381–G387. [Google Scholar]
- Lima, AA; Brito, LF; Ribeiro, HB; Martins, MC; Lustosa, AP; Rocha, EM; Lima, NL; Monte, CM; Guerrant, RL. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J. Pediatr. Gastroenterol. Nutr 2005, 40, 28–35. [Google Scholar]
- Sufia, I; Amal, KM; Ashish, KC; Nur, HA. Intestinal enzymes during malnutrition & infection in rabbits. Indian J. Med. Res 2006, 124, 313–318. [Google Scholar]
- Lunn, PG. The impact of infection and nutrition on gut function and growth in childhood. Proc. Nutr. Soc 2000, 59, 147–154. [Google Scholar]
- Oriá, RB; Vieira, CMG; Pinkerton, RC; de Castro Costa, CM; Lopes, MB; Hussaini, I; Shi, W; Brito, GAC; Lima, AAM; Guerrant, RL. Apolipoprotein E knockout mice have accentuated malnutrition with mucosal disruption and blunted insulin-like growth factor I responses to refeeding. Nutr. Res 2006, 26, 427–435. [Google Scholar]
- Rodriguez, P; Darmon, N; Chappuis, P; Candalh, C; Blaton, MA; Bouchaud, C; Heyman, M. Intestinal paracellular permeability during malnutrition in guinea pigs: Effect of high dietary zinc. Gut 1996, 39, 416–422. [Google Scholar]
- Wykes, LJ; Fiorotto, M; Burrin, DG; Del Rosario, M; Frazer, ME; Pond, WG; Jahoor, F. Chronic low protein intake reduces tissue protein synthesis in a pig model of protein malnutrition. J. Nutr 1996, 126, 1481–1488. [Google Scholar]
- Welsh, FK; Farmery, SM; MacLennan, K; Sheridan, MB; Barclay, GR; Guillou, PJ; Reynolds, JV. Gut barrier function in malnourished patients. Gut 1998, 42, 396–401. [Google Scholar]
- Brewster, DR; Manary, MJ; Menzies, IS; O’Loughlin, EV; Henry, RL. Intestinal permeability in kwashiorkor. Arch. Dis. Child 1997, 76, 236–241. [Google Scholar]
- Yang, H; Kiristioglu, I; Fan, Y; Forbush, B; Bishop, K; Antony, PA; Zhou, H; Teitelbaum, DH. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann. Surg 2002, 236, 226–234. [Google Scholar]
- Erickson, KL; Hubbard, NE. Assessing mucosal immunity with new concepts and innovative, time-honored strategies. Nutr. Rev 2009, 67, S172–S182. [Google Scholar]
- Ing, R; Su, Z; Scott, ME; Koski, KG. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. PNAS 2000, 97, 7078–7083. [Google Scholar]
- Guerrant, RL; Schorling, JB; McAuliffe, JF; de Souza, MA. Diarrhea as a cause and an effect of malnutrition: Diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am. J. Trop. Med. Hyg 1992, 47, 28–35. [Google Scholar]
- Anand, K; Sundaram, KR; Lobo, J; Kapoor, SK. Are diarrheal incidence and malnutrition related in under five children? A longitudinal study in an area of poor sanitary conditions. Indian Pediatr 1994, 31, 943–948. [Google Scholar]
- Briend, A; Hasan, KZ; Aziz, KM; Hoque, BA. Are diarrhoea control programmes likely to reduce childhood malnutrition? Observations from rural Bangladesh. Lancet 1989, 2, 319–322. [Google Scholar]
- Wierzba, TF; El-Yazeed, RA; Savarino, SJ; Mourad, AS; Rao, M; Baddour, M; El-Deen, AN; Naficy, AB; Clemens, JD. The interrelationship of malnutrition and diarrhea in a periurban area outside Alexandria, Egypt. J. Pediatr. Gastroenterol. Nutr 2001, 32, 189–196. [Google Scholar]
- Mata, L. Diarrheal disease as a cause of malnutrition. Am. J. Trop. Med. Hyg 1992, 47, 16–27. [Google Scholar]
