Vaccine Dispensing in a Section of the Private Healthcare Sector in South Africa (2017–2021)
Abstract
1. Introduction
- https://knowledgehub.health.gov.za/system/files/2024-05/VACCINE%20HESITANCY%20WEBINAR_OVERVIEW%20OF%20EPI_MAY_2024_FINAL.pdf (accessed on 15 August 2025) (government/public sector), and
- https://www.amayeza-info.co.za/wp-content/uploads/2025/03/2.-2025-Childhood-vaccine-scheduleN.pdf (accessed on 15 August 2025) (private sector).
- Birth: Bacillus Calmette–Guérin (BCG), Oral Polio Vaccine (OPV-0);
- 6 weeks: Oral Polio Vaccine (OPV-1), Rotavirus Vaccine (RV-1), Pneumococcal Conjugate Vaccine (PCV-1), Hexavalent Vaccine (DTaP-IPV-HBV-Hib)-1;
- 10 weeks: Hexavalent Vaccine (DTaP-IPV-HBV-Hib)-2;
- 14 weeks: Rotavirus Vaccine (RV-2), Pneumococcal Conjugate Vaccine (PCV-2), Hexavalent Vaccine (DTaP-IPV-HBV-Hib)-3;
- 6 months: Measles-Rubella Vaccine (MR-1);
- 9 months: Pneumococcal Conjugate Vaccine (PCV-3);
- 12 months: Measles-Rubella Vaccine (MR-2);
- 18 months: Hexavalent Vaccine (DTaP-IPV-HBV-Hib)-4;
- 6 years: Tetanus diphtheria, acellular Pertussis Vaccine (TdaP-1);
- 9–14 years: Human Papilloma Virus Vaccine (HPV) 1 + 2;
- 12 years: Tetanus diphtheria, acellular Pertussis Vaccine (TdaP-2).
- 6 months: Influenza vaccine (seasonal);
- 9 months: Meningococcal vaccine (Men-A,C,Y,W–1), Pneumococcal Conjugate Vaccine (PCV) booster;
