Neurodevelopmental Outcomes Associated with Early-Life Exposure to Heavy Metals: A Systematic Review
Abstract
1. Introduction
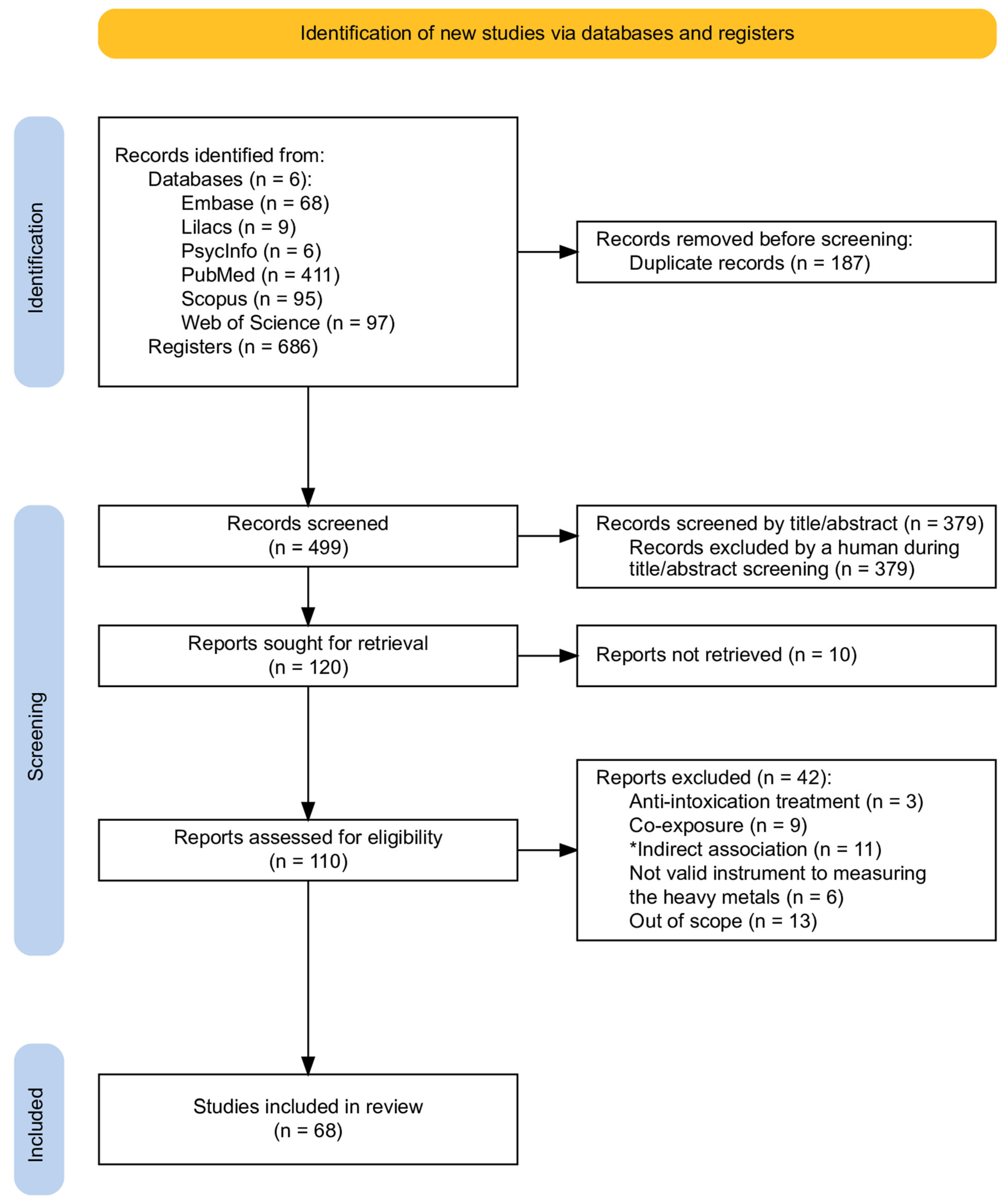
2. Method
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Screening Procedure
2.4. Data Extraction
2.5. Quality Assessment
3. Results
3.1. Characteristics of the Included Studies
3.2. Metal Analysis
3.3. Quality Assessment
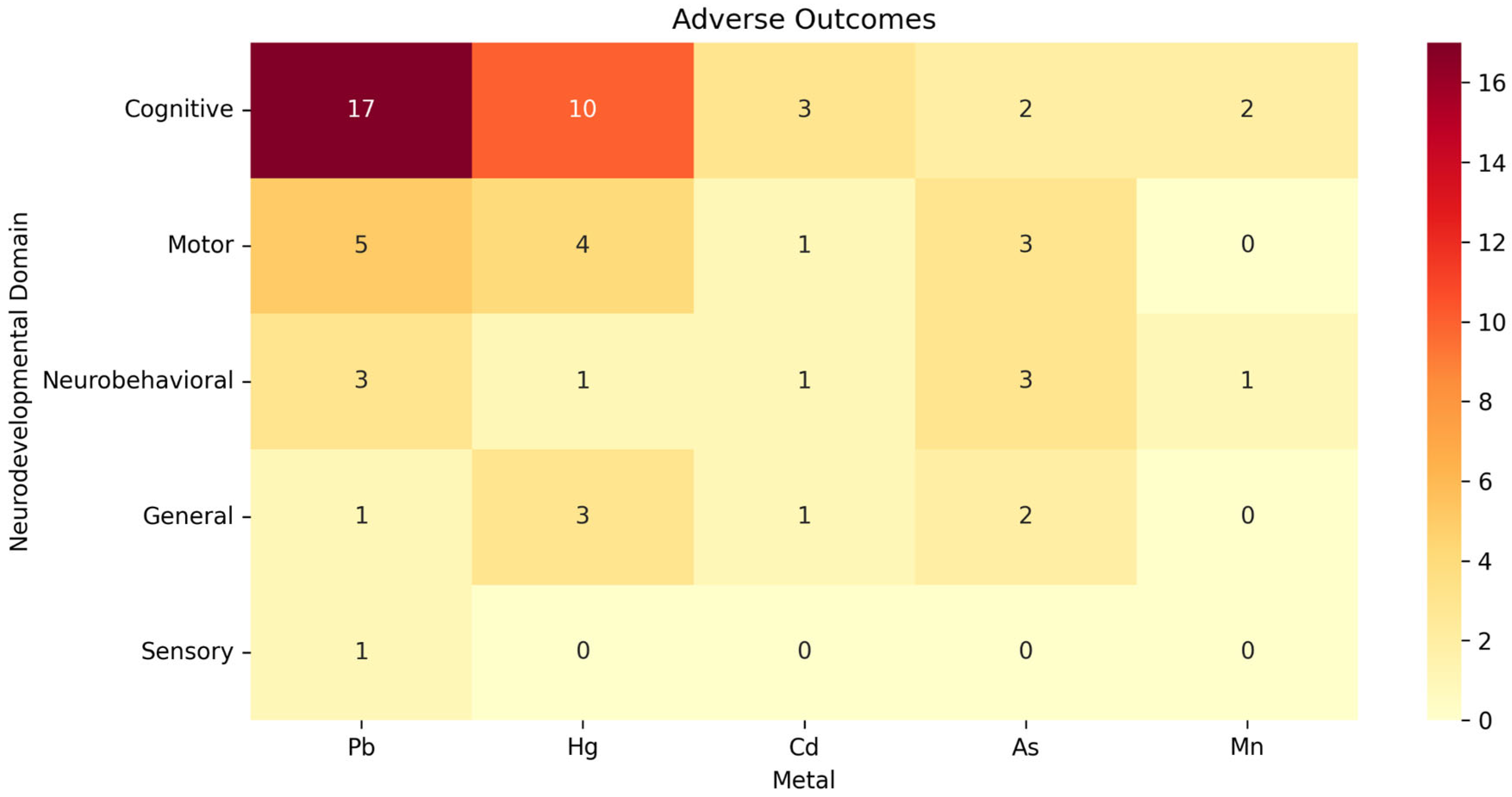
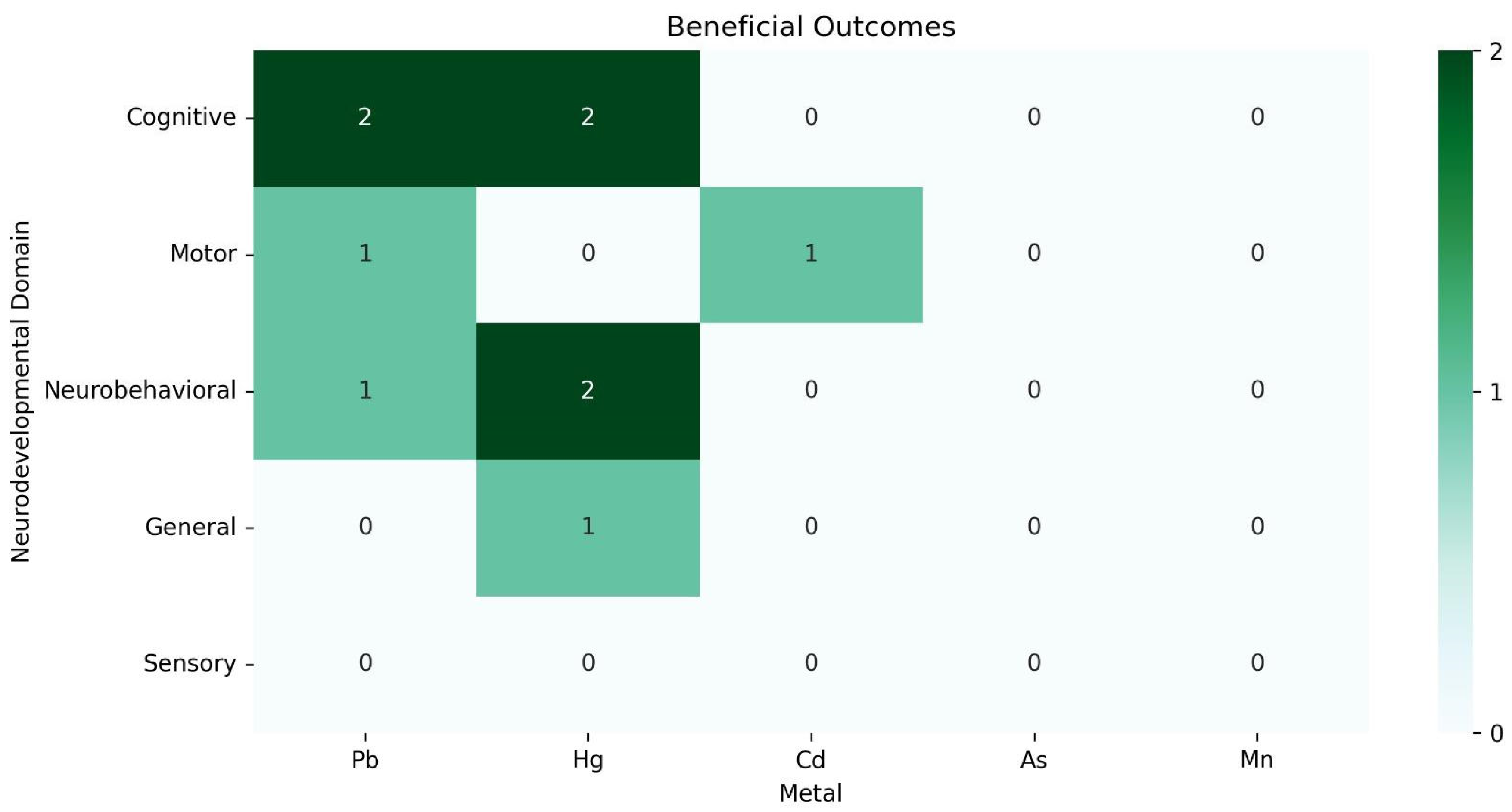
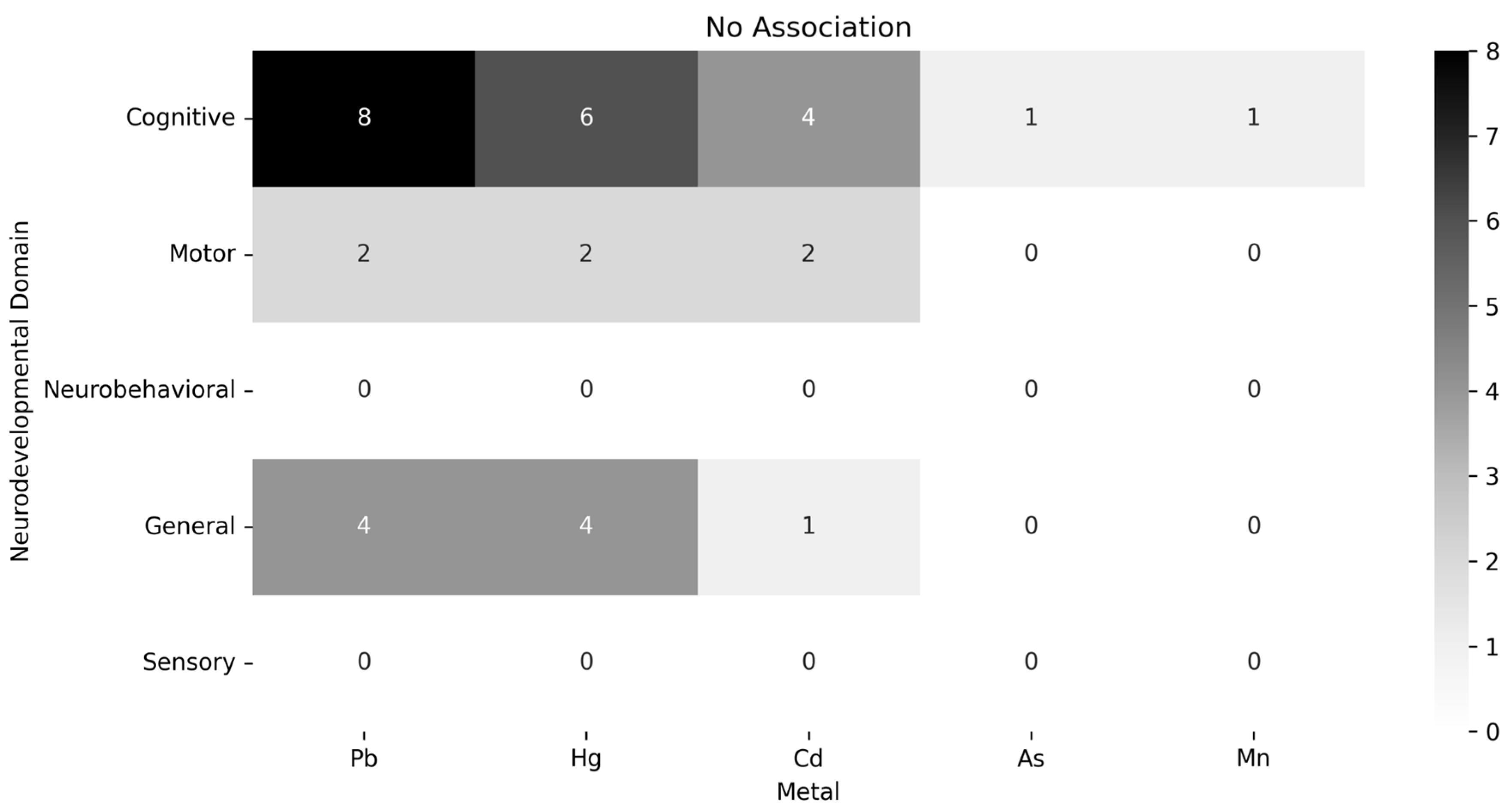
3.4. Main Associations
3.4.1. Associations with Small Effect Sizes
Hg and MeHg
Pb
As, Cd, and Mn
3.4.2. Associations with Medium Effect Sizes
3.4.3. Associations with Large Effect Sizes
| Author/Year/Country/Type Of Study | N|Age|Gender %|Neurodevelopmental Assessment | Bio Sample Type|Major Associations | Quality |
|---|---|---|---|
| Abd Wahil et al., 2022 Malaysia, Cross-sectional [19] | Typically Develop: 74 Age: 5.45 (0.83) years Males: 39 (52.70%) males ASD: 81 Age: 5.63 (0.60) years Males: 68 (84%) males Neurodevelopmental: ASD diagnoses by DSM-5 criteria and International Classification of Diseases-10 (ICD-10) | Bio sample: Child urine. Urinary Pb levels were significantly lower in children with ASD (mean 0.26 ± 0.31 μg/dL) compared to typically developing children (mean 0.58 ± 0.41 μg/dL) (p < 0.05). | *** |
| Al-Saleh(a) et al., 2016 Saudi Arabia, Cross-sectional [44] | N: 944 Age: 7.96 (2.68) months Gender: 487 (51.6%) males Neurodevelopmental: Denver Developmental Screening Test II (DDST-II) Parents’ Evaluation of Developmental Status (PEDS) | Bio sample: Maternal blood, milk and urine/Child hair, blood and urine. Neonatal hair and urinary Hg levels were positively associated with an increased risk of future learning problems (OR = 1.209, 95% CI = 1.001–1.460). Neonatal hair MeHg levels were positively associated with potential delays in overall development (OR = 1.193, 95% CI = 1.044–1.365). Neonatal hair and urinary Hg levels were positively associated with an increased risk of future behavioral problems by Parents Evaluation of Developmental Status scale (OR = 1.337, 95% CI = 1.012–1.767). | *** |
| Al-Saleh(b) et al., 2016 Saudi Arabia, Cross-sectional [47] | N: 944 Idade: 8.0 (2.7) months Gender: 487 (51.6%) males Neurodevelopmental: Denver Developmental Screening Test and II (DDST and DDST-II) Parents’ Evaluation of Developmental Status (PEDS) | Bio sample: Maternal and child Urine and hair/Maternal blood and milk. Maternal hair MeHg levels were positively associated with delayed development risk test outcomes (adjusted OR = 1.193, p = 0.01). | *** |
| Al-Saleh et al., 2020 Saudi Arabia, Longitudinal [64] | N: 82 Age: 6.81 (0.64) years Gender: 36 (43.9%) males Neurodevelopmental: Beery-Visual-Motor Integration (Beery VMI) Test Of Non-Verbal Intelligence (TONI) | Bio sample: Maternal blood, hair and urine/Child hair and urine. Urinary Hg levels in infants during breastfeeding were associated with lower visual-motor integration (VMI) scores (ß = −0.469, 95% CI = −7.541, −2.13). Urinary Hg levels in infants during breastfeeding were associated with lower non-verbal intelligence scores (ß = −0.359, 95% CI = −5.857, −0.845). No correlation was found between neurodevelopmental scores and levels of MeHg in hair, Hg in urine, or Pb in urine among children during follow-up. | ** |
| Boucher et al., 2010 Canada, Longitudinal [69] | N: 574 Age: 3–6 years Gender: 305 (53.13%) males Neurodevelopmental: Event-related potentials (ERPs) Electro-oculogram (EOG) | Bio sample: Umbilical cord and venous blood. Umbilical cord blood MeHg concentrations were not associated with children’s neurodevelopmental parameters, in the follow-up. | *** |
| Cai et al., 2019 China, Cross-sectional [56] | N: 48 Age: At birth-6 months Gender: 31 (62%) males Neurodevelopmental: Sensory Processing Measure-Hong Kong Chinese version (SPM-HKC) | Bio sample: Child blood. Children’s blood Pb levels were positively associated with the total score of sensory processing dysfunction (r = 0.168, p < 0.01). | *** |
| de Assis Araújo et al., 2021 Brazil Longitudinal [70] | N: 118 Age: 11.3 (0.57) years Gender: 41 (34.7%) males Neurodevelopmental: Denver Developmental Screening Test II (DDST-II). | Bio sample: Maternal and umbilical cord blood. Maternal blood As levels were higher in the group of children with more failures on the global neurodevelopmental test (p = 0.03). | ** |
| Deroma et al., 2013 Italy, Longitudinal [71] | N: 154 Age: 7.7 (0.7) years Gender: 77 (50%) males Neurodevelopmental: Wechsler Intelligence Scale for Children (WISC-III) | Bio sample: Maternal hair and milk/Child hair. Total Hg or MeHg levels in hair were not associated with children’s IQ scores. | *** |
| Desrochers-Couture et al., 2018 Canada, Longitudinal [49] | N: 609 Age: 3.4 (0.3) years Gender: 297 (48.8%) males Neurodevelopmental: Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III) | Bio sample: Maternal and umbilical cord blood. Cord blood Pb levels were negatively correlated with IQ in boys (b = −3.28). Umbilical cord blood Pb levels showed no significant correlation with Performance IQ in girls (β = −3.28). | *** |
| Farías et al., 2022 Mexico, Longitudinal [72] | N: 253 Age: 1–12 months Gender: 132 (52.1%) males Neurodevelopmental: Bayley Scales of Infant Development (BSID-III) | Bio sample: Maternal blood Maternal blood Pb levels were negatively associated with offspring language development (p < 0.05). | ** |
| Freire et al., 2010 Spain, Cross-sectional [45] | N: 72 Age: 51 months Gender: 72 (100%) males Neurodevelopmental: McCarthy Scales of Children’s Abilities (MSCA) General Cognitive Score (GCS) | Bio sample: Child hair Children’s total hair Hg levels (T-Hg in µg/g) showed negative associations with neurodevelopment at 4 years of age, specifically in the domain of general cognition (β = −2.09, 95% CI = −5.72 to 1.54). Children’s total hair Hg levels (T-Hg in µg/g) showed negative associations with neurodevelopment at 4 years of age in the domains of gross motor skills (β = −1.09, 95% CI: −5.25 to 3.07) and fine motor skills (β = −1.03, 95% CI: −5.46 to 3.41). | ** |
| Guo et al., 2020. China, Longitudinal [50] | N: 326 Age: 7.4 (0.4) years Gender: 186 (57%) males Neurodevelopmental: Chinese Revised-Wechsler Intelligence Scale for Children (C-WISC-IV) Verbal Intelligence Quotient (VIQ) Performance Intelligence Quotient (PIQ) Full Intelligence Quotient (FIQ), | Bio sample: Maternal urine Prenatal maternal urinary Pb levels were negatively associated with children’s total IQ at 7 years of age (β = −2.31, 95% CI: −4.13 to −0.48; p = 0.013). | ** |