- Guerrant, RL; Oriá, RB; Moore, SR; Oriá, MO; Lima, AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr. Rev 2008, 66, 487–505. [Google Scholar]
- Brown, KH; McLachlan, M; Cardosa, P; Tchibindat, F; Baker, SK. Strengthening public health nutrition research and training capacities in West Africa: Report of a planning workshop convened in Dakar, Senegal, 26–28 March 2009. Global Publ. Health 2010, 5, S1–S19. [Google Scholar]
- Jambunathan, L; Neuhoff, D; Younoszai, M. Intestinal disaccharidases in malnourished infant rats. Am. J. Clin. Nutr 1981, 34, 1879–1884. [Google Scholar]
- Mehra, R; Khambadkone, SM; Jain, MK; Ganapathy, S. Jejunal disaccharidases in protein energy malnutrition and recovery. Indian Pediatr 1994, 31, 1351–1355. [Google Scholar]
- Martorell, R; Yarbrough, C; Yarbrough, S; Klein, R. The impact of ordinary illnesses on the dietary intakes of malnourished children. Am. J. Clin. Nutr 1980, 33, 345–350. [Google Scholar]
- Akuyam, SA. A review of some metabolic changes in protein-energy malnutrition. Niger. Postgrad. Med. J 2007, 14, 155–162. [Google Scholar]
- Furuta, T; El-Omar, EM; Xiao, F; Shirai, N; Takashima, M; Sugimurra, H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 2002, 123, 92–105. [Google Scholar]
- Windle, HJ; Kelleher, D; Crabtree, JE. Childhood Helicobacter pylori infection and growth impairment in developing countries: A vicious cycle? Pediatrics 2007, 119, e754–e759. [Google Scholar]
- Bravo, LE; Mera, R; Reina, JC; Pradilla, A; Alzate, A; Fontham, E; Correa, P. Impact of Helicobacter pylori infection on growth of children: A prospective cohort study. J. Pediatr. Gastroenterol. Nutr 2003, 37, 614–619. [Google Scholar]
- Dale, A; Thomas, JF; Darboe, MK; Coward, WA; Harding, M; Weaver, LT. Helicobacter pylori infection, gastric acid secretion, and infant growth. J. Pediatr. Gastroenterol. Nutr 1998, 26, 393–397. [Google Scholar]
- Weaver, LT. Helicobacter pylori infection, nutrition and growth of West African infants. Trans. Roy. Soc. Trol. Med. Hyg 1995, 89, 347–350. [Google Scholar]
- Cook, GC; Scand, J. Infective gastroenteritis and its relationship to reduced gastric acidity. Gastroenterol. Suppl 1985, 111, 17–23. [Google Scholar]
- Torres, J; Perez, GP; Ximenez, C; Muñoz, L; Camorlinga-Ponce, M; Ramos, F; Gomez, A; Muñoz, O. The association of intestinal parasitosis and H. pylori infection in children and adults from a Mexican community with high prevalence of parasitosis. Helicobacter 2003, 8, 179–185. [Google Scholar]
- Sullivan, PB; Thomas, JE; Wight, DGD; Neale, G; Eastham, TC; Lloyd-Evans, N. Helicobacter pylori in Gambian children with chronic diarrhoea and malnutrition. Arch. Dis. Child 1990, 65, 189–191. [Google Scholar]
- Gilman, RH; Partanen, R; Brown, KH; Spira, WM; Khanam, S; Greenberg, B; Bloom, SR; Ali, A. Decreased gastric acid secretion and bacterial colonization of the stomach in severely malnourished Bangladeshi children. Gastroenterology 1988, 94, 1308–1314. [Google Scholar]
- Stockbruegger, RW; Scand, J. Bacterial overgrowth as a consequence of reduced gastric acidity. Gastroenterol. Suppl 1985, 111, 7–16. [Google Scholar]
- Lindtjorn, B. Risk factors for fatal diarrhoea: A case-control study of Ethiopian children. Scand. J. Infect. Dis 1991, 23, 207–211. [Google Scholar]
