Assessment of the Efficacy of Chelate-Assisted Phytoextraction of Lead by Coffeeweed (Sesbania exaltata Raf.)
Abstract
:Introduction
Materials and Methods
Solubility Test and Chelate Selection
Greenhouse Experiment
Metal Extraction and Analyses
Statistical Analyses
Results and Discussion
Solubility Test and Chelate Selection
Greenhouse Experiment

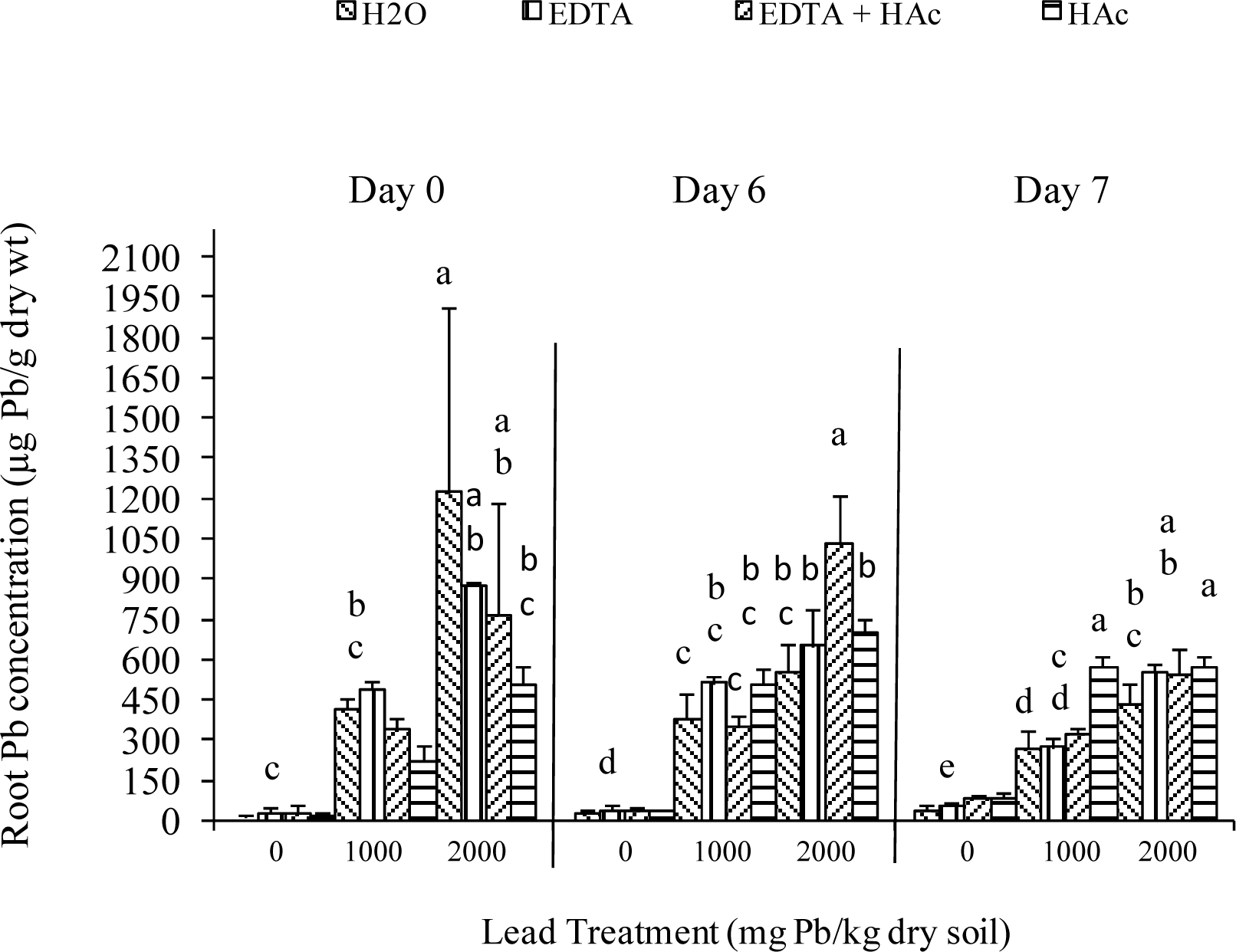
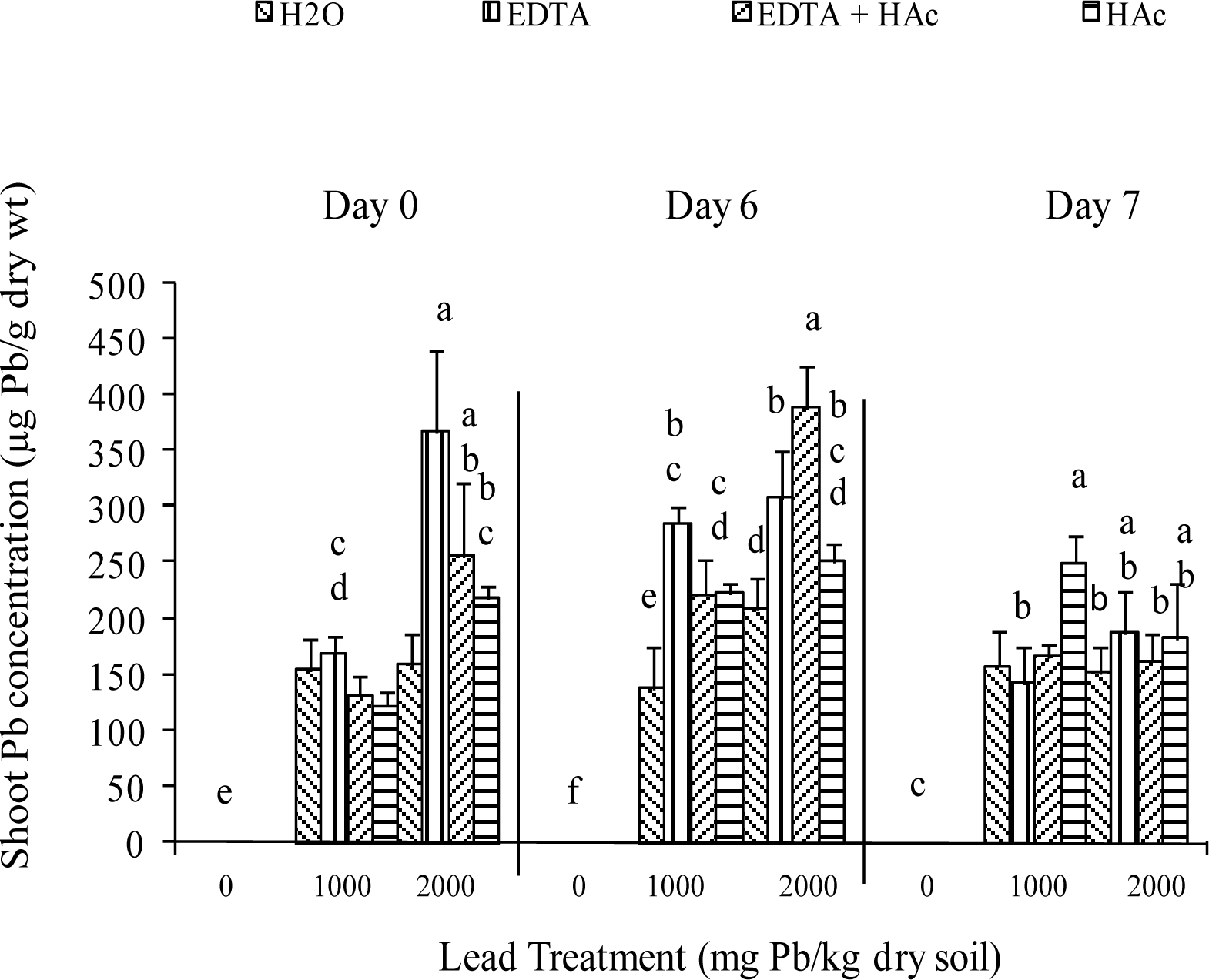
| Characteristic | Extractable Levels (lbs/acre) |
|---|---|
| Soil Acidity (pH) | 6.3 |
| Phosphorus | 130* |
| Potassium | 301* |
| Calcium | 4537 |
| Magnesium | 726** |
| Zinc | 4.2* |
| Sodium | 161 |
| CEC | 17.6 |
| % Clay | 7.50 |
| % Silt | 80.08 |
| % Sand | 12.4 |
| High* | |
| Very High** |
| Treatment | Root Dry Biomass (mg/plant) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 7 | |||||||||||
| Pb (mg/kg) | EDTA | Mean | ** | ± | SEM | Mean | ** | ± | SEM | Mean | ** | ± | SEM |
| 0 | 0 | 15.12 | c | ± | 5.2 | 34.37 | c | ± | 1.7 | 34.00 | ab | ± | 1.9 |
| 0 | 1000 | 20.25 | abc | ± | 6.9 | 37.50 | bc | ± | 6.4 | 29.62 | ab | ± | 3.2 |
| 0 | 1000* | 19.87 | abc | ± | 4.7 | 36.62 | bc | ± | 7.1 | 31.37 | ab | ± | 6.7 |
| 0 | HAc | 15.75 | abc | ± | 5.4 | 34.50 | c | ± | 5.8 | 27.75 | b | ± | 7.8 |
| 1000 | 0 | 16.25 | abc | ± | 2.0 | 36.12 | bc | ± | 5.0 | 38.37 | ab | ± | 6.7 |
| 1000 | 1000 | 17.87 | abc | ± | 3.9 | 41.52 | abc | ± | 8.5 | 26.50 | ab | ± | 3.5 |
| 1000 | 1000* | 24.12 | abc | ± | 4.4 | 47.00 | abc | ± | 1.9 | 40.50 | ab | ± | 2.1 |
| 1000 | HAc | 23.62 | abc | ± | 3.4 | 49.87 | ab | ± | 3.3 | 41.25 | ab | ± | 8.8 |
| 2000 | 0 | 29.75 | a | ± | 3.6 | 51.00 | ab | ± | 6.4 | 48.12 | a | ± | 5.3 |
| 2000 | 2000 | 24.25 | abc | ± | 3.7 | 42.37 | abc | ± | 3.6 | 47.12 | a | ± | 7.2 |
| 2000 | 2000* | 21.87 | abc | ± | 6.4 | 53.50 | a | ± | 5.7 | 47.37 | a | ± | 8.7 |
| 2000 | HAc | 28.87 | a | ± | 3.1 | 45.87 | abc | ± | 3.6 | 40.83 | ab | ± | 11.1 |
| Treatment | Shoot Dry Biomass (mg/plant) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb (mg/kg) | EDTA | Day 0 | Day 6 | Day 7 | |||||||||
| Mean | ** | ± | SEM | Mean | ** | ± | SEM | Mean | ** | ± | SEM | ||
| 0 | 0 | 133.63 | a | ± | 30.4 | 138.25 | bc | ± | 12.0 | 127.38 | b | ± | 6.6 |