| Halabicky et al., 2023 China, Longitudinal [68] | N: 417 Age: 11.51 (0.39) years Gender: 220 (52.76%) males Neurodevelopmental: Wechsler Intelligence Scale for Children-Revised (WISC-R) Working Memory Measurement Software (WM) | Bio sample: Child blood Children’s blood Pb levels were associated with lower IQ scores (β = −0.59) at 3 to 5 years of age. No association was found between Pb blood levels and worsened IQ outcomes (β = −0.59) in 12-year-old children. | ** |
| Hu et al., 2006 Mexico, Longitudinal [57] | N: 146 Age: Not applicate Gender: 76 (52.05%) males Neurodevelopmental: Bayley Scales of Infant Development II–Spanish version (BSID-IIS) | Bio sample: Umbilical cord and venous blood. Increased plasma Pb and whole blood Pb levels during the first trimester of pregnancy were associated with lower mental development scores by BSID-II scale at 24 months of age (β = −3.54, p = 0.03 and β = −2.4, p = 0.19, respectively). | *** |
| Hu et al., 2016 China, Cross-sectional [73] | N: 410 Age: Not applicable Gender: 214 (52.19%) males Neurodevelopmental: Gesell Developmental Schedules (GDS) | Bio sample: Maternal and umbilical cord blood. Umbilical cord blood Hg levels were positively associated with developmental quotients in the adaptive domain by GDS scale (β = 4.22) and the social domain (β = 4.06). | *** |
| Huang et al., 2012 Taiwan, Longitudinal [74] | N: 119 Age: 2–9 years Gender: 62 (52.1%) males Neurodevelopmental: Bayley Scales of Infant Development-II (BSID-II) Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) Wechsler Intelligence Scale for Children-version III (WISC-III) | Bio sample: Umbilical cord and venous blood. Children’s blood Pb levels were negatively associated with total IQ scores between ages 5 and 6 years (β = −5.97, SE = 2.59, p = 0.025) and between ages 2 and 9 years (β = −0.289, 95% CI: −16.9 to −1.48, p = 0.020). | *** |
| Inoue et al., 2022 Japan, Longitudinal [39] | N: 80,759 Age: Not applicable Gender: 41,190 (51%) males Neurodevelopmental: Ages and Stages Questionnaires (ASQ-III) | Bio sample: Maternal and umbilical cord blood. No significant associations were found between Pb levels in umbilical cord blood and suspected neurodevelopmental delay during the first three years of life. | ** |
| Kao et al., 2021 Taiwan, Cross-sectional [54] | N: 139 Age: 2.8 (0.4) years Gender: 66 (47%) males Neurodevelopmental: Bayley Scales of Infant Development-III (BSID-III) | Bio sample: Child hair and nail Children’s hair Pb concentrations were negatively associated with gross motor scores (β = −0.04, 95% CI: −0.07 to −0.01) after adjustment for residence near a highway. | *** |
| Kao et al., 2023 Taiwan, Longitudinal [67] | N: 152 Age: At 24 months Gender: 72 (47.36%) males Neurodevelopmental: Bayley Scales of Infant Development-III (BSID-III) | Bio sample: Child hair and nail. In low birth weight premature children, Cd concentrations in the nails were negatively associated with cognition (β = −0.63, 95% CI: −1.17 to −0.08). | *** |
| Kashala-Abotnes et al., 2016 Democratic Republic of Congo, Cross-sectional [75] | N: 89 Age: 17.5 (4.3) months Gender: 52 (58.4%) males Neurodevelopmental: Baby characteristics questionnaire (BCQ) | Bio sample: Child blood. No statistically significant differences were observed in child neurodevelopment. | *** |
| Kim et al., 2009 South Korea Cross-sectional [51] | N: 261 Age: 9.7 (0.6) years Gender: 141 (54%) males Neurodevelopmental: Korean Educational Development Institute-Wechsler Intelligence Scales (KEDI-WISC) | Bio sample: Venous blood. Pb levels were negatively associated with total IQ (β = −0.174) and verbal IQ (β = −0.187). | ** |
| Kim et al., 2018 South Korea, Longitudinal [63] | N: 1098 Age: 6–36 months Gender: 571 (52%) males Neurodevelopmental: Bayley Scales of Infant Development-II (BSID-II) to evaluate the Psychomotor Development Index (PDI) and Mental Development Index (MDI) | Bio sample: Maternal and umbilical cord blood. Prenatal exposure to Hg in early pregnancy was inversely associated with psychomotor development index (PDI) scores at 6 months (β = −0.550, p = 0.031). Prenatal exposure to Hg in early pregnancy was inversely associated with mental development index (MDI) scores at 6 months (β = −0.408, p = 0.048). | *** |
| Kippler et al., 2016 Greece, Longitudinal [76] | N: 575 Age: 4.2 (0.23) years Gender: 288 (50%) males Neurodevelopmental: McCarthy Scales of Children’s Abilities (MSCA) | Bio sample: Maternal urine. Maternal urinary Cd concentrations ≥0.8 µg/L during pregnancy were inversely associated with children’s overall cognitive scores at 4 years of age (β = −6.2, 95% CI: −12 to −0.54, p = 0.032). Maternal urinary Pb concentrations ≥0.8 µg/L during pregnancy were not associated with children’s overall cognitive scores (β = −6.2; 95% CI: −12, 0.54; p = 0.45). | *** |
| Kou et al., 2025 Spain, Longitudinal [77] | N: 400 Age: 40 days Gender: 210 (52.5%) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development-III (BSID-III) | Bio sample: Maternal urine. The mixture of heavy metals: Cd, Ni, Hg, and Pb was associated with poorer expressive language scores (β = −0.26, 95% CI: −0.44 to −0.07). Cd was the most influential contributor to lower cognitive scores (β = −1.47, p = 0.044). | *** |
| Lee et al., 2017 South Korea, Longitudinal [78] | N: 251 Age: 6–60 months Gender: Not informed Neurodevelopmental: The Korean version of Bayley Scales of Infant and Toddler Development-II (K-BSID-II) to evaluate the Psychomotor Development Index (PDI) and Mental Development Index (MDI) The Korean version of the Wechsler Preschool and Primary Scale of Intelligence–Revised (K-WPPSI-R) | Bio sample: Umbilical cord blood. Cognitive development stability appeared to be unaffected by heavy metal levels in umbilical cord blood. | ** |
| Lee et al., 2018 Taiwan, Cross-sectional [79] | N:122 Health control: N: 46 Age: 8.1 (1.2) years Gender: 31 (67.4%) males ADHD-I:N: 29 Age: 8.0 (1) years Gender: 11 (37.93%) males TDAH-H/I: N: 47 Age: 7.7 (1) years Gender: 40 (85.10%) males Neurodevelopmental: Schedule for Affective Disorders and Schizophrenia for School-Age Children, epidemiologic version (K-SADS-E) Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) Swanson, Nolan, and Pelham Version IV Scale (SNAP-IV) | Bio sample: Child urine. Cd (p < 0.05) and Pb (p < 0.01) levels were negatively correlated with the IQ. Sb (p < 0.01) and Pb (p < 0.05) levels were positively correlated with the severity of ADHD symptoms | ** |