- Bhandari, N; Bhan, MK; Sazawal, S. Mortality associated with acute watery diarrhea, dysentery and persistent diarrhea in rural north India. Acta Paediatrica. Suppl 1992, 381, 3–6. [Google Scholar]
- Bhutta, ZA; Nizami, SQ; Thobani, S; Issan, Z. Risk factors for mortality among hospitalized children with persistent diarrhoea in Pakistan. J. Trop. Pediatr 1997, 43, 330–336. [Google Scholar]
- Petri, WA, Jr; Dinesh, M; Kristine, M; Peterson, PD; Rashidul, H. Association of malnutrition with amebiasis. Nutr. Rev 2009, 67, S207–S215. [Google Scholar]
- Mondal, D; Petri, WA, Jr; Sack, RB; Kirkpatrick, BD; Haque, R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: Evidence from a prospective study. Trans. Roy. Soc. Trop. Med. Hyg 2006, 100, 1032–1038. [Google Scholar]
- Stanley, SL, Jr. Amoebiasis. Lancet 2003, 361, 1025–1034. [Google Scholar]
- Holmgren, J; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med 2005, 11, S45–S53. [Google Scholar]
- Haque, R; Mondal, D; Duggal, P; Kabir, M; Roy, S; Farr, BM; Sack, RB; Petri, WA, Jr. Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infect. Immun 2006, 74, 904–909. [Google Scholar]
- Villena, J; Barbieri, N; Salva, S; Herrera, M; Alvarez, S. Enhanced immune response to pneumococcal infection in malnourished mice nasally treated with heat-killed Lactobacillus casei. Microbiol. Immunol 2009, 53, 636–646. [Google Scholar]
- Chan, J; Tanaka, K; Mannion, C; Carroll, D; Tsang, M; Xing, Y; Lowenstein, C; Bloom, BJ. Effects of protein calorie malnutrition on mice infected with BCG. J. Nutr. Immunol 1997, 5, 11–19. [Google Scholar]
- Boehm, U; Klamp, T; Groot, M; Howard, JC. Cellular responses to interferon-gamma. Ann. Rev. Immunol 1997, 15, 749–795. [Google Scholar]
- Redmond, HP; Leon, P; Lieberman, MD; Hofmann, K; Shou, J; Reynolds, JV; Goldfine, J; Johnston, RB, Jr; Daly, JM. Impaired macrophage function in severe protein-energy malnutrition. Arch. Surg 1991, 126, 192–196. [Google Scholar]
- Reynolds, JV; Redmond, HP; Ueno, N; Steigman, C; Ziegler, MM; Daly, JM; Johnston, RB, Jr. Impairment of macrophage activation and granuloma formation by protein deprivation in mice. Cell Immunol 1992, 139, 493–504. [Google Scholar]
- Redmond, H; Gallagher, H; Shou, J; Daly, J. Antigen presentation in protein-energy malnutrition. Cell Immunol 1995, 163, 80–89. [Google Scholar]
- Haque, R; Mondal, D; Shu, J; Roy, S; Kabir, M; Davis, AN; Duggal, P; Petri, WA, Jr. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am. J. Trop. Med. Hyg 2007, 76, 340–344. [Google Scholar]
- Checkley, W; Epstein, LD; Gilman, RH; Black, RE; Cabrera, L; Sterling, CR. Effects of Cryptosporidium parvum infection in Peruvian children: Growth faltering and subsequent catch-up growth. Am. J. Epidemiol 1998, 148, 497–506. [Google Scholar]
- Steiner, TS; Lima, AAM; Nataro, JP; Guerrant, RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis 1998, 177, 88–96. [Google Scholar]
- Tarleton, JL; Haque, R; Mondal, D; Suh, J; Farr, BM; Petri, WA, Jr. The cognitive effects of diarrhea, malnutrition, and Entamoeba histolytica infection on school-age children in Dhaka Bangladesh. Am. J. Trop. Med. Hyg 2006, 74, 475–481. [Google Scholar]
