Long-Lasting Effects of Undernutrition
Abstract
:1. Introduction
2. Nutritional Transition
3. Coexistence of Undernutrition and Obesity
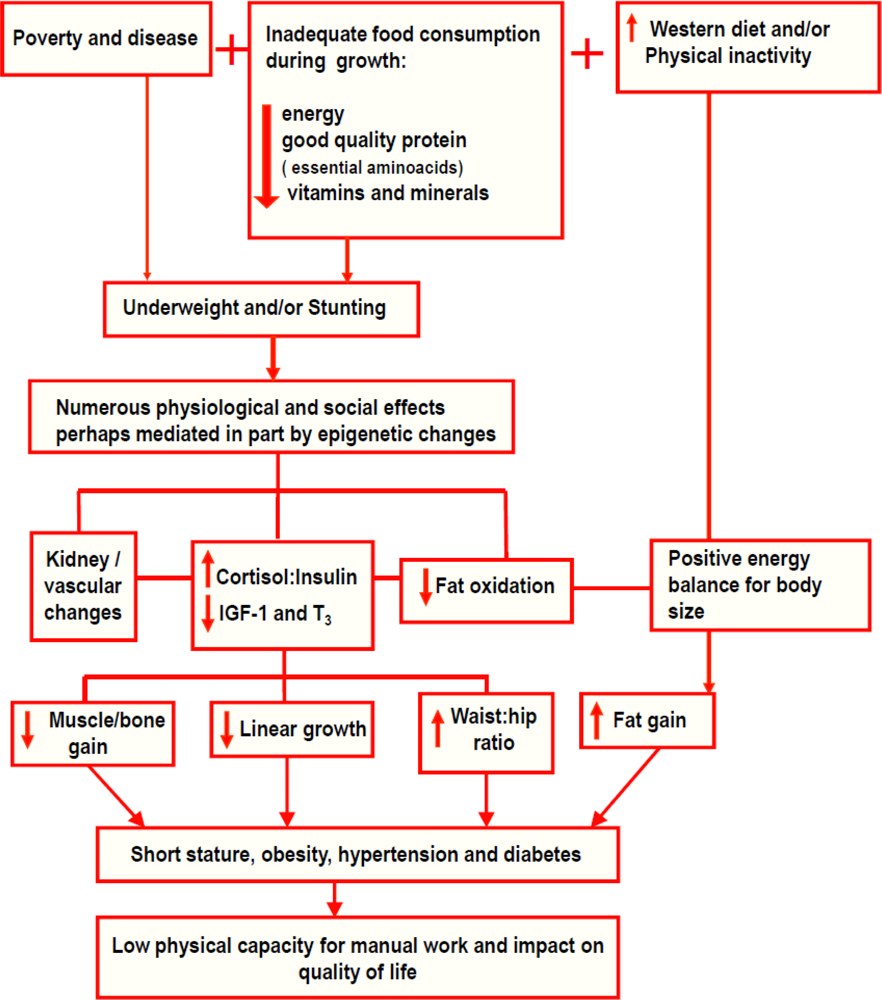
4. Aetiology of Stunting
5. Energy Expenditure
6. Body Composition
7. Hypertension
8. Glucose and Insulin Metabolism
9. Undernutrition, Stress and Impaired Mental and Living Conditions
10. Nutritional Recovery
11. Conclusions
Acknowledgments
| ACE | Angiotensin-Converting Enzyme |
| ACTH | Adrenocorticotropic Hormone |
| AH | Arterial Hypertension |
| AngII | Angiotensin II |
| BMC | Bone Mineral Content |
| BMD | Bone Mineral Density |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CREN | Center For Nutritional Recovery And Education |
| CRH | Corticotrophin Releasing Hormone |
| DALYs | Disability-Adjusted Life Years |
| DXA | Dual-Energy X-Ray Absorptiometry |
| GH | Growth Hormone |
| HAZ | Height-for-Age |
| HDL | High-Density Lipoprotein |
| HOMA | Homeostasis Model Assessment |
| HPA | Hypothalamic Pituitary Adrenocortical System |
| IGF-1 | Insulin-Like Growth Factor type 1 |
| LBM | Lean Body Mass |
| LBW | Low Birth Weight |
| LDL | Low-Density Lipoprotein |
| LRTI | Lower Respiratory Tract Infection |
| OR | Odds Ratio |
| RDA | Recommended Dietary Allowances |
| RMR | Resting Metabolic Rates |
| RQs | Respiratory Quotient |
| SAM | Sympathetic Adrenomedullary (System) |
| SBP | Systolic Blood Pressure |
| SGA | Small for Gestational Age |
| TEE | Total Energy Expenditure |
| TNF- α | Tumoral Necrosis Factor - Alpha |
| TRH | Thyrotropin Releasing Hormone |
| TSH | Thyroid Stimulating Hormone |
| URTI | Upper Respiratory Tract Infection |
| WAZ | Weight-for-Age |
| WHZ | Weight-for-Height |
References
- de Onis, M; Monteiro, C; Clugston, G. The worldwide magnitude of protein energy malnutrition: An overview from the WHO global database on child growth. Bull. World Health Organ 1993, 71, 703–712. [Google Scholar]
- Food and Agriculture Organization of the United Nations, The State of Food Insecurity in the World - Addressing Food Insecurity in Protracted Crises; FAO: Rome, Italy, 2010.
- Black, RE; Allen, LH; Bhutta, ZA; Caulfield, LE; de Onis, M; Ezzati, M; Mathers, C; Rivera, J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar]
- Repositioning Nutrition as Central to Development A Strategy for Large-Scale Action; The World Bank: Washington, DC, USA, 2006. Available online: http://siteresources.worldbank.org/NUTRITION/Resources/281846-1131636806329/NutritionStrategy.pdf (accessed on 13 September 2010).
- Emerging Societies—Coexistence of Childhood Malnutrition and Obesity; Kalhan, SC; Prentice, AM; Yajnik, CS (Eds.) Nestlé Nutrition Institute: Vevey, Switzerland, 2009; Volume 63, pp. I–XI.
- Popkin, B. The Dynamics of the Dietary Transition in the Developing World. In The Nutrition Transition: Diet and Disease in the Developing World; Caballero, B, Popkin, B, Eds.; Academic Press: London, UK, 2002; pp. 111–128. [Google Scholar]
- Poskitt, EM. Countries in transition: Underweight to obesity non-stop? Ann. Trop. Paediatr 2009, 29, 1–11. [Google Scholar]
- Martorell, R; Khan, LK; Hughes, ML; Grummer-Strawn, LM. Obesity in Latin America women and children. J. Nutr 1998, 128, 1464–1473. [Google Scholar]
- Pesquisa de Orçamentos Familiares 2002–2003: Antropometria e análise do estado nutricional de crianças e adolescentes no Brasil; IBGE: Rio de Janeiro, Brazil, 2006.