- 12 months: Measles, Mumps, Rubella (MMR), Varicella (chickenpox), Hep A (dose 1), Men-A,C,Y,W-2);
- 18 months: Hexavalent booster, Hep A (dose 2);
- 5–6 years: Diphtheria, Tetanus, acellular pertussis, and Polio vaccine, MMR booster, Varicella booster.
- To identify the general trends in vaccine claims;
- To determine the demographic distributions;
- To determine the geographical variations;
- To report on the vaccine types dispensed, with a particular focus on the impact of the COVID-19 pandemic on vaccine uptake and dispensing settings.
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Sources
2.4. Data Extraction and Analysis
2.5. Ethical Considerations
3. Results
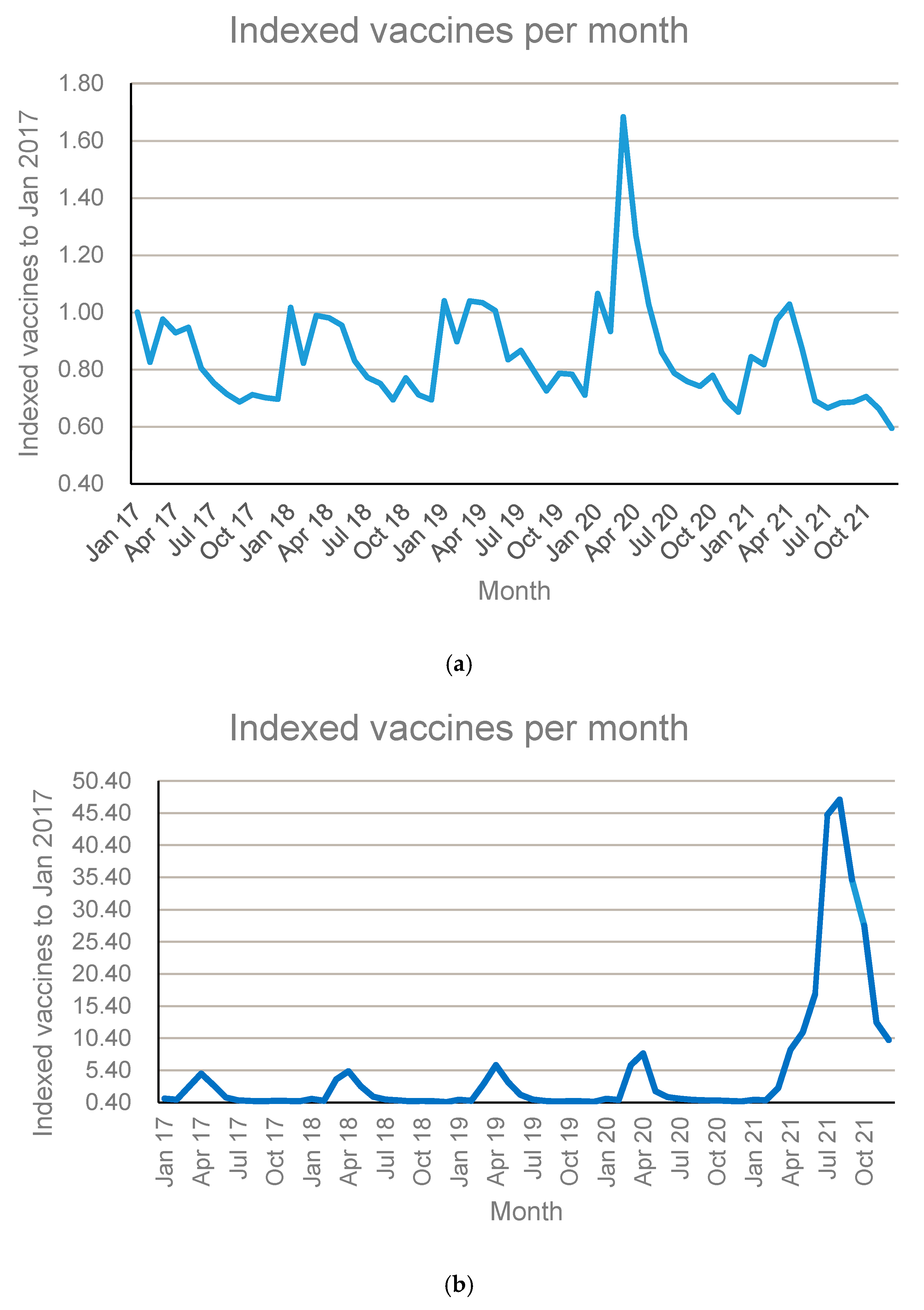
3.1. General Overview of Members and Vaccine Dispensing
3.2. Demographic Information of Patients
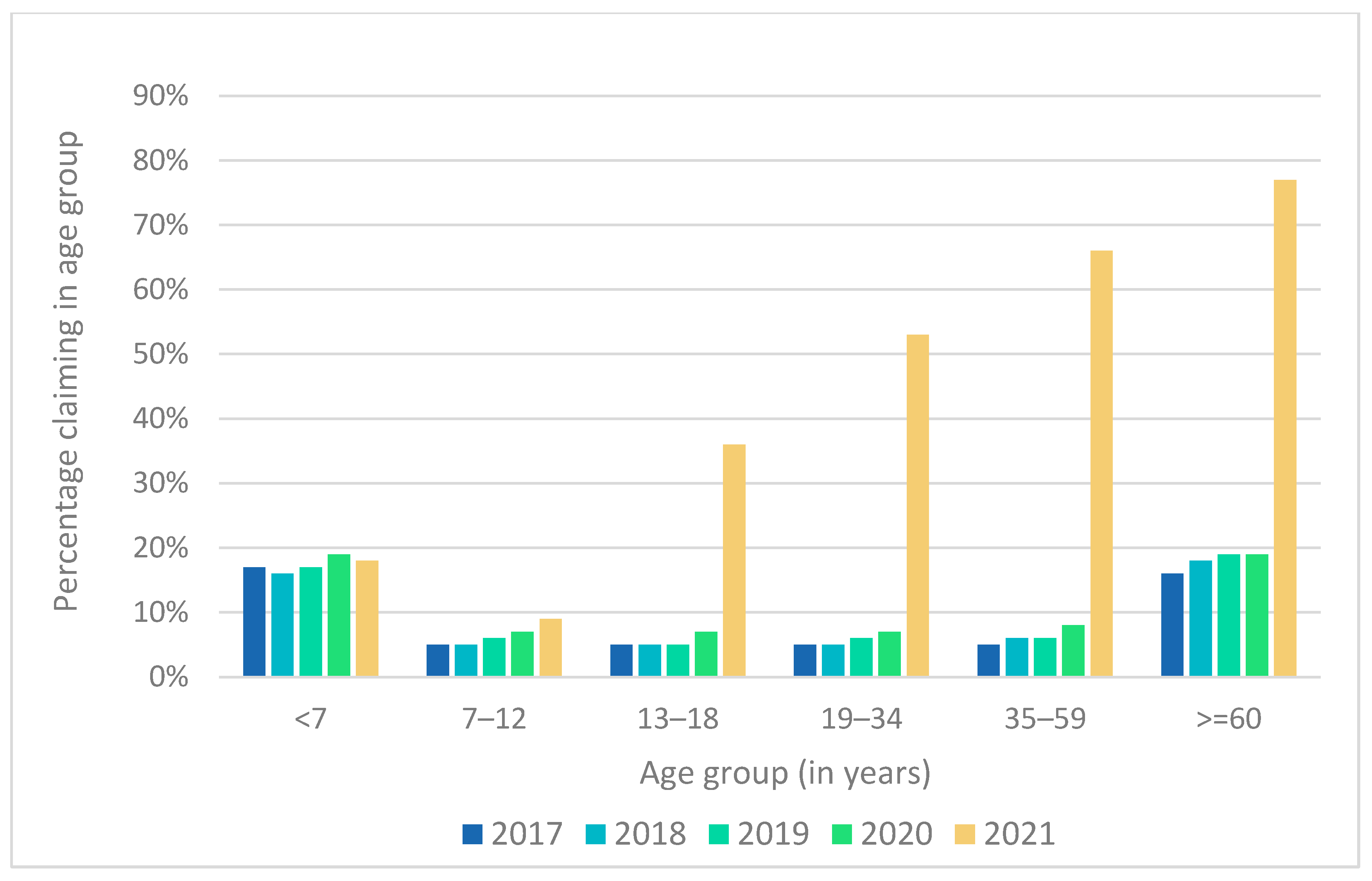
3.3. Vaccines Dispensed in the Different Age Groups
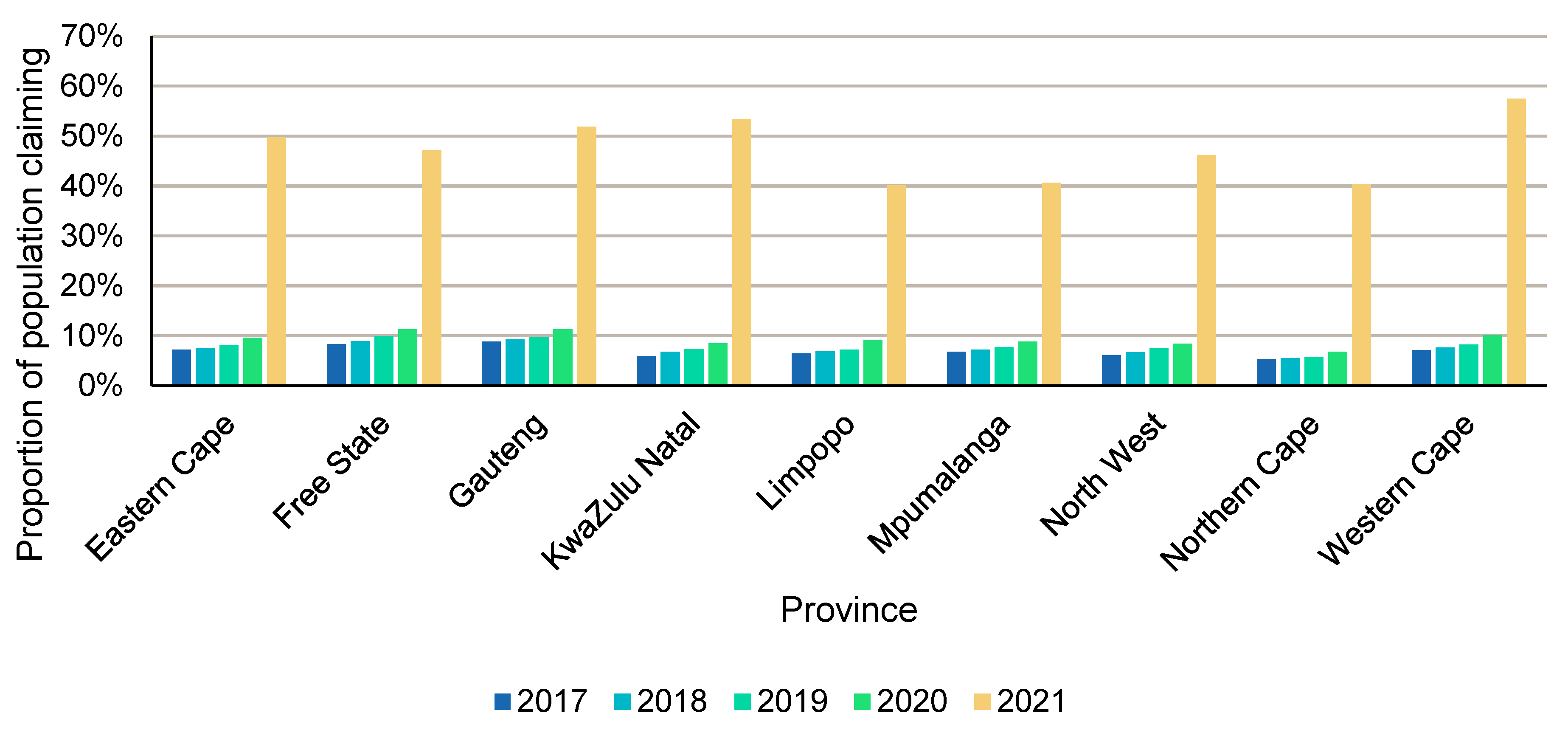
3.4. Dispensing Patterns According to Provinces
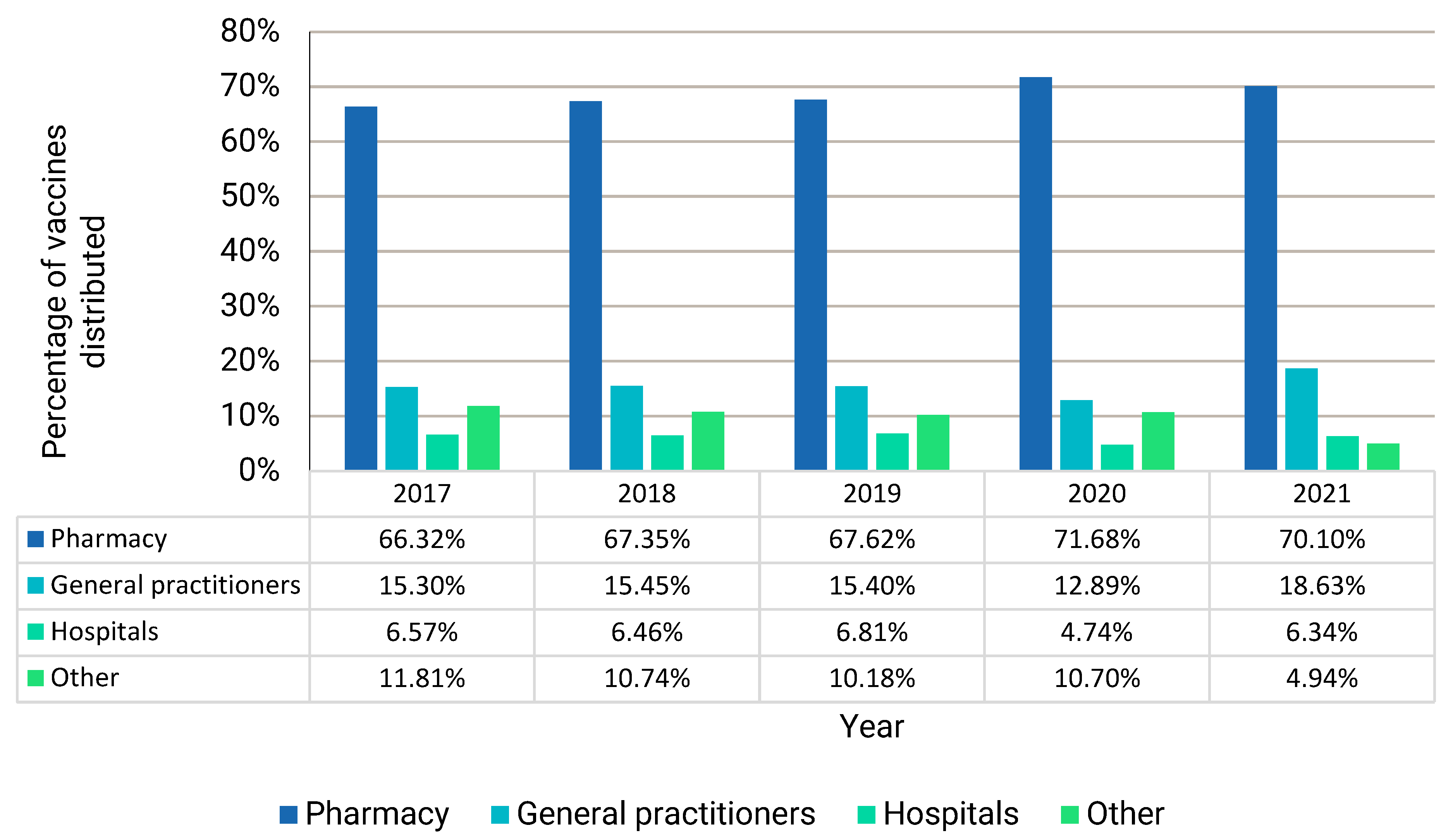
3.5. Dispensing of Specific Vaccines According to the ATC Classification System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATC | Anatomical Therapeutic Chemical |
| BCG | Bacillus Calmette–Guérin |
| COVID-19 | Coronavirus disease 2019 |
| CPT | Current Procedural Terminology |