- Qadri, F; Saha, A; Ahmed, T; Al Tarique, A; Begum, YA; Svennerholm, AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect. Immun 2007, 75, 3961–3968. [Google Scholar]
- Raj, SM; Sein, KT; Anuar, AK; Mustaffa, BE. Effect of intestinal helminthiasis on intestinal permeability of early primary schoolchildren. Trans. Roy. Soc. Trop. Med. Hyg 1996, 90, 666–669. [Google Scholar]
- Muniz, PT; Ferreira, MU; Ferreira, CS; Conde, WL; Monteiro, CA. Intestinal parasitic infections in young children in Sao Paulo, Brazil: Prevalences, temporal trends and associations with physical growth. Ann. Trop. Med. Parasitol 2002, 96, 503–512. [Google Scholar]
- Petri, WA, Jr; Miller, M; Binder, HJ; Levine, MM; Dillingham, R; Guerrant, LR. Enteric infections, diarrhea, and their impact on function and development. J. Clin. Invest 2008, 118, 1277–1290. [Google Scholar]
- Galan, JE. Salmonella interactions with host cells: Type III secretion at work. Ann. Rev. Cell Develop. Biol 2001, 17, 53–86. [Google Scholar]
- Sansonetti, PJ; Santo, JP. Debugging how bacteria manipulate the immune response. Immunity 2007, 26, 149–161. [Google Scholar]
- Hoffman-Goetz, L. Lymphokines and Monokines in Protein Energy Malnutrition. In Nutrition and Immunology; Chandra, RK, Ed.; Alan R. Liss, Inc: New York, NY, USA, 1988; Volume 11, pp. 9–23. [Google Scholar]
- Pelletier, L; Frongillo, A; Habicht, P. Nutrition and immunology. Am. J. Publ. Health 1993, 83, 1130–1133. [Google Scholar]
- González, C; Rodriguez, L; Bonilla, E; Betancourt, M; Siller, N; Zumano, E; Ortiz, R. Electrophoretic analysis of plasmatic and lymphocytes secreted proteins in malnourished children. Med. Sci. Res 1997, 25, 643–646. [Google Scholar]
- Victora, CG; Barros, FC; Kirkwood, BR; Vaughan, JP. Pneumonia, diarrhea, and growth in the first 4 y of life: A longitudinal study of 5914 urban Brazilian children. Am. J. Clin. Nutr 1990, 52, 391–396. [Google Scholar]
- Berkowitz, FE. Infections in children with severe protein-energy malnutrition. Pediatr. Infect. Dis. J 1992, 11, 750–759. [Google Scholar]
- Graham, NMH. The epidemiology of acute respiratory infections in children and adults: A global perspective. Epidemiol. Rev 1990, 12, 149–178. [Google Scholar]
- Cashat-Cruz, M; Morales-Aguirre, JJ; Mendoza-Azpiri, M. Respiratory tract infections in children in developing countries. Semin. Pediatr. Infect. Dis 2005, 16, 84–92. [Google Scholar]
- Cunha, AL. Relationship between acute respiratory infection and malnutrition in children under 5 years of age. Acta Paediatr 2000, 89, 608–609. [Google Scholar]
- Bryce, J; Boschi-Pinto, C; Shibuya, K; Black, RE. WHO child health epidemiology reference group. WHO estimates of the causes of death in children. Lancet 2005, 365, 1147–1152. [Google Scholar]
- O’Brien, KL; Wolfson, LJ; Watt, JP; Henkle, E; Deloria-Knoll, M; McCall, N; Lee, E; Mulholland, K; Levine, OS; Cherian, T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar]
- Rudan, I; Boschi-Pinto, C; Biloglav, Z; Mulholland, K; Campbell, H. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ 2008, 5, 408–416. [Google Scholar]
- Selwyn, BJ. The epidemiology of acute respiratory tract infection in young children: Comparison of findings from several developing countries. Rev. Infect. Dis 1990, 12, S870–S888. [Google Scholar]