- Deitel, M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes. Surg 2003, 13, 329–330. [Google Scholar]
- IASO. International Association for the Study of Obesity. About Obesity. 2010. Available online: http://www.iaso.org/policy/aboutobesity (accessed on 14 October 2010).
- Velásquez-Melendez, G; Schlüssel, MM; Brito, AS; Silva, AA; Lopes-Filho, JD; Kac, G. Mild but not light or severe food insecurity is associated with obesity among brazilian women. J. Nutr 2011, 141, 898–902. [Google Scholar]
- Reddy, KS. Regional Case Studies—India. In Emerging Societies—Coexistence of Childhood Malnutrition and Obesity; Kalhan, SC, Prentice, AM, Yajnik, CS, Eds.; Nestlé Nutrition Institute: Vevey, Switzerland, 2009; Volume 63, pp. 15–24. [Google Scholar]
- Yin, S. Regional Case Studies—China. In Emerging Societies—Coexistence of Childhood Malnutrition and Obesity; Kalhan, SC, Prentice, AM, Yajnik, CS, Eds.; Nestlé Nutrition Institute: Vevey, Switzerland, 2009; Volume 63, pp. 25–32. [Google Scholar]
- Prentice, AM. Regional Case Studies—Africa. In Emerging Societies—Coexistence of Childhood Malnutrition and Obesity; Kalhan, SC, Prentice, AM, Yajnik, CS, Eds.; Nestlé Nutrition Institute: Vevey, Switzerland, 2009; Volume 63, pp. 33–46. [Google Scholar]
- Sawaya, AL; Dallal, G; Solymos, G; de Sousa, MH; Ventura, ML; Roberts, SB; Sigulem, DM. Obesity and malnutrition in a Shantytown population in the city of São Paulo, Brazil. Obes. Res 1995, 3, 107s–115s. [Google Scholar]
- Kimani-Murage, EW; Kahn, K; Pettifor, JM; Tollman, SM; Dunger, DB; Gómez-Olivé, XF; Norris, SA. The prevalence of stunting, overweight and obesity, and metabolic disease risk in rural South African children. BMC Public Health 2010, 10, 158. [Google Scholar]
- Barquera, S; Peterson, KE; Must, A; Rogers, BL; Flores, M; Houser, R; Monterrubio, E; Rivera-Dommarco, JA. Coexistence of maternal central adiposity and child stunting in Mexico. Int. J. Obes. (Lond) 2007, 31, 601–607. [Google Scholar]
- Fernald, LC; Neufeld, LM. Overweight with concurrent stunting in very young children from rural Mexico: prevalence and associated factors. Eur. J. Clin. Nutr 2007, 61, 623–632. [Google Scholar]
- Wang, X; Höjer, B; Guo, S; Luo, S; Zhou, W; Wang, Y. Stunting and ‘overweight’ in the WHO Child Growth Standards—malnutrition among children in a poor area of China. Public Health Nutr 2009, 12, 1991–1998. [Google Scholar]
- Florêncio, TM; Ferreira, HS; de França, AP; Cavalcante, JC; Sawaya, AL. Obesity and undernutrition in a very-low-income population in the city of Maceió, northeastern Brazil. Br. J. Nutr 2001, 86, 277–284. [Google Scholar]
- Florêncio, TT; Ferreira, HS; Cavalcante, JC; Luciano, SM; Sawaya, AL. Food consumed does not account for the higher prevalence of obesity among stunted adults in a very-low-income population in the Northeast of Brazil (Maceió, Alagoas). Eur. J. Clin. Nutr 2003, 57, 1437–1446. [Google Scholar]
- Hoffman, DJ; Sawaya, AL; Coward, WA; Wright, A; Martins, PA; de Nascimento, C; Tucker, KL; Roberts, SB. Energy expenditure of stunted and nonstunted boys and girls living in the shantytowns of São Paulo, Brazil. Am. J. Clin. Nutr 2000, 72, 1025–1031. [Google Scholar]
- Pesquisa de Orçamentos Familiares 2002–2003: Análise da Disponibilidade Domiciliar de Alimentos e do Estado Nutricional no Brasil; IBGE: Rio de Janeiro, Brazil, 2004.
- Pesquisa de Orçamentos Familiares 2008–2009: Despesas, rendimentos, e condições de vida; IBGE: Rio de Janeiro, Brazil, 2010.