| DTP | Diphtheria, tetanus, and pertussis |
| EPI | Expanded Programme on Immunisation |
| EPI-SA | Expanded Programme on Immunisation in South Africa |
| GAVP | Global Vaccine Action Plan |
| HPV | Human papillomavirus |
| ICD-10 | 10th revision of the International Statistical Classification of Diseases and Related Health Problems |
| LMIC | Low- to middle-income countries |
| NAGI | National Advisory Group on Immunisation |
| NDoH | National Department of Health |
| PCV | Pneumococcal vaccine |
| SQL | Structured Query Language |
| WHO | World Health Organization |
References
- Remy, V.; Zöllner, Y.; Heckmann, U. Vaccination: The cornerstone of an efficient healthcare system. J. Mark. Access Health Policy 2015, 3, 27041. [Google Scholar] [CrossRef]
- Nnaji, C.A.; Wiysonge, C.S.; Lesosky, M.; Mahomed, H.; Ndwandwe, D. COVID-19 and the gaping wounds of South Africa’s suboptimal immunisation coverage: An implementation research imperative for assessing and addressing missed opportunities for vaccination. Vaccines 2021, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Wiysonge, C.S.; Ngcobo, N.J.; Jeena, P.M.; Madhi, S.A.; Schoub, B.D.; Hawkridge, A.; Shey, M.S.; Hussey, G.D. Advances in childhood immunisation in South Africa: Where to now? Programme managers’ views and evidence from systematic reviews. BMC Public