| Lee et al., 2021 South Korea, Longitudinal [80] | N: 502 Age: 4–6 years Gender: 254 (50.6%) males Neurodevelopmental: Korean Educational Developmental Institute’s Wechsler Intelligence Scale for Children (KEDI-WISC) | Bio sample: Maternal and child blood. Blood Mn levels at 4 years of age were negatively associated with children’s IQ (β = −5.99, 95% CI: −11.37 to −0.61). | *** |
| Lin et al., 2013 Taiwan, Cross-sectional [81] | N: 230 Age: At birth to 2 years Gender: 128 (55.7%) males Neurodevelopmental: Comprehensive developmental inventory for infants and toddlers (CDIIT) | Bio sample: Umbilical cord blood. General developmental quotients were significantly lower in the group with high exposure to Mn and Pb (β = −7.03, SE = 2.65, p = 0.009). High exposure to Mn and Pb was associated with lower cognitive quotients (β = −8.19, SE = 3.17, p = 0.011) and language quotients (β = −6.81, SE = 2.73, p = 0.013). No significant differences were found in language development quotients (β = −6.81, SE = 2.73, p = 0.013) in the group with high exposure to As or Hg. No significant differences were found in cognitive development quotients (β = −8.19, SE = 3.17, p = 0.011) in the group with high exposure to As or Hg. | *** |
| Liu et al., 2024 Mexico, Longitudinal [52] | N: 533 Age: 9 (9–10) years Gender: 274 (51.4%) males Neurodevelopmental: Go/NoGo Happy, Go/NoGo Neutral, Go/NoGo Letter and D-KEFS Color Word Interference Test | Bio sample: Umbilical cord blood. Pb concentrations in umbilical cord blood (β = −0.06, 95% CI: −0.11 to −0.01) and in blood at 4 years of age (β = −0.07, 95% CI: −0.12 to −0.02) were negatively associated with inhibitory control in childhood. There was a significant negative association between umbilical cord blood Pb levels and childhood hyperactivity (β = −0.117, p = 0.009). | ** |
| Liu J. et al., 2014 China, Longitudinal [58] | N: 243 Age: 6–36 months Gender: 129 (53.09%) males Neurodevelopmental: Bayley Scales of Infant Development-II (BSID-II) to evaluate the Psychomotor Development Index (PDI) and Mental Development Index (MDI) | Bio sample: Umbilical cord blood and venous blood. Significant deficit in psychomotor development index (PDI) due to prenatal Pb exposure at 36 months (β = −1.302, p = 0.041). Umbilical cord blood Pb levels were inversely associated with mental development index scores (β = −1.291, p = 0.036). | *** |
| Liu J. et al., 2014 China, Longitudinal [59] | N: 332 Age: 3 days Gender: 185 (55.72%) males Neurodevelopmental: Neonatal behavioral neurological assessments (NBNA) | Bio sample: Maternal blood. Infant exposure to Pb during the first trimester of pregnancy was positively associated with reduced neonatal development scores (β = −4.86, 95% CI: −8.831 to −0.889, p = 0.03). | *** |
| Llop et al., 2012 Spain, Longitudinal [82] | N: 1.683 Age: 14 (11–23) months Gender: 882 (52.4%) males Neurodevelopmental: Bayley Scales of Infant Development | Bio sample: Umbilical cord blood. No overall significant association was found between Hg levels and neurodevelopment. | *** |
| Llop et al., 2016 Spain, Cross-sectional [83] | N: 1362 Age: 4.8 (0.61) years Gender: 712 (52.3%) males Neurodevelopmental: McCarthy Scales of Children’s Abilities (MSCA) | Bio sample: Umbilical cord blood. Umbilical cord blood Hg concentrations were positively associated with cognitive development scale scores (β = 1.29, 95% CI: 0.28 to 2.31). | *** |
| Lu et al., 2023 China, Longitudinal [84] | N: 275 Age: 18.32 (0.68) months Gender: 140 (50.91) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development-III (BSID-III) | Bio sample: Umbilical cord blood. Umbilical cord blood Pb levels were associated with lower fine motor control scores in girls (β = −1.5, 95% CI: −2.6 to −0.4). | ** |
| Ma et al., 2021 Japan, Longitudinal [85] | N: 3545 Age: At birth to 2 years Gender: 1781 (50.2%) males Neurodevelopmental: Kyoto Scale of Psychological Development (KSPD) | Bio sample: Umbilical cord blood and maternal blood. Elevated Cd concentrations in maternal or umbilical cord blood were not significantly associated with neurodevelopmental delay. | *** |
| Marques et al., 2012 Brazil, Cross-sectional [41] | N: 668 Age: 19.82 (14.47) months Gender: Not informed Neurodevelopmental: Gesell Developmental Scores (GDS) | Bio sample: Child hair. Children’s average Hg concentration was positively correlated with neurodevelopment scores by GDS scale (r = 0.080, p = 0.035). | ** |
| Marques et al., 2015 Brazil, Longitudinal [42] | N: 294 Age: 6–24 months Gender: 105 (35.71%) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development-II (BSID-II) to evaluate the Psychomotor Development Index (PDI) and Mental Development Index (MDI) | Bio sample: Child hair. Neonatal hair Hg levels showed a significant negative association with mental development index scores in boys at 24 months (β = −0.222, 95% CI: −0.44 to −0.01, p = 0.0451). Neonatal hair Hg levels showed a positive association with age at walking onset in girls (β = 0.188, 95% CI: 0.032 to 0.344, p = 0.019). | ** |
| Masumoto et al., 2022 Japan, Longitudinal [40] | N: 96,165 Age: At birth to 3 years Gender: 49,257 (51.2%) males Neurodevelopmental: Ages and Stages questionnaires (ASQ-3) | Bio sample: Maternal blood. Blood Cd concentration was associated with gross motor function delay 1.5 years after birth (adjusted OR = 1.19, 99.7% CI: 1.01–1.40). | *** |
| Merced-Nieves et. al., 2022 Mexico, Longitudinal [86] | N: 549 Age: 6.7 (0.5) years Gender: 278 (50.6%) males Neurodevelopmental: National Center for Toxicological Research (NCTR) Operant Test Battery (OTB) | Bio sample: Maternal blood and umbilical cord blood. Umbilical cord blood Pb levels were associated with altered temporal perception in children (p = 0.02). | ** |
| Myers et al., 2004 Seychelles, Longitudinal [43] | N: 643 Age: 8.97 (0.33) years Gender: Not informed Neurodevelopmental: Child Behaviour Checklist (CBCL), WISC | Bio sample: Maternal and child hair. There was a positive association between postnatal Hg exposure and Thought Problems (p = 0.011). There was a significant negative association between prenatal Hg exposure and Social Problems scores (β = −0.029). | ** |
| Naspolini et al., 2024 Brazil, Longitudinal [65] | N: 157 Age: 3–16 months Gender: 76 (48.41) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development-III (Bayley-III). | Bio sample: Breast milk. Infants exposed to Pb showed significantly lower language performance at 10–16 months of age (β = −0.413; 95% CI: −0.653 to −0.173) compared to unexposed infants. | * |
