Effect of Butachlor on Antioxidant Enzyme Status and Lipid Peroxidation in Fresh Water African Catfish, (Clarias gariepinus)
Abstract
:Introduction
Materials and Methods
Chemicals
Fish and Treatment
Biochemical Assays
Statistics
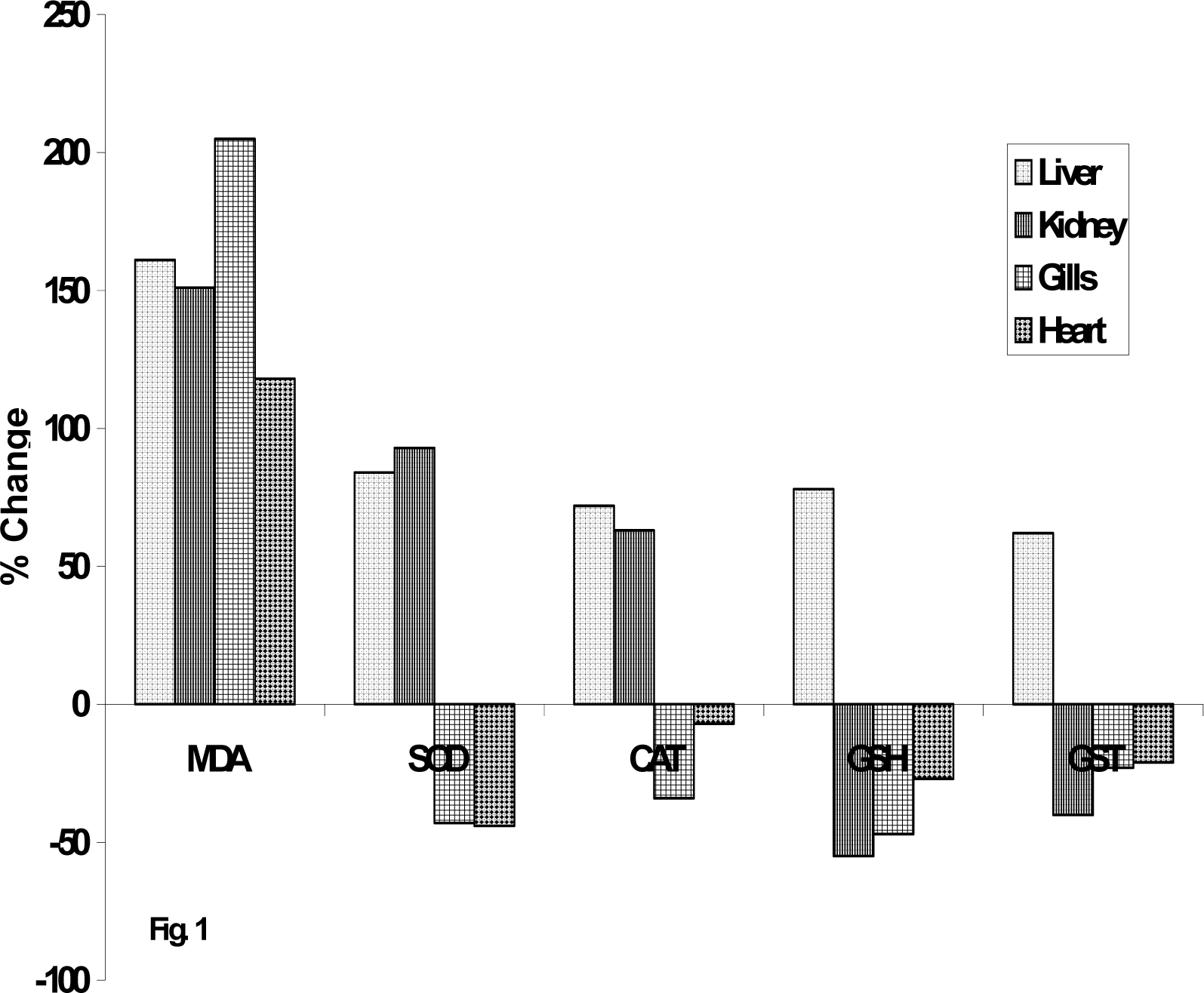
Results and Discussion
Conclusion
| Groups | Liver | Kidney | Gills | Heart |
|---|---|---|---|---|
| Control | 0.84± 0.09 | 0.86 ± 0.07 | 0.39 ± 0.11 | 0.68±0.17 |
| 1ppm Butachlor | 1.57±0.10* | 1.44±0.16* | 0.69±0.08* | 0.86±0.12* |
| 2ppm Butachlor | 1.97±0.07** | 2.06±0.03** | 0.95±0.18** | 1.29±0.12** |
| 2.5ppm Butachlor | 2.19±0.03** | 2.16±0.02** | 1.19±0.08** | 1.48±0.11** |
| Groups | Liver | Kidney | Gills | Heart |
|---|---|---|---|---|
| Control | 23.2±2.0 | 20.6±2.7 | 25.1 ± 2.4 | 18.0 ± 1.2 |
| 1ppm Butachlor | 27.4±1.8* | 25.3±2.1* | 23.6 ± 3.5§ | 16.8± 3.9§ |
| 2ppm Butachlor | 35.5±2.3** | 30.2±1.8** | 17.9±1.9** | 12.9± 2.0* |
| 2.5ppm Butachlor | 42.6±2.2** | 39.8±2.2** | 14.2±2.1** | 10.1± 2.7* |
| Groups | Liver | Kidney | Gills | Heart |
|---|---|---|---|---|
| Control | 100.2±2.4 | 106.4±1.1 | 110.0±2.7 | 92.1±3.0 |
| 1ppm Butachlor | 127.8±2.0* | 117.7±2.2* | 97.4±3.4§ | 90.0±3.7§ |
| 2ppm Butachlor | 164.3±1.9** | 167.2±1.5** | 85.0±2.3* | 88.3±4.6§ |
| 2.5ppm Butachlor | 172.6±1.4** | 174.2±2.0** | 72.4±1.1* | 85.5±3.7§ |
| Groups | Liver | Kidney | Gills | Heart |
|---|---|---|---|---|
| Control | 7.2 ± 0.4 | 5.6 ± 1.0 | 4.7 ± 0.5 | 5.9 ± 1.7 |
| 1ppm Butachlor | 9.2 ± 0.9* | 4.5 ± 1.5§ | 4.3 ± 1.8§ | 5.6 ± 1.4§ |
| 2ppm Butachlor | 11.6±0.8** | 2.9±0.3** | 2.6 ± 0.2** | 4.3 ± 2.3§ |
| 2.5ppm Butachlor | 12.8±0.6** | 2.5±0.5** | 2.5±0.3** | 4.1± 0.4* |
| Groups | Liver | Kidney | Gills | Heart |
|---|---|---|---|---|
| Control | 122.4±2.2 | 120.3±3.3 | 101 ± 3.6 | 117.5±3.1 |
| 1ppm Butachlor | 172.3±3.5** | 115.1±2.4§ | 96± 5.3§ | 115.3±2.2§ |
| 2ppm Butachlor | 188.3±2.6** | 77.1± 2.5** | 82±2.5** | 100.3±4.9§ |
| 2.5ppm Butachlor | 197.1±2.3** | 72.5± 1.7** | 78±2.4** | 92.8± 1.0* |
References
- Ateeq, B; Abul Farah, M; Ahmad, W. Detection of DNA damage by alkaline single cell gel electrophoresis in 2,4-dichlorophenoxyacetic-acid-and butachlor-exposed erythrocytes of Clarias batrachus. Ecotoxicol Environ Saf. 2005, 62, 348–54. [Google Scholar]
- Ateeq, B; Abul farah, M; Niamat Ali, M; Ahmad, W. Induction of micronuclei and erythrocyte alterations in the catfish Clarias batrachus by 2,4-dichlorophenoxyacetic acid and butachlor. Mutat Res 2002, 518, 135–44. [Google Scholar]
- Bebianno, MJ; Geret, F; Hoarau, P; Serafim, MA; Coelho, MR; Gnassia-Barelli, M; Romeo, M. Biomarkers in Ruditapes decussatus: a potential bioindicator species. Biomarkers 2004, 9, 305–30. [Google Scholar]