Health 2012, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Majiet, M.I.; Haddison, E.C.; Amponsah-Dacosta, E.; Hussey, G.D.; Muloiwa, R.; Kagina, B.M. Human vaccine and immunisation research in South Africa: A bibliometric study. S. Afr. Med. J. 2021, 111, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.R.; Maja, P. The Expanded Programme on Immunisation in South Africa: A story yet to be told. S. Afr. Med. J. 2016, 106, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.J.; Dlamini, N.; Meyer, J.C.; Fernandes, L.; Motloung, B.R.; Ndlovu, T.H.; Simango, H.A.; Kibuuka, D.K.; Dochez, C.; Montwedi, D.N.; et al. Progress towards obtaining valid vaccination coverage data in South Africa. S. Afr. J. Sci. 2019, 115, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Naseem, R.; Salam, R.A.; Siddiqui, F.; Das, J.K. The impact of the COVID-19 pandemic on immunization campaigns and programs: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 988. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L. What Is Pharmacoepidemiology? In Pharmacoepidemiology, 6th ed.; Strom, B.L., Kimmel, S.E., Hennessy, S., Eds.; wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- ATC/DDD Index 2023. Oslo: WHO Collaborating Centre for Drug Statistics Methodology. Available online: https://atcddd.fhi.no/ (accessed on 26 July 2023).