- Martins, PA; Hoffman, DJ; Fernandes, MT; Nascimento, CR; Roberts, SB; Sesso, R; Sawaya, AL. Stunted children gain less lean body mass and more fat mass than their non-stunted counterparts: a prospective study. Br. J. Nutr 2004, 92, 819–825. [Google Scholar]
- Attanasio, AF; Howell, S; Bates, PC; Blum, WF; Frewer, P; Quigley, C; Shalet, SM. Confirmation of severe GH deficiency after final height in patients diagnosed as GH deficient during childhood. Clin. Endocrinol. (Oxf) 2002, 56, 503–507. [Google Scholar]
- Reynolds, JM; Wood, AJ; Eminson, DM; Postlethwaite, RJ. Short stature and chronic renal failure: What concerns children and parents? Arch. Dis. Child 1995, 73, 36–42. [Google Scholar]
- Trip-Hoving, M; van Alfen-Van der Velden, JA; Otten, BJ. Psychosocial deprivation as a cause of growth retardation in children. Ned. Tijdschr. Geneeskd 2009, 153, A455, [Abstract in English]. [Google Scholar]
- Amigo, H; Bustos, P. Factores condicionantes de la estatura en escolares de alta vulnerabilidad social; Maigret: Santiago, Chile, 1994. [Google Scholar]
- Merchant, AT; Jones, C; Kiure, A; Kupka, R; Fitzmaurice, G; Herrera, MG; Fawzi, WW. Water and sanitation associated with improved child growth. Eur. J. Clin. Nutr 2003, 57, 1562–1568. [Google Scholar]
- Allen, LH; Gillespie, SR. What Works? A Review of the Efficacy and Effectiveness of Nutrition Interventions; United Nations Administrative Committee on Coordination/Sub-Committee on Nutrition: Geneva, Switzerland, 2001; pp. 1–123. [Google Scholar]
- Sawaya, AL; Martins, P; Hoffman, D; Roberts, SB. The link between childhood undernutrition and risk of chronic diseases in adulthood: A case study of Brazil. Nutr. Rev 2003, 61, 168–175. [Google Scholar]
- Soliman, AT; Hassan, AE; Aref, MK; Hintz, RL; Rosenfeld, RG; Rogol, AD. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr. Res 1986, 20, 1122–1130. [Google Scholar]
- Zamboni, G; Dufillot, D; Antoniazzi, F; Valentini, R; Gendrel, D; Tato, L. Growth hormone-binding proteins and insulin-like growth factor-binding proteins in protein-energy malnutrition, before and after nutritional rehabilitation. Pediatr. Res 1996, 39, 410–414. [Google Scholar]
- Thissen, JP; Underwood, LE; Ketelslegers, JM. Regulation of insulin-like growth factor-I in starvation and injury. Nutr. Rev 1999, 57, 167–176. [Google Scholar]
- Haspolat, K; Ece, A; Gürkan, F; Atamer, Y; Tutanç, M; Yolbaş, I. Relationships between leptin, insulin, IGF-1 and IGFBP-3 in children with energy malnutrition. Clin. Biochem 2007, 40, 201–205. [Google Scholar]
- Ketelslegers, JM; Maiter, D; Maes, M; Underwood, LE; Thissen, JP. Nutritional regulation of insulin-like growth factor-I. Metabolism 1995, 44, 50–57. [Google Scholar]
- Doherty, CP; Crofton, PM; Sarkart, MAK; Shakurt, MS; Wade, JC; Kelnar, CJH; Elminger, MW; Ranke, MB; Cutting, WA. Malnutrition, zinc supplementation and catch-up growth: changes in insulin-like growth factor I, its binding proteins, bone formation and collagen turnover. Clin. Endocrinol 2002, 57, 391–399. [Google Scholar]
- Hay, WW. Nutrition-gene interactions during intrauterine life and lactation. Nutr. Rev 1999, 57, S20–S30. [Google Scholar]
- Yakar, S; Liu, JL; Le, Roith, D. The growth hormone/insulin-like growth factor-I system: implications for organ growth and development. Pediatr. Nephrol 2000, 14, 544–549. [Google Scholar]
- Jahreis, G; Zander, R; Ranft, U; Kauf, E; Hennig, A; Schubert, H. Insulin-like growth factor I--a connecting link between nutrition and growth. Z Ernahrungswiss 1992, 31, 62–69, [Abstract in English]. [Google Scholar]
- Soares-Wynter, SY; Walker, SP. Resting metabolic rate and body composition in stunted and nonstunted children. Am. J. Clin. Nutr 1996, 64, 137–141. [Google Scholar]
- Hoffman, DJ; Sawaya, AL; Verreschi, I; Tucker, KL; Roberts, SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am. J. Clin. Nutr 2000, 72, 702–707. [Google Scholar]
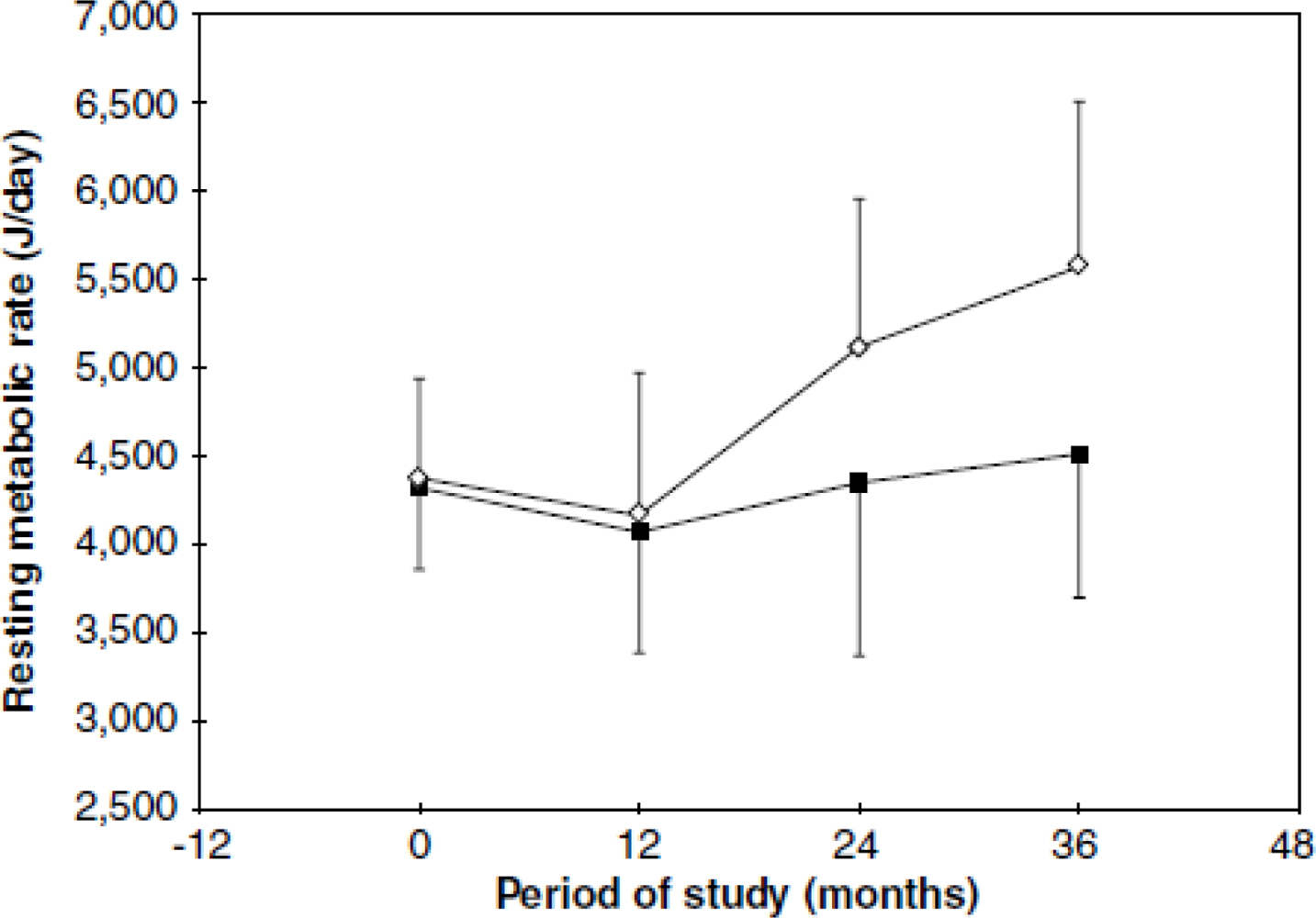
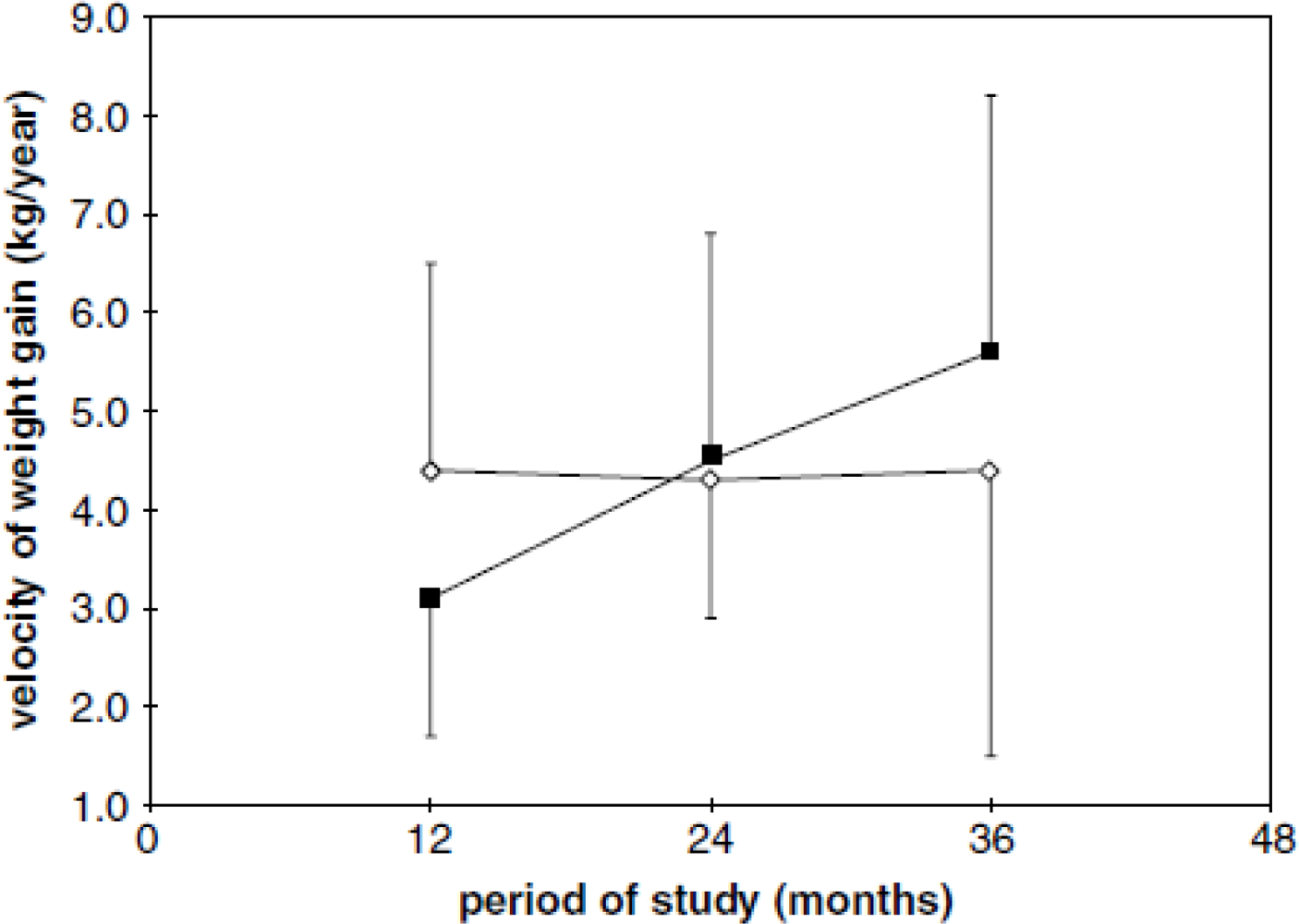