- Berman, S. Epidemiology of acute respiratory infections in children of developing countries. Rev. Infect. Dis 1991, 13, S454–S462. [Google Scholar]
- Shann, F. Etiology of severe pneumonia in children in developing countries. Pediatr. Infect. Dis. J 1986, 5, 247–252. [Google Scholar]
- Chisti, MJ; Tebruegge, M; La Vincente, S; Graham, SM; Duke, T. Pneumonia in severely malnourished children in developing countries—mortality risk, aetiology and validity of WHO clinical signs: A systematic review. Trop. Med. Int. Health 2009, 14, 1173–1189. [Google Scholar]
- Bogaert, D; de Groot, R; Hermans, PWM. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis 2004, 4, 144–154. [Google Scholar]
- Silvermen, M; Strattton, D; Diallo, A; Egler, J. Diagnosis of acute bacterial pneumonia in Nigerian children. Arch. Dis. Child 1977, 52, 925–931. [Google Scholar]
- Halfon-Yaniv, I; Dagan, R. Epidemiology of invasive Haemophilus influenzae type b infection in Bedoins and Jews in Southern Israel. Pediatr. Infect. Dis. J 1990, 9, 321–326. [Google Scholar]
- Shimeles, D; Lulseged, S. Clinical profile and pattern of infection in Ethiopian children with severe protein-energy malnutrition. East Afr. Med. J 1994, 71, 264–267. [Google Scholar]
- Hammerschmidt, S; Bethe, G; Remane, PH; Chatwal, GS. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun 1999, 67, 1683–1687. [Google Scholar]
- Loeb, M; High, K. The effect of malnutrition on risk and outcome of community-acquired pneumonia. Resp. Care Clin. North Am 2005, 11, 99–108. [Google Scholar]
- Tupasi, TE; Velmonte, MA; Sanctivores, MEG; Abraham, L; De Leon, LE; Tan, SA; Miguel, CA; Saniel, MC. Determinants of morbidity and mortality due to acute respiratory infections: Implications for intervention. J. Infect. Dis 1988, 157, 615–623. [Google Scholar]
- Rice, AL; Sacco, L; Hyder, A; Black, RE. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ 2000, 78, 1207–1221. [Google Scholar]
- Chisti, MJ; Ahmed, T; Faruque, AS; Abdus, SM. Clinical and laboratory features of radiologic pneumonia in severely malnourished infants attending an urban diarrhea treatment center in Bangladesh. Pediatr. Infect. Dis. J 2010, 29, 174–177. [Google Scholar]
- Adegbola, RA; Falade, AG; Sam, BE; Aidoo, M; Baldeh, I; Hazlett, D; Whittle, H; Greenwood, BM; Mulholland, EK. The etiology of pneumonia in malnourished and well-nourished Gambian children. Pediatr. Infect. Dis. J 1994, 13, 975–982. [Google Scholar]
- Tupasi, TE; de Leon, LE; Lupisan, S; Torres, CU; Leonor, ZA; Sunico, ES; Mangubat, NV; Miguel, CA; Medalla, F; Tan, ST. Patterns of acute respiratory tract infection in children: A longitudinal study in a depressed community in Metro Manila. Rev. Infect. Dis 1990, 12, S940–S949. [Google Scholar]
- Nantanda, R; Hildenwall, H; Peterson, S; Kaddu-Mulindwa, D; Kalyesubula, I; Tumwine, JK. Bacterial aetiology and outcome in children with severe pneumonia in Uganda. Ann. Trop. Paediatr 2008, 28, 253–260. [Google Scholar]
- WHO. Clinical management of acute respiratory infections in children a WHO memorandum. Bull. World Health Organ 1981, 59, 707–716. [Google Scholar]