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision, 5th ed.; World Health Organization: Geneva, Switzerland, 2016; Available online: https://apps.who.int/iris/handle/10665/246208 (accessed on 4 August 2025).
- Dotson, P. CPT® Codes: What are they, why are they necessary, and how are they developed? Adv. Wound Care 2013, 2, 583–587. [Google Scholar] [CrossRef] [PubMed]
- NAPPI®. Sandton: MediKredit. 2023. Available online: https://www.medikredit.co.za/products-and-services/nappi/ (accessed on 4 August 2025).
- South African Government. No. 4 of 2013: Protection of Personal Information Act, 2013; Government Gazette Vol. 581 No. 37067; South African Government: Cape Town, South Africa, 2013. [Google Scholar]
- COVID-19 Vaccination Messaging Guideline. Government Communication and Information System, National Department of Health. 2023. Available online: https://www.gcis.gov.za/vaccine-guideline (accessed on 30 July 2025).
- Statistics South Africa (Stats SA). Mid-Year Population Estimates 2019. Statistical Release P0302; Republic of South Africa, Department of Statistics: Pretoria, South Africa, 2019. [Google Scholar]
- Dzinamarira, T.; Nachipo, B.; Phiri, B.; Musuka, G. COVID-19 vaccine roll-out in South Africa and Zimbabwe: Urgent need to address community preparedness, fears and hesitancy. Vaccines 2021, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Truter, I. Exploratory study of the dispensing patterns of vaccines by a sample of community pharmacies in Southern Africa. Exp. Rev. Vaccines 2018, 17, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Ser, J.; Sim, U.; Cho, H. Promising expectations for pneumococcal vaccination during COVID-19. Vaccines 2021, 9, 1507. [Google Scholar] [CrossRef] [PubMed]
- Phasha, M. Foreword by the President of Council. In South African Pharmacy Council 2021 Annual Report; South African Pharmacy Council: Pretoria, South Africa, 2021; Available online: https://static.pmg.org.za/AR2021_web_version_2.pdf (accessed on 3 July 2025).

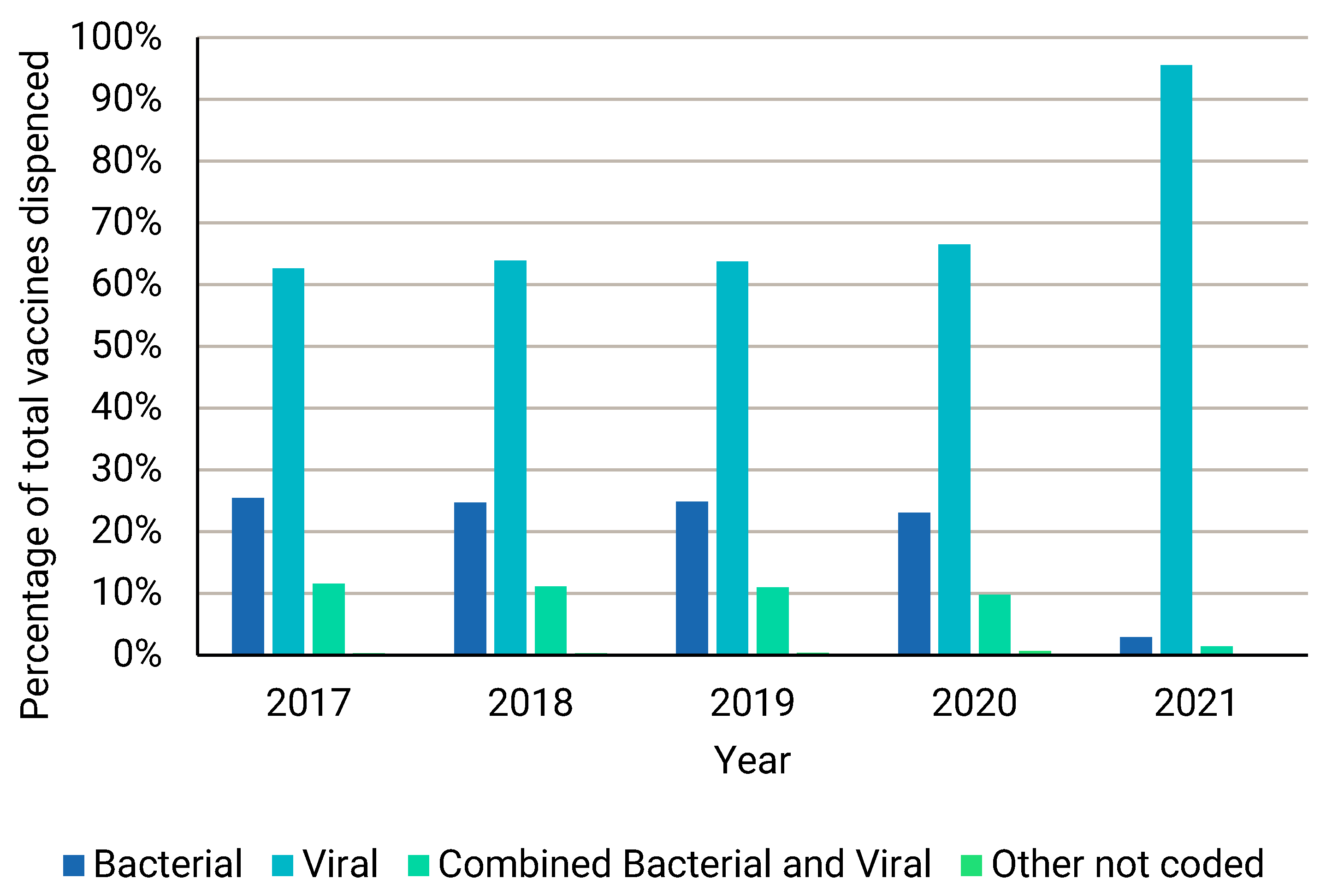
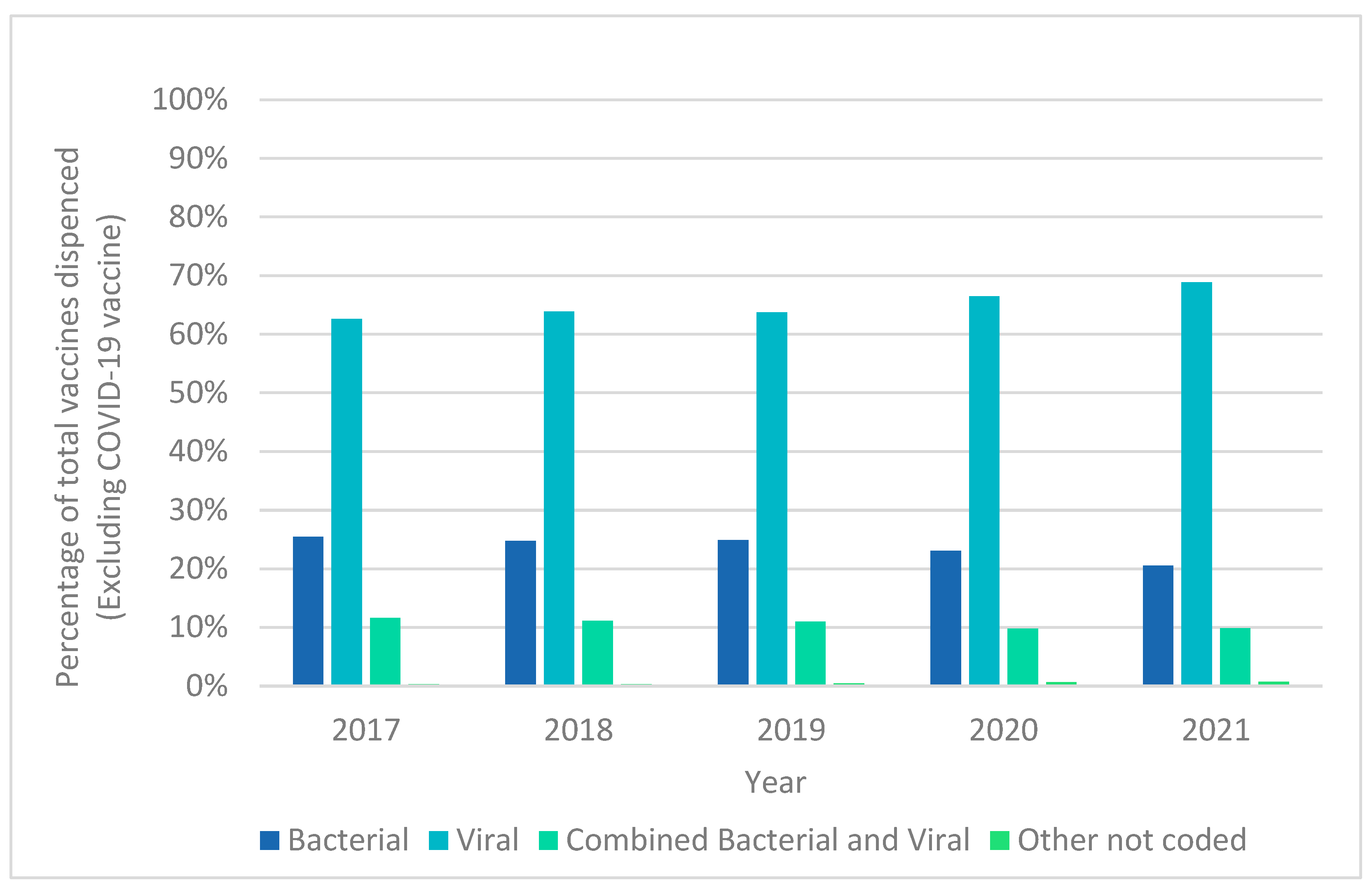
| Year | Total Vaccines | Total Vaccine Patients | Total Vaccine Families |
|---|---|---|---|
| 2017 | 464,766 | 293,464 | 221,213 |
| 2018 | 489,858 | 319,758 | 238,850 |
| 2019 | 512,799 | 339,863 | 253,128 |
| 2020 | 592,169 | 393,496 | 268,129 |
| 2021 | 3,831,794 | 1,980,538 | 1,270,080 |
| Baseline Information | Year * | ||||
|---|---|---|---|---|---|
| 2017 * | 2018 | 2019 | 2020 | 2021 | |