- Grillo, LP; Siqueira, AF; Silva, AC; Martins, PA; Verreschi, IT; Sawaya, AL. Lower resting metabolic rate and higher velocity of weight gain in a prospective study of stunted vs nonstunted girls living in the shantytowns of Sao Paulo, Brazil. Eur. J. Clin. Nutr 2005, 59, 835–842. [Google Scholar]
- Grover, Z; Ee, LC. Protein energy malnutrition. Pediatr. Clin. North. Am 2009, 56, 1055–1068. [Google Scholar]
- Sawaya, AL; Martins, PA; Martins, VJ; Florêncio, TT; Hoffman, D; do Carmo, PFM; das Neves, J. Malnutrition, long-term health and the effect of nutritional recovery. In Emerging Societies—Coexistence of Childhood Malnutrition and Obesity; Kalhan, SC, Prentice, AM, Yajnik, CS, Eds.; Nestlé Nutrition Institute: Vevey, Switzerland, 2009; Volume 63, pp. 95–105. [Google Scholar]
- Sawaya, AL. Transição: Desnutrição Energético-protéica e Obesidade. In Desnutrição Urbana no Brasil em um Período de Transição; Cortez editor: São Paulo, Brasil, 1997; pp. 35–61. [Google Scholar]
- Bénéfice, E; Garnier, D; Simondon, KB; Malina, RM. Relationship between stunting in infancy and growth and fat distribution during adolescence in Senegalese girls. Eur. J. Clin. Nutr 2001, 55, 50–58. [Google Scholar]
- Hoffman, DJ; Martins, PA; Roberts, SB; Sawaya, AL. Body fat distribution in stunted compared with normal-height children from the shantytowns of São Paulo, Brazil. Nutrition 2007, 23, 640–646. [Google Scholar]
- Phillips, DI; Walker, BR; Reynolds, RM; Flanagan, DE; Woodm, PJ; Osmond, C; Barker, DJ; Whorwood, CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 2000, 35, 1301–1306. [Google Scholar]
- Kajantie, E; Phillips, DI; Andersson, S; Barker, DJ; Dunkel, L; Forsén, T; Osmond, C; Tuominen, J; Wood, PJ; Eriksson, J. Size at birth, gestational age and cortisol secretion in adult life: foetal programming of both hyper- and hypocortisolism? Clin. Endocrinol. (Oxf) 2002, 57, 635–641. [Google Scholar]
- Kajantie, E; Eriksson, J; Barker, DJ; Forsén, T; Osmond, C; Wood, PJ; Andersson, S; Dunkel, L; Phillips, DI. Birthsize, gestational age and adrenal function in adult life: Studies of dexamethasone suppression and ACTH1-24 stimulation. Eur. J. Endocrinol 2003, 149, 569–575. [Google Scholar]
- Björntorp, P. Do stress reactions cause abdominal obesity and comorbidities? Obes. Rev 2001, 2, 73–86. [Google Scholar]
- Drapeau, V; Therrien, F; Richard, D; Tremblay, A. Is visceral obesity a physiological adaptation to stress? Panminerva Med 2003, 45, 189–195. [Google Scholar]
- Wallerius, S; Rosmond, R; Ljung, T; Holm, G; Bjorntorp, P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J. Endocrinol. Invest 2003, 26, 616–619. [Google Scholar]
- Dimitriou, T; Maser-Gluth, C; Remer, T. Adrenocortical activity in healthy children is associated with fat mass. Am. J. Nutr 2003, 77, 731–736. [Google Scholar]
- Kruger, HS; Pretorius, R; Schutte, AE. Stunting, adiposity, and low-grade inflammation in African adolescents from a township high school. Nutrition 2010, 26, 90–99. [Google Scholar]
- Richard, D; Chapdelaine, S; Deshaies, Y; Pepin, MC; Barden, N. Energy balance and lipid metabolism in transgenic mice bearing an antisense GCR gene construct. Am. J. Physiol 1993, 265, R146–R150. [Google Scholar]
- Masuzaki, H; Paterson, J; Shinyama, H; Morton, NM; Mullins, JJ; Seckl, JR; Flier, JS. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001, 294, 2166–2170. [Google Scholar]
- Fernandes, MTB; Sesso, R; Martins, PA; Sawaya, AL. Increased blood pressure in adolescents of socioeconomic status with short stature. Pediatr. Nephrol 2003, 18, 435–439. [Google Scholar]
- Florêncio, TT; Ferreira, HS; Cavalcante, JC; Sawaya, AL. Short stature, obesity and arterial hypertension in a very low income population in North-eastern Brazil. Nutr. Metab. Cardiovasc. Dis 2004, 14, 26–33. [Google Scholar]
- Ferreira, HS; Moura, FA; Cabral, CR, Jr; Florêncio, TM; Vieira, RC; de Assunção, ML. Short stature of mothers from an area endemic for undernutrition is associated with obesity, hypertension and stunted children: A population-based study in the semi-arid region of Alagoas, Northeast Brazil. Br. J. Nutr 2009, 101, 1239–1245. [Google Scholar]