| 0 | 1000 | 190.75 | a | ± | 47.65 | 146.75 | abc | ± | 27.3 | 114.75 | b | ± | 8.9 |
| 0 | 1000* | 143.75 | a | ± | 18.0 | 141.38 | bc | ± | 14.1 | 374.13 | a | ± | 25.94 |
| 0 | HAc | 143.50 | a | ± | 20.7 | 99.0 | c | ± | 15.8 | 150.00 | ab | ± | 16.1 |
| 1000 | 0 | 128.38 | a | ± | 24.9 | 100.88 | c | ± | 19.2 | 140.50 | ab | ± | 30.3 |
| 1000 | 1000 | 145.00 | a | ± | 9.0 | 220.13 | a | ± | 68.9 | 163.75 | ab | ± | 15.6 |
| 1000 | 1000* | 187.75 | a | ± | 33.7 | 167.0 | abc | ± | 18.6 | 143.50 | ab | ± | 25.0 |
| 1000 | HAc | 191.38 | a | ± | 16.6 | 189.63 | ab | ± | 6.1 | 152.88 | ab | ± | 30.2 |
| 2000 | 0 | 190.50 | a | ± | 21.7 | 197.0 | ab | ± | 22.7 | 208.75 | ab | ± | 35.6 |
| 2000 | 2000 | 141.50 | a | ± | 36.5 | 174.88 | abc | ± | 23.7 | 181.88 | ab | ± | 19.1 |
| 2000 | 2000* | 140.38 | a | ± | 15.7 | 186.25 | ab | ± | 15.6 | 178.50 | ab | ± | 23.4 |
| 2000 | HAc | 190.38 | a | ± | 18.0 | 158.0 | abc | ± | 16.7 | 207.83 | ab | ± | 73.3 |
Acknowledgments
References
- Kumar, PBAN; Dushenkov, V; Motto, H; Raskin, I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995, 29(5), 1232–1238. [Google Scholar]
- Cunningham, SD; Ow, WD. Promises prospects of phytoremediation. Plant Physiology 1996, 110, 715–719. [Google Scholar]
- Blaylock, MJ; Salt, DE; Dushenkov, S; Zakharova, O; Gussman, C; Kapulnik, Y; Ensley, BD; Raskin, I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997, 31(3), 860–865. [Google Scholar]
- Friedland, AJ. Heavy metal tolerance in plants: Evolutionary aspects; Shaw, AJ, Ed.; CRC Press: Boca Raton, FL, 1990. [Google Scholar]
- Glass, DJ. Economic potential of phytoremediation. In Phytoremediation of toxic metals: Using plants to clean up the environment; Raskin, I, Ensley, DD, Eds.; John Wiley & Sons, Inc: New York, 2000; pp. 1–14. [Google Scholar]
- McGrath, SP; Sidoli, CMD; Baker, AJM; Reeves, RD. The potential for the use of metal-accumulating plants for the in situ decontamination of metal-polluted soils. In Integrated soil sediment research: A basis for proper protection; Eijascker, HJP, Hamers, T, Eds.; Academic Publ: Dordrecht, Netherlands, 1993; pp. 673–677. [Google Scholar]
- Raskin, I; Kumar, PBAN; Dushenkov, V; Salt, DE. Bioconcentration of heavy metals by plants. Current Opinions in Biotechnology 1994, 5, 285–290. [Google Scholar]