- Aderele, WI; Osinusi, K; Johnson, WB; Rotowa, NA. Staphylococcal lower respiratory infection in children. West Afr. J. Med 1994, 3, 7–12. [Google Scholar]
- Ambrus, JL, Sr; Ambrus, JL, Jr. Nutrition and infectious diseases in developing countries and problems of acquired immunodeficiency syndrome. Exp. Biol. Med 2004, 229, 464–472. [Google Scholar]
- Hedlund, J. Community-acquired pneumonia requiring hospitalization. Factors of importance for the short-and long term prognosis. Scand. J. Infect. Dis 1995, 97, 1–60. [Google Scholar]
- Jones, GE. Cellular signaling in macrophage migration and chemotaxis. J. Leukoc. Biol 2000, 68, 593–602. [Google Scholar]
- Paton, JC; Andrew, PW; Boulnois, GJ; Mitchell, TJ. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: The role of pneumococcal proteins. Ann. Rev. Microbiol 1993, 47, 89–115. [Google Scholar]
- Gordon, SB; Irving, GR; Lawson, RA; Lee, ME; Read, RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect. Immun 2000, 68, 2286–2293. [Google Scholar]
- Gingles, NA; Alexander, JE; Kadioglu, A; Andrew, PW; Kerr, A; Mitchell, TJ; Hopes, E; Denny, P; Brown, S; Jones, HB; et al. Role of genetic resistance in invasive pneumococcal infection: Identification and study of susceptibility and resistance in inbred mouse strains. Infect. Immun 2001, 69, 426–434. [Google Scholar]
- Anttila, M; Voutilainen, M; Jantti, V; Eskola, J; Kayhty, H. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol 1999, 118, 402–407. [Google Scholar]
- Bruyn, GA; van Furth, R. Pneumococcal polysaccharide vaccines: indications, efficacy and recommendations. Eur. J. Clin. Microbiol. Infect. Dis 1991, 10, 897–910. [Google Scholar]
- Cripps, AW; Otczyk, DC; Barker, J; Lehmann, D; Alpers, MP. The relationship between undernutrition and humoral immune status in children with pneumonia in Papua New Guinea. PNG Med. J 2008, 51, 120–130. [Google Scholar]
- Hagel, I; Lynch, R; Di Prisco, C; Sanchez, J; Pérez, M. Nutritional status and the IgE response against Ascaris lumbricoides in children from a tropical slum. Trans. Roy. Soc. Trop. Med. Hyg 1995, 89, 562–565. [Google Scholar]
- Mizgerd, JP; Meek, BB; Kutkoski, GJ; Bullard, DC; Beaudet, AL; Doerschuk, CM. Selectins and neutrophil traffic: Margination and Streptococcus pneumoniae-induced emigration in murine lungs. J. Exp. Med 1996, 184, 639–645. [Google Scholar]
- Keusch, GT; Farthing, MJ. Nutrition and infection. Ann. Rev. Nutr 1986, 6, 131–154. [Google Scholar]
- Honda, Y; Takahashi, K; Naito, M; Fujiyama, S. The role of macrophage colony-stimulating factor in the differentiation and proliferation of Kupffer cells in the liver of protein-deprived mice. Lab. Invest 1995, 72, 696–706. [Google Scholar]
- Olusi, O; McFarlane, H; Ade-Serrano, M; Osunkoya, O; Adesina, H. Complement components in children with protein-calorie malnutrition. Trop. Geogr. Med 1976, 28, 323–328. [Google Scholar]
- Jagadeesan, V; Reddy, V. Serum complement levels in malnourished children. Indian J. Med. Res 1979, 70, 745–749. [Google Scholar]
- Chandra, RK. Serum complement and immunoconglutinin in malnutrition. Arch. Dis. Child 1975, 50, 225–229. [Google Scholar]
- Infante-Duarte, C; Kamradt, T. Th1/Th2 balance in infection. Springer Semin. Immunopathol 1999, 21, 317–338. [Google Scholar]