| Nie et al., 2011 USA, Cross-sectional [66] | N: 11 Age: 11 (1.6) years Gender: 55% males Neurodevelopmental: Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) Behavior Rating Inventory of Executive Function (BRIEF) Conners ADHD/DSM-IV Scale (CADS-IV) | Bio sample: Blood. Blood Pb levels were associated with increased externalizing problems, internalizing problems, and behavioral symptoms (r = 0.943, r = 0.648, and r = 0.853, respectively). Blood Pb levels showed a positive association with Verbal Comprehension, Working Memory, and IQ (r = 0.746, r = 0.853, and r = 0.823, respectively). | *** |
| Notario-Barandiaran et al., 2024 Spain, Cross-sectional [87] | N: 962 Age: 4.45 (4–6.4) years Gender: 502 (52.2%) males Neurodevelopmental: McCarthy Scales of Children’s Abilities (MSCA) | Bio sample: Urine. Exposure to the mixture of Cu Se, Pb, and Zn was associated with poorer verbal executive function (β = −1.88, 95% CI: −3.17 to −0.59). Exposure to the mixture of inorganic and organic As was associated with poorer gross motor function (β = −1.41, 95% CI: −2.36 to −0.46). | *** |
| Nozadi et al., 2021 USA, Cross-sectional [60] | N: 327 Age: 10–13 months Gender: 163 (49.8%) males Neurodevelopmental: Ages and Stages Questionnaire Inventory (ASQ:I) | Bio sample: Maternal urine and blood. Problem-solving ability showed negative correlations with urinary Mo, Sb, and As levels (r = −0.001, r = −0.106, and r = −0.124, respectively). Fine motor skills showed negative correlations with urinary Ba and As levels (r = −0.132 and r = −0.156, respectively). Social behavior showed negative correlations with urinary As and urinary U levels (r = −0.100 and r = −0.01, respectively). Fine motor skills showed positive correlations with blood Cd, urinary Cs, urinary Mo, urinary Sr, and urinary W levels (r = 0.070, r = 0.038, r = 0.065, r = 0.021, and r = 0.120, respectively). Gross motor skills showed a positive correlation with urinary Sr levels (r = 0.113). Urinary W levels were positively correlated with communication development (r = 0.136). Problem-solving ability showed positive correlations with urinary Cs, Sn, Sr, Tl, and W levels (r = 0.039, r = 0.073, r = 0.089, r = 0.120, and r = 0.079, respectively). | ** |
| Nyanza et al., 2021 Tanzania, Longitudinal [46] | N: 439 Age: 7.92 (1.77) months Gedner: 213 (48.6%) males Neurodevelopmental: Malawi Developmental Assessment Tool (MDAT). | Bio sample: Maternal blood and urine. Higher prenatal As exposure was positively associated with an increased risk of social impairment in girls (adjusted prevalence ratio [aPR] = 1.01, 95% CI: 1.00–1.02, p < 0.05). Higher prenatal Hg exposure was positively associated with global neurodevelopmental impairment in girls (adjusted prevalence ratio [aPR] = 1.10, 95% CI: 1.06–1.14, p < 0.001). Prenatal exposure to As and Cd was positively associated with global neurodevelopmental impairment in boys (adjusted prevalence ratios [aPR] = 1.03, 95% CI: 1.02–1.048, p < 0.001; and aPR = 2.55, 95% CI: 1.33–4.87, p < 0.05, respectively). Higher prenatal Hg exposure was positively associated with language impairment in boys (adjusted prevalence ratio [aPR] = 1.05, 95% CI: 1.04–1.07) and girls (aPR = 1.21, 95% CI: 1.16–1.27, p < 0.001). | ** |
| Orenstein et al., 2014 USA, Cross-sectional [88] | N: 393 Age: 8.1 (0.6) years Gender: 196 (49.9%) males Neurodevelopmental: Wide Range Assessment of Memory and Learning (WRAML) | Bio sample: Maternal hair. The figure memorization capacity index (visual memory) was negatively associated with MeHg concentration (β = −3.1). | *** |
| Parajuli et al., 2013 Nepal, Cross-sectional [89] | N = 100 Age: 17.4 (3.3) hours Gender: Not informed Neurodevelopmental: Brazelton neonatal behavioral assessment scale (NBAS III) | Bio sample: Umbilical cord blood. Pb and As levels in umbilical cord blood were negatively associated with motor function scores (β = −2.29, 95% CI: −4.35 to −0.24; β = −3.03, 95% CI: −6.05 to −0.01, respectively) at neonatal stage. | ** |
| Parajuli et al., 2014 Nepal, Cross-sectional [90] | N: 100 Age: At birth-6 months Gender: 47 (47%) males Neurodevelopmental: Bayley Scale of Infant Development (BSID II) | Bio sample: Umbilical cord blood. There was no association between Pb levels and infant development scale scores at 6 months. | *** |
| Parajuli et al., 2015 Nepal, Longitudinal [91] | N: 100 Age: 36.9 (0.4) months Gender: 47 (47%) males Neurodevelopmental: Bayley Scale of Infant Development, Second Edition (BSID II) | Bio sample: Umbilical cord blood. Cord blood toxic element levels (Pb and As) were not associated with any developmental delay scores at 36 months. | *** |
| Park et al., 2016 South Korea, Cross-sectional [55] | ADHD sample: 114 Age: 8.79 (1.57) years Gender: 83 (72.8%) males Controls sample: 114 Age: 8.73 (1.65) years Gender: 81 (71.1%) males Neurodevelopmental: Kiddie-Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K-SADS-PL-K) Continuous Performance Test (CPT) | Bio sample: Child blood. Children with ADHD showed significantly higher blood Pb concentrations compared to controls (p = 0.003). Total blood Pb concentration was associated with an increased risk of ADHD (odds ratio [OR] = 1.60, 95% CI: 1.04–2.45, p < 0.05). | ** |
| Polanska et al., 2018 Poland, Longitudinal [92] | N: 402 Age: At birth-24 months Gender: 195 (48.5%) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development-III (BSID III) | Bio sample: Maternal and umbilical cord blood. Higher umbilical cord blood Pb levels were associated with lower cognitive function scores in boys (β = −2.07, 95% CI = −4.07 to −0.06, p = 0.04). | ** |
| Qiu et al., 2024 China, Longitudinal [93] | N: 854 Age: 36 (0.3) months Gender: 416 (48.7%) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development- III (BISD-III) | Bio sample: Maternal urine. Higher levels of the metal mixture—V, Cu, Zn, Sb, Ce, and U—were associated with a 2.37-fold increased risk of suboptimal gross motor development (95% CI: 1.15–4.86, p = 0.012). | *** |
| Rodrigues et al., 2016 Bangladesh, Longitudinal [48] | N: 525 Age: 2.3 (1.7–3.3) years Gender: 261 (47,9%) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) | Bio sample: Child blood. Pb levels were negatively associated with cognitive development (β = −0.17). | *** |
| Rosa et al., 2024 USA, Longitudinal [94] | N: 326 Age: 6.53 (2.15) years Gender: 182 (55.8%) males Neurodevelopmental: NIH Toolbox Cognition Battery (NIHTB-CB). | Bio sample: Maternal urine. There was no evidence of an association between the mixture of metals and overall cognitive functioning scores in children. | ** |