- Chaney, RL; Malik, M; Li, YM; Brown, SL; Brewer, EP; Angle, JS; Baker, AJM. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar]
- Salt, DE; Blaylock, M; Kumar, PBAN; Sushenkov, V; Ensley, BD; Chet, I; Raskin, I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 1995, 13, 468–475. [Google Scholar]
- Salt, DE; Smith, RD; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar]
- Shen, ZG; Zhao, EJ; McGrath, SP. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the nonhyperaccumulator Thlaspi ochroleucum. Plant Cell Environment 1997, 20, 898–906. [Google Scholar]
- Keltjens, WG; van Beusichem, ML. Phytochelatins as biomarkers for heavy metal toxicity in maize: Single metal effects of copper and cadmium. J. Plant Nutr. 1998, 21(4), 635–648. [Google Scholar]
- Lim, TT; Tay, JH; Wang, JY. Chelating-agent-enhanced heavy metal extraction from a contaminated acidic soil. J. Environ. Eng. 2004, 130, 59–66. [Google Scholar]
- Ghosh, S; Rhyne, C. Influence of EDTA on Pb uptake in two weed species, Sesbania and Ipomoea, in hydroponic culture. J. Miss. Acad. Sci. 1999, 44(1), 11. [Google Scholar]
- McBride, MB. Environmental chemistry of soils; Oxford University Press, 1994. [Google Scholar]
- Kinniburgh, DG; Jackson, ML; Syers, JK. Adsorption of alkaline-earth, transition, and heavy-metal cations by hydrous oxide gels of iron and aluminum. Soil Science Society American Journal 1976, 40, 796–799. [Google Scholar]
- Ruby, MV; Schoof, R; Brattin, W; Goldade, M; Post, G; Harnois, M; Mosby, DE; Casteel, SW; Berti, W; Carpenter, M; Edwards, D; Cragin, D; Chappell, W. Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ. Sci. Technol. 1999, 32, 3697–3705. [Google Scholar]
- Grcman, H; Vodnik, D; Velikonja-Bolta, S; Lestan, D. Heavy metals in the environment: Ethylenediaminedissuccinate as a new chelate for environmentlaly safe enhanced lead phytoextraction. J. Environ. Qual. 2003, 32, 500–506. [Google Scholar]
- Jorgensen, SE. Removal of heavy metals from compost and soil by ecotechnological methods. Ecol. Eng. 1993, 2, 89–100. [Google Scholar]
- Huang, JW; Chen, J; Berti, WR; Cunningham, SD. Phytoremediation of lead-contaminated soils: Role of synthetic chelates in lead phytoextraction. Environ. Sci. Technol. 1997, 31(3), 800–805. [Google Scholar]