- Buege, JA; Aust, SD. Microsomal lipid peroxidation. Methods Enzymol 1978, 52, 302–10. [Google Scholar]
- Chandra, J; Samali, A; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000, 29, 323–33. [Google Scholar]
- Clairborne, A. activity. In Handbook of methods for oxygen Radical Research; CRC Press: Florida, 1995. [Google Scholar]
- Coleman, S; Linderman, R; Hodgson, E; Rose, RL. Comparative metabolism of chloroacetamide herbicides and selected metabolites in human and rat liver microsomes. Environ Health Perspect 2000, 108, 1151–7. [Google Scholar]
- Dautremepuits, C; Paris-Palacios, S; Betoulle, S; Vernet, G. Modulation in hepatic and head kidney parameters of carp (Cyprinus carpio L.) induced by copper and chitosan. Comp Biochem Physiol C Toxicol Pharmacol 2004, 137, 325–33. [Google Scholar]
- Dearfield, KL; McCarroll, NE; Protzel, A; Stack, HF; Jackson, MA; Waters, MD. A survey of EPA/OPP and open literature on selected pesticide chemicals. II. Mutagenicity and carcinogenicity of selected chloroacetanilides and related compounds. Mutat Res 1999, 443, 183–221. [Google Scholar]
- Geng, BR; Yao, D; Xue, QQ. Acute toxicity of the pesticide dichlorvos and the herbicide butachlor to tadpoles of four anuran species. Bull Environ Contam Toxicol 2005, 75, 343–9. [Google Scholar]
- Hsu, KY; Lin, HJ; Lin, JK; Kuo, WS; Ou, YH. Mutagenicity study of butachlor and its metabolites using Salmonella typhimurium. J Microbiol Immunol Infect 2005, 38, 409–16. [Google Scholar]
- Lash, LH; Putt, DA; Hueni, SE; Horwitz, BP. Molecular markers of trichloroethylene-induced toxicity in human kidney cells. Toxicol Appl Pharmacol 2005, 206, 157–68. [Google Scholar]
- Lopes, PA; Pinheiro, T; Santos, MC; da Luz Mathias, M; Collares-Pereira, MJ; Viegas-Crespo, AM. Response of antioxidant enzymes in freshwater fish populations (Leuciscus alburnoides complex) to inorganic pollutants exposure. Sci Total Environ 2001, 280, 153–63. [Google Scholar]
- Natarajan, AT. An overview of the results of testing of known or suspected aneugens using mammalian cells in vitro. Mutat Res 1993, 287, 113–8. [Google Scholar]
- Olaifa, FG; Olaifa, A; Onwude, T. Lethal and sub lethal effects of copper to the African Cat fish (Clarias garienpinus). Afr. J. Biomed. Res 2004, 7, 65–70. [Google Scholar]
- Ou, YH; Chung, PC; Chang, YC; Ngo, FQ; Hsu, KY; Chen, FD. Butachlor, a suspected carcinogen, alters growth and transformation characteristics of mouse liver cells. Chem Res Toxicol 2000, 13, 1321–5. [Google Scholar]
- Ou, YH; Lin, JK. Biotransformation of butachlor through mercapturic acid pathway in rat tissue homogenates. J. Toxicol Environ Health. 1992, 35, 19–28. [Google Scholar]