| Rothenberg et al., 2016 China, Longitudinal [95] | N: 270 Age: At birth-12 months Gender: 127 (47%) males Neurodevelopmental: Bayley Scales of Infant Development (BSID)-II. | Bio sample: Maternal blood and hair. Higher hair Hg concentration was negatively associated with the mental development index (β = −4.9). Higher blood Pb concentration was negatively associated with the psychomotor development index (β = −11). | *** |
| Ruiz-Castell et al., 2012 Bolivia, Longitudinal [96] | N: 246 Age: 10.5–12.5 months Gender: 129 (52.44%) males Neurodevelopmental: Bayley Scales of Infant Development (BSID) | Bio sample: Maternal blood. Maternal blood Pb levels were positively associated with mental development indices (β = 2.27, p = 0.034). Maternal blood cesium levels were positively associated with infant psychomotor development (β = 2.20, p = 0.015). | *** |
| Shah-Kulkarni et al., 2020 South Korea, Longitudinal [97] | N: 523 Age: At birth-6 months Gender: 283 (54.1%) males Neurodevelopmental: Korean version of Bayley Scales of Infant Development II (KBSID-II) | Bio sample: Maternal and umbilical cord blood. Exposure to mixtures of Pb, Hg, and Cd in early pregnancy and umbilical cord blood did not significantly affect mental development scores at 6 months. Exposure to mixtures of Pb, Hg, and Cd in early pregnancy and umbilical cord blood did not significantly affect psychomotor development scores at 6 months. | ** |
| Shekhawat et al., 2021 India, Longitudinal [98] | N: 167 Age: At birth-6 months Gender: 80 (48%) males Neurodevelopmental: The Bayley Scale of Infants Developments-III (BSID-III) | Bio sample: Umbilical cord blood. Umbilical cord blood Pb concentrations between 5.0 and 10.5 μg/dL were negatively associated with gross motor skills subscale scores (β = −0.29, 95% CI: −5.00 to 0.11, p = 0.042) at a mean age of 6.5 months. | *** |
| Skogheim et al., 2021 Norway, Longitudinal [61] | ADHD: 705 Age: From 2 years Gender: 520 (73.8%) males ASD: 397 Age: From 2 years Gender: 336 (84.6%) males Controls: 1034 Age: From 2 years Gender: 705 (68.2%) males Neurodevelopmental: Adult ADHD Self-Report Scale | Bio sample: Maternal blood. The risk of ADHD in children exposed to the highest quartile of Cd compared to the lowest quartile was increased [OR = 1.59 (95% CI: 1.15, 2.18)]. Elevated risk of ASD was observed in children in the second quartile of As [OR = 1.77 (95% CI: 1.26, 2.49)] and in the highest quartiles of Cd [OR = 1.57 (95% CI: 1.07, 2.31)] and Mn [OR = 1.84 (95% CI: 1.30, 2.59)] compared to the first quartile (reference) | *** |
| Tatsuta et al., 2014 Japan, Longitudinal [99] | N: 387 Age: 42.1 (40–45) months Gender: 202 (52.2%) males Neurodevelopmental: Kaufman Assessment Battery for Children (K-ABC) | Bio sample: Umbilical cord blood. No significant associations were found between prenatal exposure to total Hg (THg) or Pb and children’s intelligence scores. | *** |
| Tatsuta et al., 2020 Japan, Longitudinal [53] | N: 289 Age: 12 (11.1–12.8) years Gender: 148 (51.21%) males Neurodevelopmental: Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) Boston Naming Test (BNT) | Bio sample: Umbilical cord and venous blood. Among boys, IQ was associated with child-blood Pb (B = −16.362, p = 0.033), but there was no association with Pb in cord blood (B = −6.844, p = 0.309). Umbilical cord blood Pb levels showed no significant correlation with any developmental outcomes among girls. | *** |
| Taylor et al., 2018 United Kingdom, Longitudinal [100] | N: 1558 Age: 7 years Gender: 780 (50.06%) males Neurodevelopmental: ALSPAC Coordination Test | Bio sample: Maternal blood. There was no evidence of associations between prenatal exposure to Pb, Cd, or Hg and motor skills measured at 7 years of age. Furthermore, no associations were found with probable developmental coordination disorder. | *** |
| Tong et al., 2022 China, Longitudinal [101] | N: 2164 Age: 55.6 (6.9) months Gender: 1117 (51.6%) males Neurodevelopmental: Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition (WPPSI-IV). | Bio sample: Maternal blood and umbilical cord blood. Higher maternal serum Tl levels during pregnancy were associated with lower IQ in children (β = −1.51, 95% CI: −2.68 to −0.35, p = 0.01). | *** |
| Tong et al., 2023 China, Longitudinal [102] | N: 2164 Age: 4.6 (0.6) years Gender: 1117 (51.6%) males Neurodevelopmental: Chinese version of the Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition (WPPSI-IV) | Bio sample: Maternal blood and umbilical cord blood. Elevated maternal serum Ba levels during pregnancy were significantly associated with reduced childhood intellectual function, as indicated by lower global IQ scores [−3.76 (95% CI: −6.19, −1.33)]. | *** |
| Valent et al., 2013 Italy, Longitudinal [103] | N: 606 Age: 0–18 months Gender: 307 (50.7) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development-III (BSID-III). | Bio sample: Maternal hair/blood and umbilical cord blood. Maternal total hair Hg levels were positively associated with language development scores in girls (β = 1.5291, p = 0.0445). Total Hg in maternal hair was not associated with positive scores on the language development scale in boys (β = 0.3551, p = 0.6278). | ** |
| Valeri et al., 2017 India, Longitudinal [62] | N: 825 Age: At birth-40 months Gender: 419 (50.78%) males Neurodevelopmental: Bayley Scales of Infant and Toddler Development-III (BSID-III) | Bio sample: Umbilical cord blood. Higher levels of Mn and As were associated with decreased cognitive scores (β = −0.206, 95% CI: −0.39 to −0.02, p < 0.05). | *** |
| Vejrup et al., 2018 Norway, Longitudinal [104] | N: 2239 Age: At birth-5 years Gender: 1187 (53%) males Neurodevelopmental: Ages and Stages Communication scale (ASQ) Neurodevelopmental: Speech and Language Assessment Scale (SLAS) Language-Related Difficulties list (language 20) | Bio sample: Maternal blood. The analysis showed that maternal blood Hg concentration was not significantly associated with language and communication scale scores. | *** |
| Xue et al., 2020 China, Longitudinal [105] | N: 456 Dyslexic: 228 Age: 9.76 (1.29) years Gender: 171 (75%) males Non-dyslexic: 228 Age: 9.69 (1.25) years Gender: 171 (75%) males Neurodevelopmental: Dyslexia Checklist for Chinese Children (DCCC) Pupil Rating Scale-Revised Screening (PRS) | Bio sample: Child urine. Children with dyslexia showed higher concentrations of Sr (p = 0.028), Ag (p = 0.014), and U (p = 0.005). | *** |
4. Discussion
5. Conclusions
Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. 10 Chemicals of Public Health Concern; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/photo-story/detail/10-chemicals-of-public-health-concern (accessed on 1 August 2025).
- Santos, A.D.S.E.; Saraiva, R.D.D.S.; Oliveira, A.P.N.D.; Costa, M.A.; Alonzo, H.G.A.; Campolina, D.; André, L.C.; Peixoto, S.V.; Cãmara, H.G.; Asmus, C.I.R.F.; et al. Metal exposure in a child population after a mine tailings dam failure. Projeto Bruminha. Rev. Bras. Epidemiol. 2023, 26, e230017. [Google Scholar] [CrossRef]
- Teasdale, A.; Ulman, K.; Domoradzki, J.; Walsh, P. Establishing limits for dermal absorption of elemental impurities. Pharm. Technol. 2015, 39, 44–51. [Google Scholar]
- Choi, E.H. Skin Barrier Function in Neonates and Infants. Allergy Asthma Immunol. Res. 2025, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, M.; Bruccoleri, R.; Woolf, A.D. An Update on Childhood Lead Poisoning. Clin. Pediatr. Emerg. Med. 2017, 18, 181–192. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, L.A.; Ropar, R.; Brown, M.B.; Fitzpatrick, K. Heavy Metals in Children: The Developmental Impact and Detection Strategies. J. Pediatr. Neuropsychol. 2011, 1, 15–30. [Google Scholar] [CrossRef]
- Botelho, R.M.; Silva, A.L.M.; Borbely, A.U. The Autism Spectrum Disorder and Its Possible Origins in Pregnancy. Int. J. Environ. Res. Public Health 2024, 21, 244. [Google Scholar] [CrossRef]
- Bondi, B.C.; Tassone, V.K.; Bucsea, O.; Desrocher, M.; Pepler, D.J. A Systematic Review of Neurodevelopmental Assessments in Infancy and Early Childhood: Developing a Conceptual Framework, Repository of Measures, and Clinical Recommendations. Neuropsychol. Rev. 2024, 35, 337–353. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, M.; Strodl, E.; Yin, X.; Wen, G.; Sun, D.; Xian, D.; Zhao, Y.; Zheng, Y.; Liu, F.; et al. The effects of early childhood probiotic intake on the association between prenatal micronutrient supplementation and neurobehavioral development in preschool children: A four-way decomposition analysis. Front. Nutr. 2025, 12, 1614820. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Payne-Sturges, D.C.; Taiwo, T.K.; Ellickson, K.; Mullen, H.; Tchangalova, N.; Anderko, L.; Chen, A.; Swanson, M. Disparities in Toxic Chemical Exposures and Associated Neurodevelopmental Outcomes: A Scoping Review and Systematic Evidence Map of the Epidemiological Literature. Environ. Health Perspect. 2023, 131, 096001. [Google Scholar] [CrossRef]
- Barbiero, F.; Rosolen, V.; Consonni, D.; Mariuz, M.; Parpinel, M.; Ronfani, L.; Brumatti, L.V.; Bin, M.; Castriotta, L.; Valent, F.; et al. Copper and zinc status in cord blood and breast milk and child’s neurodevelopment at 18 months: Results of the Italian PHIME cohort. Int. J. Hyg. Environ. Health 2025, 263, 114485. [Google Scholar] [CrossRef]
- Lee, K.S.; Min, W.K.; Choi, Y.J.; Jin, S.; Park, K.H.; Kim, S. The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea. Medicina 2023, 59, 951. [Google Scholar] [CrossRef]
- Schildroth, S.; Kordas, K.; Bauer, J.A.; Wright, R.O.; Claus Henn, B. Environmental Metal Exposure, Neurodevelopment, and the Role of Iron Status: A Review. Curr. Environ. Health Rep. 2022, 9, 758–787. [Google Scholar] [CrossRef]
- Guo, T.; Najafi, M.L.; Zhang, J. A systematic review of exposure to toxic elements and neurocognitive development in children. Ecotoxicol. Environ. Saf. 2025, 291, 117792. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Davis, A.C.; Wertlieb, D.; Boo, N.-Y.; Nair, M.K.C.; Halpern, R.; Kuper, H.; Breinbauer, C.; De Vries, P.J.; Gladstone, M.; et al. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob. Health 2018, 6, e1100–e1121. [Google Scholar] [CrossRef]
- Olabumuyi, O.O.; Uchendu, O.C.; Green, P.A. Prevalence, Pattern and Factors Associated with Developmental Delay amongst Under-5 Children in Nigeria: Evidence from Multiple Indicator Cluster Survey 2011–2017. Niger. Postgrad. Med. J. 2024, 31, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Yevoo, P.; Sadahiro, M.; Austin, C.; Amarasiriwardena, C.; Awawda, M.; Arora, M.; Dudley, J.T.; Morishita, H. Integrative bioinformatics identifies postnatal lead (Pb) exposure disrupts developmental cortical plasticity. Sci. Rep. 2018, 8, 16388. [Google Scholar] [CrossRef]
- Abd Wahil, M.S.; Ja’afar, M.H.; Md Isa, Z. Assessment of Urinary Lead (Pb) and Essential Trace Elements in Autism Spectrum Disorder: A Case-Control Study Among Preschool Children in Malaysia. Biol. Trace Elem. Res. 2022, 200, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Ravenscroft, J.; Cao, Y.; McLean, E.V. Lead Exposure in Low and Middle-Income Countries: Perspectives and Lessons on Patterns, Injustices, Economics, and Politics. Int. J. Environ. Res. Public Health 2018, 15, 2351. [Google Scholar] [CrossRef]
- Shi, J.; Du, P.; Luo, H.; Chen, J.; Zhang, Y.; Wu, M.; Xu, G. Characteristics and Risk Assessment of Soil Polluted by Lead around Various Metal Mines in China. Int. J. Environ. Res. Public Health 2021, 18, 4598. [Google Scholar] [CrossRef]
- Moulatlet, G.M.; Yacelga, N.; Rico, A.; Mora, A.; Hauser-Davis, R.A.; Cabrera, M.; Capparelli, M.V. A systematic review on metal contamination due to mining activities in the Amazon basin and associated environmental hazards. Chemosphere 2023, 339, 139700. [Google Scholar] [CrossRef]
- Surenbaatar, U.; Lee, S.; Kwon, J.-Y.; Lim, H.; Kim, J.-J.; Kim, Y.-H.; Hong, Y.-S. Bioaccumulation of Lead, Cadmium, and Arsenic in a Mining Area and Its Associated Health Effects. Toxics 2023, 11, 519. [Google Scholar] [CrossRef]
- Wirtu, Y.D. A review of environmental and health effects of synthetic cosmetics. Front. Environ. Sci. 2024, 12, 1402893. [Google Scholar] [CrossRef]
- Gyamfi, O.; Aboko, J.; Ankapong, E.; Marfo, J.T.; Awuah-Boateng, N.Y.; Sarpong, K.; Dartey, E. A systematic review of heavy metals contamination in cosmetics. Cutan. Ocul. Toxicol. 2024, 43, 5–12. [Google Scholar] [CrossRef]
- Hudson, K.M.; Shiver, E.; Yu, J.; Mehta, S.; Jima, D.D.; Kane, M.A.; Patisaul, H.B.; Cowley, M. Transcriptomic, proteomic, and metabolomic analyses identify candidate pathways linking maternal cadmium exposure to altered neurodevelopment and behavior. Sci. Rep. 2021, 11, 16302. [Google Scholar] [CrossRef]