| Total vaccine doses claimed | 1.00 | 1.05 | 1.10 | 1.27 | 8.24 |
| Total vaccine patients | 1.00 | 1.09 | 1.16 | 1.34 | 6.75 |
| Total vaccine claiming families | 1.00 | 1.08 | 1.14 | 1.21 | 5.74 |
| Total scheme members | 1.00 | 1.02 | 1.03 | 1.02 | 1.02 |
| Total scheme families | 1.00 | 1.03 | 1.04 | 1.03 | 1.06 |
| Year | Proportion of Scheme Members Who Claimed for Vaccines | Proportion of Scheme Families Who Claimed for Vaccines |
|---|---|---|
| 2017 | 7.89% | 12.37% |
| 2018 | 8.41% | 13.01% |
| 2019 | 8.86% | 13.61% |
| 2020 | 10.40% | 14.58% |
| 2021 | 52.04% | 66.96% |
| Patient and Member Proportions | Years | ||||
|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | |
| Proportion of patients claiming for vaccines | |||||
| Females | 0.53 | 0.53 | 0.53 | 0.54 | 0.53 |
| Males | 0.47 | 0.47 | 0.47 | 0.46 | 0.47 |
| Both genders | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Proportion of total scheme members overall | |||||
| Females | 0.52 | 0.52 | 0.52 | 0.52 | 0.52 |
| Males | 0.48 | 0.48 | 0.48 | 0.48 | 0.48 |
| Both genders | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Statistics on Ages of Patients Claiming for Vaccines | Years | ||||
|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | |
| Average age (in years) | |||||
| Females | 36.52 | 38.23 | 38.63 | 38.35 | 43.45 |
| Males | 34.45 | 36.47 | 37.05 | 36.72 | 42.64 |
| Both genders | 35.54 | 37.40 | 37.89 | 37.60 | 43.07 |
| Standard deviation of age (in years) | |||||
| Females | 27.48 | 27.03 | 26.95 | 25.77 | 19.49 |
| Males | 27.57 | 27.26 | 27.44 | 26.86 | 19.16 |
| Both genders | 27.54 | 27.16 | 27.19 | 26.29 | 19.34 |
| Median age (in years) | |||||
| Females | 35.00 | 37.00 | 37.00 | 38.00 | 42.00 |
| Males | 33.00 | 36.00 | 37.00 | 38.00 | 42.00 |
| Both genders | 34.00 | 37.00 | 37.00 | 38.00 | 42.00 |
| Vaccine Type | ATC Code | ATC Description | YEAR | |||||
|---|---|---|---|---|---|---|---|---|
| 2017 (n = 464,766) | 2018 (n = 489,858) | 2019 (n = 512,799) | 2020 (n = 592,169) | 2021 (n = 3,831,794) | Average | |||
| J07A—Bacterial vaccines | ||||||||
| Cholera vaccines | J07AE01 | Cholera, inactivated, whole cell | 0.01 | 0.05 | 0.04 | 0.00 | 0.00 | 0.02 |
| Hemophilus influenzae B vaccines | J07AG01 | Hemophilus influenzae B, purified-antigen-conjugated | 0.07 | 0.10 | 0.11 | 0.03 | 0.00 | 0.06 |
| Meningococcal vaccines | J07AH03 | Meningococcus A,C, bivalent purified polysaccharides antigen | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 |