- Grimble, R. Nutrition and cytokine action. Nutr. Res. Rev 1990, 3, 193–210. [Google Scholar]
- Dai, G; McMurray, DN. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect. Immun 1998, 66, 3562–3568. [Google Scholar]
- Palacio, A; López, M; Pérez-Bravo, F; Monkeberg, F; Schlesinger, L. Leptin levels are associated with immune response in malnourished infants. J. Clin. Endocrinol. Metabol 2002, 87, 3040–3046. [Google Scholar]
- Doherty, JF; Golden, MH; Remick, DG; Griffin, GE. Production of interleukin-6 and tumour necrosis factor-alpha in vitro is reduced in whole blood of severely malnourished children. Clin. Sci 1994, 86, 347–351. [Google Scholar]
- Malavé, I; Vethercourt, M; Chacón, R; Quiñones, D; Rebrij, C; Bolívar, G. Production of interleukin-6 in cultures of peripheral blood mononuclear cells from children with primary protein-calorie malnutrition and from eutrophic control. Ann. Nutr. Metabol 1998, 42, 266–273. [Google Scholar]
- Cederholm, T; Wretlind, B; Hellström, K; Andersson, B; Engström, L; Brismar, K; Scheynius, A; Forslid, J; Palmblad, J. Enhanced generation of interleukins 1 beta and 6 may contribute to the cachexia of chronic disease. Am. J. Clin. Nutr 1997, 65, 876–882. [Google Scholar]
- Chandra, RK. 1990 McCollum Award lecture. Nutrition and immunity: Lessons from the past and new insights into the future. Am. J. Clin. Nutr 1991, 53, 1087–1101. [Google Scholar]
- Chalmers, H; Janossy, G; Contreras, M; Navarrete, C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood 1998, 92, 11–18. [Google Scholar]
- Moore, K; Vieira, P; Fiorentino, F; Trounstine, L; Khan, A; Mosmann, R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein Barr virus gene BCRFI. Science 1990, 248, 1230–1234. [Google Scholar]
- Moore, K; deWaal, MR; Coffman, R; O’Garra, A. Interleukin-10 and the Interleukin-10 receptor. Ann. Rev. Immunol 2001, 19, 683–765. [Google Scholar]
- Abo-Shousha, S; Hussein, M; Rashwan, A; Salama, M. Production of proinflammatory cytokines: Granulocyte-macrophage colony stimulating factor, interleukin-8 and interleukin-6 by peripheral blood mononuclear cells of protein energy malnourished children. Egypt J. Immunol 2005, 12, 125–131. [Google Scholar]
- Deshmukh, PR; Dongre, AR; Sinha, N; Garg, BS; Nayar, S. Acute childhood morbidities in rural Wardha: Some epidemiological correlates and health care seeking. Indian J. Med. Sci 2009, 63, 345–354. [Google Scholar]
- Gainsford, T; Willson, TA; Metcalf, D; Handman, E; McFarlane, C; Ng, A; Nicola, NA; Alexander, WS; Hilton, DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. PNAS USA 1996, 93, 14564–14568. [Google Scholar]
- Loffreda, SK; Yang, SQ; Lin, HZ; Karp, CL; Brengman, ML; Wang, DJ; Klein, AS; Bulkley, GB; Bao, C; Noble, PW; Lane, MD; Diehl, AM. Leptin regulates proinflammatory immune responses. FASEB J 1998, 12, 57–65. [Google Scholar]
- Lord, M; Matarese, G; Howard, J; Baker, R; Bloom, S; Lechler, R. Leptin modulates the T-Cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar]
- Matarese, G. Leptin and the immune system: How nutritional status influences the immune response. Eur. Cytokine. Netw 2000, 11, 7–13. [Google Scholar]