- Goodpaster, BH; Thaete, FL; Simoneau, JA; Kelley, DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997, 46, 1579–1585. [Google Scholar]
- Lamarche, B; Lemieux, S; Dagenais, GR; Despres, JP. Visceral obesity and the risk of ischaemic heart disease: insights from the Quebec Cardiovascular Study. Growth Horm. IGF Res 1998, 8, 1–8. [Google Scholar]
- Nicklas, BJ; Cesari, M; Penninx, BW; Kritchevsky, SB; Ding, J; Newman, A; Kitzman, DW; Kanaya, AM; Pahor, M; Harris, TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J. Am. Geriatr. Soc 2006, 54, 413–420. [Google Scholar]
- Franco, MC; Casarini, DE; Carneiro-Ramos, MS; Sawaya, AL; Barreto-Chaves, ML; Sesso, R. Circulating renin-angiotensin system and catecholamines in childhood: is there a role for birthweight? Clin. Sci. (Lond) 2008, 114, 375–380. [Google Scholar]
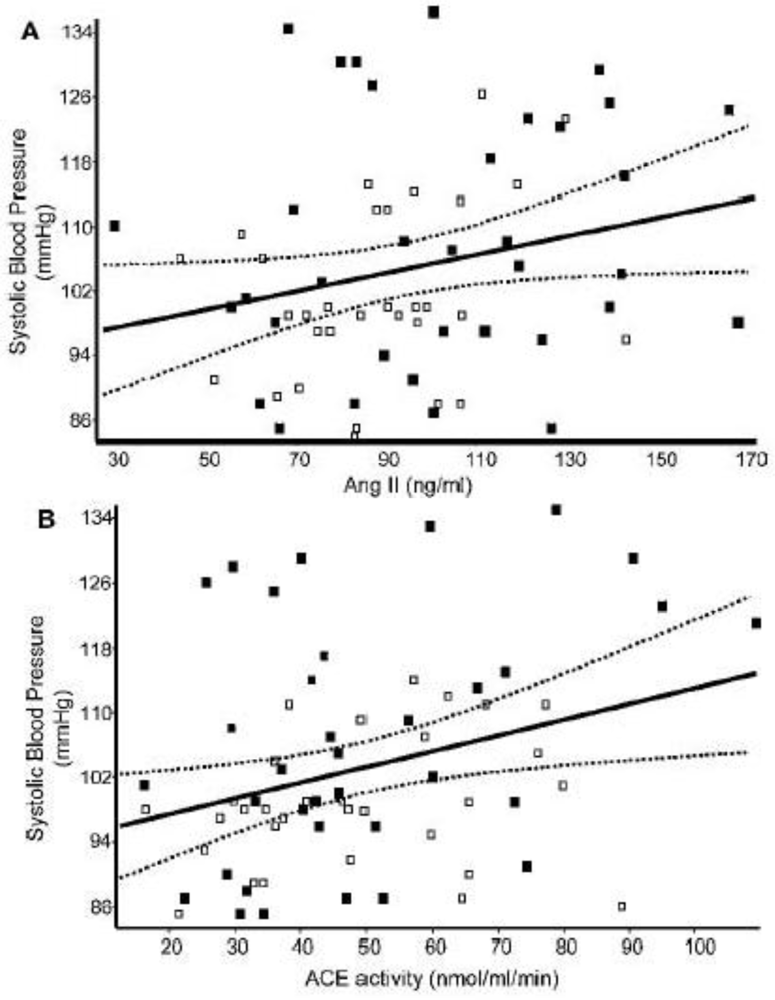
- Febba, A; Sesso, R; Barreto, GP; Liboni, CS; Franco, MC; Casarini, DE. Stunting growth: Association of the blood pressure levels and ACE activity in early childhood. Pediatr. Nephrol 2009, 24, 379–386. [Google Scholar]
- Hinchliffe, AS; Lynch, MR; Sargent, PH; Howard, CV; Van Velzen, D. The effect of intrauterine growth retardation on the development of renal nephrons. Br. J. Obstet. Gynaecol 1992, 99, 296–301. [Google Scholar]
- Konje, JC; Bell, SC; Morton, JJ; DeChazal, R; Taylor, DJ. The renal development in human intrauterine growth retardation. Clin. Sci 1996, 91, 169–175. [Google Scholar]
- Franco, MdoC; Arruda, RM; Fortes, ZB; de Oliveira, SF; Carvalho, MH; Tostes, RC; Nigro, D. Severe nutritional restriction in pregnant rats aggravates hypertension, altered vascular reactivity, and renal development in spontaneously hypertensive rats offspring. J. Cardiovasc. Pharmacol 2002, 39, 369–377. [Google Scholar]
- Wild, S; Roglic, G; Green, A; Sicree, R; King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar]
- Yajnik, CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J. Nutr 2004, 134, 205–210. [Google Scholar]
- Fekadu, S; Yigzaw, M; Alemu, S; Dessie, A; Fieldhouse, H; Girma, T; Trimble, ER; Phillips, DI; Parry, EH. Insulin-requiring diabetes in Ethiopia: Associations with poverty, early undernutrition and anthropometric disproportion. Eur. J. Clin. Nutr 2010, 64, 1192–1198. [Google Scholar]
- Gonzalez-Barranco, J; Rios-Torres, JM; Castillo-Martinez, L; Lopez-Alvarenga, JC; Guilar-Salinas, CA; Bouchard, C; Depres, JP; Tremblay, A. Effect of malnutrition during the first year of life on adult plasma insulin and glucose tolerance. Metabolism 2003, 52, 1005–1011. [Google Scholar]
- Martins, PA; Sawaya, AL. Evidence for impaired insulin production and higher sensitivity in stunted children living in slums. Br. J. Nutr 2006, 95, 996–1001. [Google Scholar]
- Ravelli, GP; Stein, Z; Susser, M. Obesity in young mean after famine exposure in utero and early infancy. N. Engl. J. Med 1976, 259, 349–353. [Google Scholar]
- Bréant, B; Gesina, E; Blondeau, B. Nutrition, glucocorticoids and pancreas development. Horm. Res 2006, 65, 98–104. [Google Scholar]
- Romero, LM; Dickens, MJ; Cyr, NE. The Reactive Scope Model—A new model integrating homeostasis, allostasis, and stress. Horm. Behav 2009, 55, 375–389. [Google Scholar]
- Bedi, M; Babbar, R; Chakrabarty, AS; Sachdev, HP. Comparative study of autonomic nervous system activity in malnourished and normal children in India. Ann. Trop. Paediatr 1999, 19, 185–189. [Google Scholar]
- Waterlow, JC. Protein Energy Malnutrition; Edward Arnold: London, UK, 1992. [Google Scholar]
- Manary, MJ; Muglia, LJ; Vogt, SK; Yarasheski, KE. Cortisol and its action on the glucocorticoid receptor in malnutrition and acute infection. Metabolism 2006, 55, 550–554. [Google Scholar]
- Lunn, PG; Whitehead, RG; Hay, RW; Baker, BA. Progressive changes in serum cortisol, insulin and growth hormone concentrations and their relationship to the distorted amino acid pattern during the development of kwashiorkor. Br. J. Nutr 1973, 29, 399–422. [Google Scholar]