| J07AH08 | Meningococcus A,C,Y,W-135, tetravalent-purified-polysaccharide-antigen-conjugated | 5.27 | 5.59 | 5.96 | 5.09 | 0.73 | 4.53 | |
| Pertussis vaccines | J07AJ52 | Pertussis, purified antigen, combinations with toxoids | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 |
| Pneumococcal vaccines | J07AL01 | Pneumococcus, purified polysaccharides antigen | 1.04 | 1.09 | 1.22 | 1.26 | 0.16 | 0.95 |
| J07AL02 | Pneumococcus, purified-polysaccharide-antigen-conjugated | 7.61 | 6.98 | 6.67 | 8.84 | 0.87 | 6.19 | |
| Tetanus vaccines | J07AM01 | Tetanus toxoid | 10.34 | 9.77 | 9.48 | 6.94 | 1.03 | 7.51 |
| J07AM51 | Tetanus toxoid, combinations with diphtheria toxoid | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | |
| Tuberculosis vaccines | J07AN01 | Tuberculosis, live-attenuated (BCG vaccine) | 0.43 | 0.50 | 0.77 | 0.75 | 0.10 | 0.51 |
| Typhoid vaccines | J07AP03 | Typhoid, purified polysaccharide antigen | 0.66 | 0.64 | 0.62 | 0.14 | 0.02 | 0.41 |
| J07B—Viral vaccines | ||||||||
| Influenza vaccines | J07BB02 | Influenza, inactivated, split virus or surface antigen | 32.94 | 38.13 | 39.70 | 44.45 | 6.51 | 32.35 |
| Hepatitis vaccines | J07BC01 | Hepatitis B, purified antigen | 1.02 | 0.92 | 1.12 | 0.80 | 0.10 | 0.79 |
| J07BC02 | Hepatitis A, inactivated, whole virus | 3.25 | 2.49 | 0.40 | 4.24 | 0.70 | 2.22 | |
| J07BC20 | Hepatitis vaccines, combinations | 0.97 | 1.02 | 1.33 | 0.35 | 0.04 | 0.74 | |
| Measles vaccines | J07BD01 | Measles, live-attenuated | 2.64 | 1.70 | 1.27 | 0.70 | 0.11 | 1.28 |
| J07BD52 | Measles, combinations with mumps and rubella, live-attenuated | 5.65 | 6.14 | 6.26 | 5.63 | 0.81 | 4.90 | |
| J07BD54 | Measles, combinations with mumps, rubella, and varicella, live-attenuated | 2.14 | 0.10 | 0.03 | 0.03 | 0.01 | 0.46 | |
| Poliomyelitis vaccines | J07BF03 | Poliomyelitis, trivalent, inactivated, whole virus | 0.16 | 0.12 | 0.13 | 0.12 | 0.01 | 0.11 |
| J07BF04 | Poliomyelitis oral, bivalent, live-attenuated | 0.51 | 0.61 | 0.79 | 0.61 | 0.03 | 0.51 | |
| Rabies vaccines | J07BG01 | Rabies, inactivated, whole virus | 1.18 | 1.07 | 1.39 | 0.97 | 0.17 | 0.96 |
| Rotavirus diarrhoea vaccines | J07BH01 | Rotavirus, live-attenuated | 2.34 | 1.93 | 2.29 | 2.21 | 0.31 | 1.82 |
| J07BH02 | Rota virus, pentavalent, live, reassorted | 1.76 | 1.96 | 1.21 | 0.82 | 0.11 | 1.17 | |