- Gu, Q.; Liu, J.; Zhang, X.; Huang, A.; Yu, X.; Wu, K.; Huang, Y. Association between heavy metals exposure and risk of attention deficit hyperactivity disorder (ADHD) in children: A systematic review and meta-analysis. Eur. Child. Adolesc. Psychiatry 2025, 34, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Rezaei, N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 340–368. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, S.; Amirabadizadeh, A.; Farnia, V.; Ali Azadi, N.; Mansouri, B.; Radmehr, F. Association Between Biological Lead Concentrations and Autism Spectrum Disorder (ASD) in Children: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2023, 201, 1567–1581. [Google Scholar] [CrossRef]
- Tian, Y.; Hou, Q.; Zhang, M.; Gao, E.; Wu, Y. Exposure to arsenic and cognitive impairment in children: A systematic review. PLoS ONE 2025, 20, e0319104. [Google Scholar] [CrossRef]
- Sharma, B.M.; Sáňka, O.; Kalina, J.; Scheringer, M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ. Int. 2019, 125, 300–319. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, B.; Zhang, F.; Zhang, B.; Guo, Y.; Pang, M.; Huang, L.; Wang, T. Toxic and essential metals: Metabolic interactions with the gut microbiota and health implications. Front. Nutr. 2024, 11, 1448388. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, n71. [Google Scholar] [CrossRef] [PubMed]
- Boivin, M.J.; Kakooza, A.M.; Warf, B.C.; Davidson, L.L.; Grigorenko, E.L. Reducing neurodevelopmental disorders and disability through research and interventions. Nature 2015, 527, S155–S160. [Google Scholar] [CrossRef]
- Chakraborty, R.; Vijay Kumar, M.J.; Clement, J.P. Critical aspects of neurodevelopment. Neurobiol. Learn. Mem. 2021, 180, 107415. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic Statistical Manual of Mental Disorders: D.S.M.-5.-T.R, 5th ed.; Text Revision; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Mastrangelo, M. Clinical approach to neurodegenerative disorders in childhood: An updated overview. Acta Neurol. Belg. 2019, 119, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Kolaski, K.; Logan, L.R.; Ioannidis, J.P.A. Guidance to best tools and practices for systematic reviews. Syst. Rev. 2023, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Sanefuji, M.; Sonoda, Y.; Ogawa, M.; Hamada, N.; Shimono, M.; Suga, R.; Nakayama, S.F.; Taniguchi, Y.; Kusuhara, K.; et al. No association between prenatal lead exposure and neurodevelopment during early childhood in the Japan Environment and Children’s Study. Sci. Rep. 2022, 12, 15305. [Google Scholar] [CrossRef]
- Masumoto, T.; Amano, H.; Otani, S.; Kamijima, M.; Yamazaki, S.; Kobayashi, Y.; Kurozawa, Y. Association between prenatal cadmium exposure and child development: The Japan Environment and Children’s study. Int. J. Hyg. Environ. Health 2022, 243, 113989. [Google Scholar] [CrossRef]
- Marques, R.C.; Dórea, J.G.; Leão, R.S.; dos Santos, V.G.; Bueno, L.; Marques, R.C.; Brandão, K.G.; Palermo, E.F.A.; Guimarães, J.R.D. Role of Methylmercury Exposure (from Fish Consumption) on Growth and Neurodevelopment of Children Under 5 Years of Age Living in a Transitioning (Tin-Mining) Area of the Western Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2012, 62, 341–350. [Google Scholar] [CrossRef]
- Marques, R.C.; Bernardi, J.V.E.; Abreu, L.; Dórea, J.G. Neurodevelopment Outcomes in Children Exposed to Organic Mercury from Multiple Sources in a Tin-Ore Mine Environment in Brazil. Arch. Environ. Contam. Toxicol. 2015, 68, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J.; Davidson, P.W.; Shamlaye, C.; Cox, C.; Kost, J.; Beck, C.; Huang, L.-S.; Weiss, B. The Seychelles Child Development Study of methyl mercury from fish consumption: Analysis of subscales from the Child Behaviour Checklist at age 107 months in the main cohort. NeuroToxicology 2020, 81, 331–338. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Nester, M.; Abduljabbar, M.; Al-Rouqi, R.; Eltabache, C.; Al-Rajudi, T.; Elkhatib, R. Mercury (Hg) exposure and its effects on Saudi breastfed infant’s neurodevelopment. Int. J. Hyg. Environ. Health 2016, 219, 129–141. [Google Scholar] [CrossRef]
- Freire, C.; Ramos, R.; Lopez-Espinosa, M.-J.; Díez, S.; Vioque, J.; Ballester, F.; Fernández, M.-F. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ. Res. 2010, 110, 96–104. [Google Scholar] [CrossRef]
- Nyanza, E.C.; Bernier, F.P.; Martin, J.W.; Manyama, M.; Hatfield, J.; Dewey, D. Effects of prenatal exposure and co-exposure to metallic or metalloid elements on early infant neurodevelopmental outcomes in areas with small-scale gold mining activities in Northern Tanzania. Environ. Int. 2021, 149, 106104. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Elkhatib, R.; Al-Rouqi, R.; Abduljabbar, M.; Eltabache, C.; Al-Rajudi, T.; Nester, M. Alterations in biochemical markers due to mercury (Hg) exposure and its influence on infant’s neurodevelopment. Int. J. Hyg. Environ. Health 2016, 219, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Bellinger, D.C.; Valeri, L.; Hasan, M.O.S.I.; Quamruzzaman, Q.; Golam, M.; Kile, M.L.; Christiani, D.C.; Wright, R.O.; Mazumdar, M. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ. Health 2016, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Desrochers-Couture, M.; Oulhote, Y.; Arbuckle, T.E.; Fraser, W.D.; Séguin, J.R.; Ouellet, E.; Forget-Dubois, N.; Ayotte, P.; Boivin, M.; Lanphear, B.P.; et al. Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environ. Int. 2018, 121, 1235–1242. [Google Scholar] [CrossRef]
- Guo, J.; Wu, C.; Zhang, J.; Qi, X.; Lv, S.; Jiang, S.; Zhou, T.; Lu, D.; Feng, C.; Chang, X.; et al. Prenatal exposure to mixture of heavy metals, pesticides and phenols and IQ in children at 7 years of age: The SMBCS study. Environ. Int. 2020, 139, 105692. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, B.-N.; Hong, Y.-C.; Shin, M.-S.; Yoo, H.-J.; Kim, J.-W.; Bhang, S.-Y.; Cho, S.-C. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. NeuroToxicology 2009, 30, 564–571. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, Y.; Bellinger, D.; de Water, E.; Horton, M.; Téllez-Rojo, M.M.; Wright, R. Pre-natal and early life lead exposure and childhood inhibitory control: An item response theory approach to improve measurement precision of inhibitory control. Environ. Health 2024, 23, 71. [Google Scholar] [CrossRef]
- Tatsuta, N.; Nakai, K.; Kasanuma, Y.; Iwai-Shimada, M.; Sakamoto, M.; Murata, K.; Satoh, H. Prenatal and postnatal lead exposures and intellectual development among 12-year-old Japanese children. Environ. Res. 2020, 189, 109844. [Google Scholar] [CrossRef]