- Paisey, RB; Angers, M; Frenk, S. Plasma cortisol levels in malnourished children with and without superimposed acute stress. Arch. Dis. Child 1973, 48, 714–716. [Google Scholar]
- Fernald, LC; Grantham-McGregor, SM; Manandhar, DS; Costello, A. Salivary cortisol and heart rate in stunted and nonstunted Nepalese school children. Eur. J. Clin. Nutr 2003, 57, 1458–1465. [Google Scholar]
- Ray, A; Prefontaine, KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 752–756. [Google Scholar]
- Stevens, A; Begum, G; Cook, A; Connor, K; Rumball, C; Oliver, M; Challis, J; Bloomfield, F; White, A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 2010, 151, 3652–3664. [Google Scholar]
- Stevens, A; Begum, G; White, A. Epigenetic changes in the hypothalamic proopiomelanocortin gene: A mechanism linking maternal undernutrition to obesity in the offspring? Eur. J. Pharmacol 2011, 660, 194–201. [Google Scholar]
- Lillycrop, KA; Phillips, ES; Jackson, AA; Hanson, MA; Burdge, GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J. Nutr 2005, 135, 1382–1386. [Google Scholar]
- Strike, PC; Steptoe, A. Psychosocial factors in the development of coronary artery disease. Prog. Cardiovasc. Dis 2004, 46, 337–347. [Google Scholar]
- Steptoe, A; Wardle, J; Marmot, M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc. Natl. Acad. Sci. USA 2005, 102, 6508–6512. [Google Scholar]
- McEwen, BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev 2007, 87, 873–904. [Google Scholar]
- Gunnar, M; Quevedo, K. The neurobiology of stress and development. Annu. Rev. Psychol 2007, 58, 145–173. [Google Scholar]
- Lesage, J; Dufourny, L; Laborie, C; Bernet, F; Blondeau, B; Avril, I; Bréant, B; Dupouy, JP. Perinatal malnutrition programs sympathoadrenal and hypothalamic-pituitary-adrenal axis responsiveness to restraint stress in adult male rats. J. Neuroendocrinol 2002, 14, 135–143. [Google Scholar]
- Molendi-Coste, O; Laborie, C; Scarpa, MC; Montel, V; Vieau, D; Breton, C. Maternal perinatal undernutrition alters postnatal development of chromaffin cells in the male rat adrenal medulla. Neuroendocrinology 2009, 90, 54–66. [Google Scholar]
- de Rooij, SR; Painter, RC; Phillips, DI; Osmond, C; Tanck, MW; Bossuyt, PM; Roseboom, TJ. Cortisol responses to psychological stress in adults after prenatal exposure to the Dutch famine. Psychoneuroendocrinology 2006, 31, 1257–1265. [Google Scholar]
- Grantham McGregor, S; Fernald, LC. Effects of health and nutrition on cognitive and behavioural development in children in the first three years of life. Part 1: Small for gestational age, breastfeeding and protein-energy malnutrition. Food Nutr. Bull 1999, 20, 53–75. [Google Scholar]
- Stein, Z; Susser, M; Saenger, G; Marolla, F. Nutrition and mental performance. Science 1972, 178, 708–713. [Google Scholar]
- de Groot, RH; Stein, AD; Jolles, J; van Boxtel, MP; Blauw, GJ; van de Bor, M; Lumey, L. Prenatal famine exposure and cognition at age 59 years. Int. J. Epidemiol 2011, 40, 327–337. [Google Scholar] [Green Version]
- Lumey, LH; Stein, AD; Susser, E. Prenatal famine and adult health. Annu. Rev. Public Health 2011, 32, 237–262. [Google Scholar]
- Crookston, BT; Penny, ME; Alder, SC; Dickerson, TT; Merrill, RM; Stanford, JB; Porucznik, CA; Dearden, KA. Children who recover from early stunting and children who are not stunted demonstrate similar levels of cognition. J. Nutr 2010, 140, 1996–2001. [Google Scholar]
- Pryer, JA. Body mass index and work-disabling morbidity: results from a Bangladeshi case study. Eur. J. Clin. Nutr 1993, 47, 653–657. [Google Scholar]
- Durnin, JV. Low body mass index, physical work capacity and physical activity levels. Eur J Clin Nutr 1994, 48, S39–43, discussion S43–44. [Google Scholar]
- Florêncio, TT; Ferreira, HS; Cavalcante, JC; Assunção, ML; Sawaya, AL. Short stature and food habits as determining factors for the low productivity of sugarcane labourers in the State of Alagoas, north-eastern Brazil. Arch. Latinoam. Nutr 2008, 58, 33–39. [Google Scholar]
- Kabir, I; Rahman, MM; Haider, R; Mazumder, RN; Khaled, MA; Mahalanabis, D. Increased height gain of children fed a high-protein diet during convalescence from shigellosis: A six-month follow-Up study. J. Nutr 1998, 128, 1688–1691. [Google Scholar]