| Rubella vaccines | J07BJ01 | Rubella, live-attenuated | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Varicella zoster vaccines | J07BK01 | Varicella, live-attenuated | 4.59 | 4.47 | 4.33 | 3.57 | 0.54 | 3.50 |
| J07BK03 | Zoster, purified antigen | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Yellow fever vaccines | J07BL01 | Yellow fever, live-attenuated | 0.99 | 1.10 | 1.04 | 0.21 | 0.04 | 0.68 |
| Papillomavirus vaccines | J07BM01 | Papillomavirus (human types 6, 11, 16, 18) | 2.47 | 2.11 | 2.44 | 1.78 | 0.35 | 1.83 |
| Other viral vaccines | J07BX03 | COVID-19 vaccines | 0.00 | 0.00 | 0.00 | 0.00 | 85.70 | 17.14 |
| J07C—Bacterial and viral vaccines, combined | ||||||||
| Bacterial and viral vaccines, combined | J07CA02 | Diphtheria–pertussis–poliomyelitis–tetanus | 3.06 | 3.75 | 3.93 | 3.48 | 0.51 | 2.95 |
| J07CA05 | Diphtheria–hepatitis B–pertussis–tetanus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| J07CA06 | Diphtheria–haemophilus influenzae B–pertussis–poliomyelitis–tetanus | 8.44 | 6.78 | 6.20 | 6.09 | 0.85 | 5.67 | |
| J07CA09 | Diphtheria–haemophilus influenzae B–pertussis–poliomyelitis–tetanus–hepatitis B | 0.09 | 0.59 | 0.82 | 0.20 | 0.05 | 0.35 | |
| J07CA10 | Typhoid–hepatitis A | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Other vaccines not coded | Other * | 0.33 | 0.27 | 0.41 | 0.68 | 0.12 | 0.36 | |
| TOTAL | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truter, I.; Hugo, J.; Smith, H.; Bansal, S.; Vira, A. Vaccine Dispensing in a Section of the Private Healthcare Sector in South Africa (2017–2021). Int. J. Environ. Res. Public Health 2025, 22, 1329. https://doi.org/10.3390/ijerph22091329
Truter I, Hugo J, Smith H, Bansal S, Vira A. Vaccine Dispensing in a Section of the Private Healthcare Sector in South Africa (2017–2021). International Journal of Environmental Research and Public Health. 2025; 22(9):1329. https://doi.org/10.3390/ijerph22091329
Chicago/Turabian StyleTruter, Ilse, Johan Hugo, Hank Smith, Shailav Bansal, and Alykhan Vira. 2025. "Vaccine Dispensing in a Section of the Private Healthcare Sector in South Africa (2017–2021)" International Journal of Environmental Research and Public Health 22, no. 9: 1329. https://doi.org/10.3390/ijerph22091329
APA StyleTruter, I., Hugo, J., Smith, H., Bansal, S., & Vira, A. (2025). Vaccine Dispensing in a Section of the Private Healthcare Sector in South Africa (2017–2021). International Journal of Environmental Research and Public Health, 22(9), 1329. https://doi.org/10.3390/ijerph22091329