- Vassil, AD; Kapulnik, Y; Raskin, I; Salt, DE. The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiology 1998, 117, 447–453. [Google Scholar]
- Wu, J; Hsu, FC; Cunningham, SD. Chelate-assisted Pb phytoextraction: Pb availability uptake, and translocation constraints. Environ. Sci. Technol. 1999, 33, 1898–1905. [Google Scholar]
- Begonia, GB; Begonia, MFT; Miller, GS; Kambhampati, M. Phytoremediation of metal-contaminated soils: Jackson State University research initiatives. In Metal ions in biology and medicine; Centeno, JA, Collery, P, Vernet, G, Finkelman, RB, Gibb, H, Etienne, JC, Eds.; 2000; Volume 6, pp. 682–684. [Google Scholar]
- Begonia, GB; Miller, GS; Begonia, MFT; Burks, C. Chelate-enhanced phytoextraction of lead-contaminated soils using coffeeweed (Sesbania exaltata raf.). Bull. Environ. Contam. Toxicol. 2002, 69(5), 624–654, 37. [Google Scholar]
- Shen, ZG; Li, XD; Wang, CC; Chen, HM; Chua, H. Lead phytoextraction from contaminated soil with high-biomass plant species. J. Environ. Qual. 2002, 31, 1893–1900. [Google Scholar]
- Turpeinen, R; Salminen, J; Kairesalo, T. Mobility bioavailability of lead in contaminated boreal forest soil. Environ. Sci. Technol. 2000, 34, 5152–5156. [Google Scholar]
- Cao, X; Ma, LQ; Chen, M; Donald, W; Hardison, J; Harris, WG. Weathering of lead bullets and their environmental effects at outdoor shooting ranges. J. Environ. Qual. 2003, 32, 526–534. [Google Scholar]
- Dushenkov, V; Kumar, PBAN; Motto, H; Raskin, I. Rhizofiltration: The use of plants to remove heavy metals from aqueous streams. Environ. Sci. Technol. 1995, 29, 1239–1245. [Google Scholar]
- Lasat, MM. Phytoextraction of toxic metals: A review of biological mechanisms. J Environ Qual. 2002, 31, 109–120. [Google Scholar]
- Lasat, MM; Baker, AJM; Kochian, LV. Altered Zn compartmentation in the root symplasm and simulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens. Plant Physiology 1998, 118, 875–883. [Google Scholar]
- Kayser, A; Wenger, K; Keller, A; Attinger, W; Felix, HR; Gupta, SK; Schulin, R. Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of NTA and sulfur amendments. Environ. Sci. Technol. 2000, 34, 1778–1783. [Google Scholar]