- Pandey, S; Ahmad, I; Parvez, S; Bin-Hafeez, B; Haque, R; Raisuddin, S. Effect of endosulfan on antioxidants of freshwater fish Channa punctatus Bloch: 1. Protection against lipid peroxidation in liver by copper preexposure. Arch Environ Contam Toxicol 2001, 41, 345–52. [Google Scholar]
- Pandey, S; Parvez, S; Sayeed, I; Haque, R; Bin-Hafeez, B; Raisuddin, S. Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 2003, 309, 105–15. [Google Scholar]
- Shen, HM; Liu, ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med 2006, 40, 928–39. [Google Scholar]
- Thake, DC; Iatropoulos, MJ; Hard, GC; Hotz, KJ; Wang, CX; Williams, GM; Wilson, AG. A study of the mechanism of butachlor-associated gastric neoplasms in Sprague-Dawley rats. Exp Toxicol Pathol 1995, 47, 107–16. [Google Scholar]
- Tjalkens, RB; Valerio, LG, Jr; Awasthi, YC; Petersen, DR. Association of glutathione S-transferase isozyme-specific induction and lipid peroxidation in two inbred strains of mice subjected to chronic dietary iron overload. Toxicol Appl Pharmacol 1998, 151, 174–81. [Google Scholar]
- Ueda, S; Masutani, H; Nakamura, H; Tanaka, T; Ueno, M; Yodoi, J. Redox control of cell death. Antioxid Redox Signal 2002, 4, 405–14. [Google Scholar]
- Uner, N; Oruc, EO; Canli, M; Sevgiler, Y. Effects of cypermethrin on antioxidant enzyme activities and lipid peroxidation in liver and kidney of the freshwater fish, Oreochromis niloticus and Cyprinus carpio (L.). Bull Environ Contam Toxicol 2001, 67, 657–64. [Google Scholar]
© 2008 MDPI All rights reserved.
Share and Cite
Farombi, E.O.; Ajimoko, Y.R.; Adelowo, O.A. Effect of Butachlor on Antioxidant Enzyme Status and Lipid Peroxidation in Fresh Water African Catfish, (Clarias gariepinus). Int. J. Environ. Res. Public Health 2008, 5, 423-427. https://doi.org/10.3390/ijerph5050423
Farombi EO, Ajimoko YR, Adelowo OA. Effect of Butachlor on Antioxidant Enzyme Status and Lipid Peroxidation in Fresh Water African Catfish, (Clarias gariepinus). International Journal of Environmental Research and Public Health. 2008; 5(5):423-427. https://doi.org/10.3390/ijerph5050423
Chicago/Turabian StyleFarombi, E. O., Y. R. Ajimoko, and O. A. Adelowo. 2008. "Effect of Butachlor on Antioxidant Enzyme Status and Lipid Peroxidation in Fresh Water African Catfish, (Clarias gariepinus)" International Journal of Environmental Research and Public Health 5, no. 5: 423-427. https://doi.org/10.3390/ijerph5050423
APA StyleFarombi, E. O., Ajimoko, Y. R., & Adelowo, O. A. (2008). Effect of Butachlor on Antioxidant Enzyme Status and Lipid Peroxidation in Fresh Water African Catfish, (Clarias gariepinus). International Journal of Environmental Research and Public Health, 5(5), 423-427. https://doi.org/10.3390/ijerph5050423