- Vieira, AMF; Ferraro, AA; Souza, NMH; Fernandes, MT; Sawaya, AL. Height and weight gains in a nutrition rehabilitation day-care service. Public Health Nutr 2010, 13, 1505–1510. [Google Scholar]
- Das Neves, J; Martins, PA; Sesso, R; Sawaya, AL. Malnourished children treated in day-hospital or outpatient clinics exhibit linear catch-up and normal body composition. J. Nutr 2006, 136, 648–655. [Google Scholar]
- Martins, VJB; Martins, PA; Neves, J; Sawaya, AL. Children recovered from malnutrition exhibit normal insulin production and sensitivity. Br. J. Nutr 2008, 99, 297–302. [Google Scholar]
 ) and nonstunted children (□) (overall group) between the two study visits. (a) fat mass; (b) lean mass; (c) fat mass percentage; (d) lean mass percentage. The boxes represent the interquartile ranges, which contain 50% of values; the whiskers are the highest and lowest values (excluding outliers), and the line across each box indicates the median. Reprinted with permission from Brit. J. Nutr. [26].
) and nonstunted children (□) (overall group) between the two study visits. (a) fat mass; (b) lean mass; (c) fat mass percentage; (d) lean mass percentage. The boxes represent the interquartile ranges, which contain 50% of values; the whiskers are the highest and lowest values (excluding outliers), and the line across each box indicates the median. Reprinted with permission from Brit. J. Nutr. [26].
 ) and nonstunted children (□) (overall group) between the two study visits. (a) fat mass; (b) lean mass; (c) fat mass percentage; (d) lean mass percentage. The boxes represent the interquartile ranges, which contain 50% of values; the whiskers are the highest and lowest values (excluding outliers), and the line across each box indicates the median. Reprinted with permission from Brit. J. Nutr. [26].
) and nonstunted children (□) (overall group) between the two study visits. (a) fat mass; (b) lean mass; (c) fat mass percentage; (d) lean mass percentage. The boxes represent the interquartile ranges, which contain 50% of values; the whiskers are the highest and lowest values (excluding outliers), and the line across each box indicates the median. Reprinted with permission from Brit. J. Nutr. [26].
| Boys | Girls | |||
|---|---|---|---|---|
| Eutrophic (n = 13) | Undernourished (n = 12) | Eutrophic (n = 17) | Undernourished (n = 8) | |
| Bone mineral content | ||||
| Arms (g) | 46.44 ± 16.90 | 21.63 ± 9.99 ** | 48.77 ± 21.31 | 30.74 ± 13.40 * |
| Legs (g) | 154.03 ± 45.03 | 35.65 ± 165.06 * | 127.17 ± 42.79 | 73.83 ± 18.05 ** |
| Trunk (g) | 135.34 ± 103.64 | 67.62 ± 22.79 * | 169.14 ± 66.95 | 82.43 ± 75.06 * |
| Total (g) | 557.86 ± 206.66 | 275.34 ± 107.69 ** | 570.87 ± 211.26 | 330.85 ± 133.78 * |
| Bone mineral density | ||||
| Arms (g/cm2) | 0.054 ± 0.047 | 0.027 ± 0.026 | 0.067 ± 0.042 | 0.037 ± 0.042 |
| Legs (g/cm2) | 0.209 ± 0.080 | 0.108 ± 0.057 * | 0.184 ± 0.062 | 0.094 ± 0.030 ** |
| Total (g/cm2) | 0.099 ± 0.059 | 0.038 ± 0.043 * | 0.114 ± 0.058 | 0.051 ± 0.048* |
| BMI (kg/m2) | Number of individuals n (%) | Average (±SD) productivity (ton/day) | Average (±SD) energy intake (kJ/day) | Average (±SD) daily nutrient intake (g/kg body weight) | ||
|---|---|---|---|---|---|---|
| Proteins | Carbohydrates | Lipids | ||||
| <21.5 | 25(40.3%) | 7.48 ± 1.5 | 12,380 ± 4,184 | 2.0 ± 0.5 | 7.4 ± 3.6 | 0.8 ± 0.6 |
| 21.5–25 | 30 (48.4%) | 9.12 ± 1.5 * | 16,506 ± 6,360 * | 2.1 ± 1.5 | 9.8 ± 4.1 * | 1.7 ± 0.7 * |
| >25 | 7 (11.3%) | 7.80 ± 1.7 | 13,215 ± 1,251 | 2.0 ± 0.5 | 8.5 ± 1.4 | 1.2 ± 0.3 |
| Height (cm) | Number of individuals n (%) | Average age (years) | Average productivity (ton/day) | Average daily energy intake (kJ/day) | Average daily protein intake (g/kg body weight) | Body fat composition | ||
|---|---|---|---|---|---|---|---|---|
| Initial (a) (%) | Final (b) (%) | Loss Δ = a−b | ||||||
| 158–159.9 | 12 (19.4%) | 42 ± 12.3 | 7.14 ± 1.6 * | 14,292 ± 4,300 | 2.2 ± 0.7 | 17.7 ± 4.3 | 16.6 ± 5.7 | 1.1 |