- Moshyedi, AK; Josephs, MD; Abdalla, EK; MacKay, SLD; Edwards, CK, III; Copeland, EM, III; Moldawer, LL. Increased leptin expression in mice with bacterial peritonitis is partially regulated by tumor necrosis factor. Infect. Immu 1998, 66, 1800–1802. [Google Scholar]
- Faggioni, R; Feingold, KR; Grunfeld, C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J 2001, 15, 2565–2571. [Google Scholar]
- Fantuzzi, G; Faggioni, R. Leptin in the regulation of immunity, inflamation, and hematopoiesis. J. Leukoc. Biol 2000, 68, 437–446. [Google Scholar]
- Soliman, A; Zalabany, M; Salama, M; Ansari, B. Serum leptin concentrations during severe protein-energy malnutrition: Correlation with growth parameters and endocrine function. Metabolism 2000, 49, 819–825. [Google Scholar]
- Sánchez-Margalet, V; Martín-Romero, C; Santos-Alvarez, J; Goberna, R; Najib, S; González-Yanes, C. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin. Exp. Immunol 2003, 133, 11–19. [Google Scholar]
- Howard, JK; Lord, GM; Matarese, G; Vendetti, S; Ghatei, MA; Ritter, MA; Lechler, RI; Bloom, SR. Leptin protects mice from starvationinduced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Invest 1999, 104, 1051–1059. [Google Scholar]
- Faggioni, R; Jones-Carson, J; Reed, DA; Dinarello, CA; Feingold, KR; Grunfeld, C; Fantuzzi, G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: Role of tumor necrosis factor and IL-18. PNAS USA 2000, 97, 2367–2372. [Google Scholar]
- Bornstein, SR; Licinio, J; Tauchnitz, R; Engelmann, L; Negrao, AB; Gold, P; Chrousos, GP. Plasma leptin levels are increased in survivors of acute sepsis: Associated loss of diurnal rhythm in cortisol and leptin secretion. J. Clin. Endocrinol. Metabol 1998, 83, 280–283. [Google Scholar]
- Rodríguez, L; Graniel, J; Ortiz, R. Effect of leptin on activation and cytokine synthesis in peripheral blood lymphocytes of malnourished infected children. Clin. Exp. Immunol 2007, 148, 478–485. [Google Scholar]
- Mancuso, P; Gottschalk, A; Phare, SM; Peters-Golden, M; Lukacs, NW; Huffnagle, GB. Leptin-Deficient Mice Exhibit Impaired Host Defense in Gram-Negative Pneumonia. J. Immunol 2002, 168, 4018–4024. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rodríguez, L.; Cervantes, E.; Ortiz, R. Malnutrition and Gastrointestinal and Respiratory Infections in Children: A Public Health Problem. Int. J. Environ. Res. Public Health 2011, 8, 1174-1205. https://doi.org/10.3390/ijerph8041174
Rodríguez L, Cervantes E, Ortiz R. Malnutrition and Gastrointestinal and Respiratory Infections in Children: A Public Health Problem. International Journal of Environmental Research and Public Health. 2011; 8(4):1174-1205. https://doi.org/10.3390/ijerph8041174
Chicago/Turabian StyleRodríguez, Leonor, Elsa Cervantes, and Rocío Ortiz. 2011. "Malnutrition and Gastrointestinal and Respiratory Infections in Children: A Public Health Problem" International Journal of Environmental Research and Public Health 8, no. 4: 1174-1205. https://doi.org/10.3390/ijerph8041174
APA StyleRodríguez, L., Cervantes, E., & Ortiz, R. (2011). Malnutrition and Gastrointestinal and Respiratory Infections in Children: A Public Health Problem. International Journal of Environmental Research and Public Health, 8(4), 1174-1205. https://doi.org/10.3390/ijerph8041174