- Fuhrmann, M; Lasat, MM; Ebbs, SD; Kochian, LV; Cornish, J. Plant environmental interactions: Uptake of cesium-137 and strontium-90 from contaminated soil by three plant species; application to phytoremediation. J. Environ. Qual. 2002, 31, 904–909. [Google Scholar]
- Toxnet Hazardous substances data bank. http://toxnet.nlm.nih.gov. Available 08-15-06.
- Ebbs, SD; Lasat, MM; Brady, DJ; Cornish, J; Gordon, R; Kochian, LV. Phytoextraction of cadmium and zinc from a contaminated soil. J. Environ. Qual. 1997, 26, 1424–1430. [Google Scholar]
- Lombi, E; Zhao, FJ; Dunham, DJ; McGrath, SP. Phytoremediation of heavy metal-contaminated soils: Natural hyperaccumulation versus chemically enhanced phytoextraction. J. Environ. Qual. 2001, 30, 1919–1926. [Google Scholar]
- Grill, E; Winnacker, EL; Zenk, MH. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science (Washington, D. C., 1883) 1985, 230, 674–676. [Google Scholar]
- Grill, E; Winnacker, EL; Zenk, MH. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA. 1987, 84, 439–443. [Google Scholar]
© 2008 MDPI All rights reserved.
Share and Cite
Miller, G.; Begonia, G.; Begonia, M.; Ntoni, J.; Hundley, O. Assessment of the Efficacy of Chelate-Assisted Phytoextraction of Lead by Coffeeweed (Sesbania exaltata Raf.). Int. J. Environ. Res. Public Health 2008, 5, 428-435. https://doi.org/10.3390/ijerph5050428
Miller G, Begonia G, Begonia M, Ntoni J, Hundley O. Assessment of the Efficacy of Chelate-Assisted Phytoextraction of Lead by Coffeeweed (Sesbania exaltata Raf.). International Journal of Environmental Research and Public Health. 2008; 5(5):428-435. https://doi.org/10.3390/ijerph5050428
Chicago/Turabian StyleMiller, Gloria, Gregorio Begonia, Maria Begonia, Jennifer Ntoni, and Oscar Hundley. 2008. "Assessment of the Efficacy of Chelate-Assisted Phytoextraction of Lead by Coffeeweed (Sesbania exaltata Raf.)" International Journal of Environmental Research and Public Health 5, no. 5: 428-435. https://doi.org/10.3390/ijerph5050428
APA StyleMiller, G., Begonia, G., Begonia, M., Ntoni, J., & Hundley, O. (2008). Assessment of the Efficacy of Chelate-Assisted Phytoextraction of Lead by Coffeeweed (Sesbania exaltata Raf.). International Journal of Environmental Research and Public Health, 5(5), 428-435. https://doi.org/10.3390/ijerph5050428