| 160–164.9 | 16 (25.8%) | 35 ± 10.7 | 7.90 ± 1.7 | 11,593 ± 4,449 | 1.8 ± 0.7 | 13.7 ± 2.2 | 12.9 ± 2.0 | 0.8 |
| 165–169.9 | 12 (19.4%) | 37 ± 10.3 | 7.95 ± 1.5 | 12,041 ± 3,969 | 1.9 ± 0.7 | 13.3 ± 3.6 | 12.4 ± 4.3 | 0.9 |
| 170–174.9 | 12 (19.4%) | 30 ± 8.6 | 9.01 ± 1.5 * | 15,388 ± 5,217 | 2.4 ± 0.5 | 12.3 ± 3.5 | 11.4 ± 2.5 | 0.9 |
| =175 | 10 (16.1%) | 26 ± 7.4 | 8.65 ± 1.4 | 15,551 ± 6,758 | 2.2 ± 0.7 | 9.8 ± 2.5 | 9.5 ± 1.9 | 0.3 |
| Increment of WAZ at discharge | P | Increment of HAZ at discharge | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0 (n 21) | 0.01–0.50 (n 44) | 0.51–1.00 (n 22) | >1.00 (n 19) | ≤0 (n 21) | 0.01–0.50 (n 36) | 0.51–1.00 (n 28) | >1.00 (n 21) | |||
| Age at admission (months), median * | 17.4 | 17.5 | 20.9 | 24.2 | 0.527 | 10.8 | 21.4 | 23.2 | 19.1 | 0.338 |
| Gender (% male) † | 57.1 | 47.7 | 59.1 | 36.8 | 0.464 | 66.7 | 50.0 | 42.9 | 42.9 | 0.343 |
| Duration of treatment (months), mean ‡ | 10.1 | 14.8 | 19.7 | 23.0 | <0.001 | 9.2 | 12.9 | 22.2 | 21.7 | 0.957 |
| Fetal development (% premature) † | 20.0 | 18.4 | 25.0 | 29.4 | 0.811 | 21.1 | 25.9 | 20.0 | 21.1 | 0.957 |
| Birth weight (kg), mean ‡ | 2.593 | 2.648 | 2.550 | 2.317 | 0.103 | 2.670 | 2.619 | 2.604 | 2.295 | 0.045 |
| Small for gestational age (%) § | 27.8 | 21.4 | 19.1 | 50.0 | 0.129 | 21.1 | 18.2 | 23.1 | 52.6 | 0.026 |
| WAZ at admission, mean ‡ | −2.04 | −2.08 | −2.49 | −2.52 | <0.001 | −2.22 | −2.07 | −2.26 | −2.51 | 0.041 |
| HAZ at admission, mean ‡ | −1.92 | −1.91 | −2.36 | −2.26 | 0.061 | −1.63 | −1.78 | −2.27 | −2.71 | <0.001 |
| Frequent URTI (rate 10–3) * | 55.3 | 26.2 | 44.1 | 33.4 | 0.011 | 22.9 | 36.8 | 37.3 | 39.3 | 0.226 |
| Frequent LRTI (rate 10–3) * | 5.8 | 2.4 | 1.3 | 0.5 | 0.857 | 1.8 | 3.7 | 1.3 | 1.7 | 0.712 |
| Frequent diarrhoea (rate 10–3) §* | 11.2 | 5.6 | 6.0 | 6.0 | 0.215 | 7.1 | 10.7 | 3.5 | 6.0 | 0.201 |
| Maternal education (years), mean ‡ | 2.1 | 2.4 | 2.3 | 2.3 | 0.767 | 2.5 | 2.3 | 2.1 | 2.3 | 0.347 |
| Family income (R$), median* | 300 | 300 | 352 | 268 | 0.454 | 350 | 300 | 300 | 300 | 0.721 |
| Boys | p † | Girls | p | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Recovered | Control | Recovered | |||||||||||
| n | Mean | SE | n | Mean | SE | n | Mean | SE | n | Mean | SE | |||
| Insulin (pmol/L) * | 15 | 3.62 | 0.40 | 28 | 3.76 | 0.28 | NS | 9 | 3.78 | 0.26 | 30 | 3.52 | 0.09 | NS |
| Glucose (mg/dL) | 15 | 78,90 | 5.92 | 27 | 73.28 | 4.23 | NS | 9 | 75.54 | 4.38 | 29 | 79.04 | 1.65 | NS |
| HOMA-B (%)* | 15 | 4.71 | 0.308 | 27 | 4.92 | 0.220 | NS | 9 | 4.84 | 0.206 | 29 | 4.59 | 0.076 | NS |
| HOMA-S (%)* | 15 | 4.85 | 0.403 | 27 | 4.75 | 0.288 | NS | 10 | 4.73 | 0.264 | 30 | 4.96 | 0.098 | NS |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martins, V.J.B.; Toledo Florêncio, T.M.M.; Grillo, L.P.; Do Carmo P. Franco, M.; Martins, P.A.; Clemente, A.P.G.; Santos, C.D.L.; Vieira, M.d.F.A.; Sawaya, A.L. Long-Lasting Effects of Undernutrition. Int. J. Environ. Res. Public Health 2011, 8, 1817-1846. https://doi.org/10.3390/ijerph8061817
Martins VJB, Toledo Florêncio TMM, Grillo LP, Do Carmo P. Franco M, Martins PA, Clemente APG, Santos CDL, Vieira MdFA, Sawaya AL. Long-Lasting Effects of Undernutrition. International Journal of Environmental Research and Public Health. 2011; 8(6):1817-1846. https://doi.org/10.3390/ijerph8061817
Chicago/Turabian StyleMartins, Vinicius J. B., Telma M. M. Toledo Florêncio, Luciane P. Grillo, Maria Do Carmo P. Franco, Paula A. Martins, Ana Paula G. Clemente, Carla D. L. Santos, Maria de Fatima A. Vieira, and Ana Lydia Sawaya. 2011. "Long-Lasting Effects of Undernutrition" International Journal of Environmental Research and Public Health 8, no. 6: 1817-1846. https://doi.org/10.3390/ijerph8061817
APA StyleMartins, V. J. B., Toledo Florêncio, T. M. M., Grillo, L. P., Do Carmo P. Franco, M., Martins, P. A., Clemente, A. P. G., Santos, C. D. L., Vieira, M. d. F. A., & Sawaya, A. L. (2011). Long-Lasting Effects of Undernutrition. International Journal of Environmental Research and Public Health, 8(6), 1817-1846. https://doi.org/10.3390/ijerph8061817