- Kao, C.-S.; Wang, Y.-L.; Chuang, T.-W.; Jiang, C.-B.; Hsi, H.-C.; Liao, K.-W.; Chien, L.-C. Effects of soil lead exposure and land use characteristics on neurodevelopment among children under 3 years of age in northern Taiwan. Environ. Pollut. 2021, 286, 117288. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, J.-H.; Hong, Y.-S.; Kim, Y.-M.; Kang, J.-W.; Yoo, J.-H.; Chueh, H.W.; Lee, J.H.; Kwak, M.J.; Kim, J.; et al. Blood lead concentrations and attention deficit hyperactivity disorder in Korean children: A hospital-based case control study. BMC Pediatr. 2016, 16, 156. [Google Scholar] [CrossRef]
- Cai, H.; Xu, X.; Zhang, Y.; Cong, X.; Lu, X.; Huo, X. Elevated lead levels from e-waste exposure are linked to sensory integration difficulties in preschool children. NeuroToxicology 2019, 71, 150–158. [Google Scholar] [CrossRef]
- Hu, H.; Téllez-Rojo, M.M.; Bellinger, D.; Smith, D.; Ettinger, A.S.; Lamadrid-Figueroa, H.; Schwartz, J.; Schnaas, L.; Mercado-García, A.; Hernández-Avila, M. Fetal Lead Exposure at Each Stage of Pregnancy as a Predictor of Infant Mental Development. Environ. Health Perspect. 2006, 114, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Gao, D.; Jing, J.; Hu, Q. Prenatal and postnatal lead exposure and cognitive development of infants followed over the first three years of life: A prospective birth study in the Pearl River Delta region, China. NeuroToxicology 2014, 44, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, D.; Chen, Y.; Jing, J.; Hu, Q.; Chen, Y. Lead exposure at each stage of pregnancy and neurobehavioral development of neonates. NeuroToxicology 2014, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nozadi, S.S.; Li, L.; Luo, L.; MacKenzie, D.; Erdei, E.; Du, R.; Roman, C.W.; Hoover, J.; O’donald, E.; Burnette, C.; et al. Prenatal Metal Exposures and Infants’ Developmental Outcomes in a Navajo Population. Int. J. Environ. Res. Public Health 2021, 19, 425. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Valeri, L.; Mazumdar, M.M.; Bobb, J.F.; Claus Henn, B.; Rodrigues, E.; Sharif, O.I.; Kile, M.L.; Quamruzzaman, Q.; Afroz, S.; Golam, M.; et al. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ. Health Perspect. 2017, 125, 067015. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, E.-H.; Park, H.; Ha, M.; Kim, Y.; Hong, Y.-C.; Lee, E.J.; Kim, H.; Chang, N.; Kim, B.-N. Prenatal mercury exposure, fish intake and neurocognitive development during first three years of life: Prospective cohort mothers and Children’s environmental health (MOCEH) study. Sci. Total Environ. 2018, 615, 1192–1198. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Moncari, L.; Jomaa, A.; Elkhatib, R.; Al-Rouqi, R.; Eltabache, C.; Al-Rajudi, T.; Alnuwaysir, H.; Nester, M.; Aldhalaan, H. Effects of early and recent mercury and lead exposure on the neurodevelopment of children with elevated mercury and/or developmental delays during lactation: A follow-up study. Int. J. Hyg. Environ. Health 2020, 230, 113629. [Google Scholar] [CrossRef]
- Naspolini, N.F.; Vanzele, P.A.R.; Tótolo, P.; Schüroff, P.A.; Fatori, D.; Vicentini Neto, S.A.; Barata-Silva, C.; dos Santos, L.M.G.; Fujita, A.; Passos-Bueno, M.R.; et al. Lead contamination in human milk affects infants’ language trajectory: Results from a prospective cohort study. Front. Public Health 2024, 12, 1450570. [Google Scholar] [CrossRef]
- Nie, L.H.; Wright, R.O.; Bellinger, D.C.; Hussain, J.; Amarasiriwardena, C.; Chettle, D.R.; Pejović-Milić, A.; Woolf, A.; Shannon, M. Blood lead levels and cumulative blood lead index (CBLI) as predictors of late neurodevelopment in lead poisoned children. Biomarkers 2011, 16, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-S.; Fan, Y.-T.; Chien, L.-C.; Liao, K.-W.; Chang, J.-H.; Hsu, C.-H.; Chen, Y.-J.; Jiang, C.-B. Effects of preterm birth and postnatal exposure to metal mixtures on neurodevelopment in children at 24 months of age. Environ. Sci. Pollut. Res. 2023, 30, 86856–86865. [Google Scholar] [CrossRef] [PubMed]
- Halabicky, O.M.; Pinto-Martin, J.A.; Compton, P.; Liu, J. Low level lead exposure in early childhood and parental education on adolescent IQ and working memory: A cohort study. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 168–176. [Google Scholar] [CrossRef]
- Boucher, O.; Bastien, C.H.; Saint-Amour, D.; Dewailly, É.; Ayotte, P.; Jacobson, J.L.; Jacobson, S.W.; Muckle, G. Prenatal exposure to methylmercury and PCBs affects distinct stages of information processing: An event-related potential study with Inuit children. NeuroToxicology 2010, 31, 373–384. [Google Scholar] [CrossRef] [PubMed]
- De Assis Araujo, M.S.; Froes-Asmus, C.I.R.; De Figueiredo, N.D.; Camara, V.M.; Luiz, R.R.; Prata-Barbosa, A.; Martins, M.M.; Jacob, S.d.C.; Santos, L.M.G.d.; Vicentini Neto, S.A.; et al. Prenatal Exposure to Metals and Neurodevelopment in Infants at Six Months: Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA Project). Int. J. Environ. Res. Public Health 2022, 19, 4295. [Google Scholar] [CrossRef]
- Deroma, L.; Parpinel, M.; Tognin, V.; Channoufi, L.; Tratnik, J.; Horvat, M.; Valent, F.; Barbone, F. Neuropsychological assessment at school-age and prenatal low-level exposure to mercury through fish consumption in an Italian birth cohort living near a contaminated site. Int. J. Hyg. Environ. Health 2013, 216, 486–493. [Google Scholar] [CrossRef]
- Farías, P.; Hernández-Bonilla, D.; Moreno-Macías, H.; Montes-López, S.; Schnaas, L.; Texcalac-Sangrador, J.L.; Ríos, C.; Riojas-Rodríguez, H. Prenatal Co-Exposure to Manganese, Mercury, and Lead, and Neurodevelopment in Children during the First Year of Life. Int. J. Environ. Res. Public Health 2022, 19, 13020. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Wang, C.; Zhou, Y.; Zhang, Y.; Wang, Y.; Shi, R.; Gao, Y.; Tian, Y. Prenatal low-level mercury exposure and infant neurodevelopment at 12 months in rural northern China. Environ. Sci. Pollut. Res. 2016, 23, 12050–12059. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-C.; Su, P.-H.; Chen, H.-Y.; Huang, H.-B.; Tsai, J.-L.; Huang, H.-I.; Wang, S.-L. Childhood blood lead levels and intellectual development after ban of leaded gasoline in Taiwan: A 9-year prospective study. Environ. Int. 2012, 40, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kashala-Abotnes, E.; Mumbere, P.P.; Mishika, J.M.; Ndjukendi, A.O.; Mpaka, D.B.; Bumoko, M.-M.G.; Kayembe, T.K.; Tshala-Katumbay, D.; Kazadi, T.K.; Okitundu, D.L. Lead exposure and early child neurodevelopment among children 12–24 months in Kinshasa, the Democratic Republic of Congo. Eur. Child. Adolesc. Psychiatry 2016, 25, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Bottai, M.; Georgiou, V.; Koutra, K.; Chalkiadaki, G.; Kampouri, M.; Kyriklaki, A.; Vafeiadi, M.; Fthenou, E.; Vassilaki, M.; et al. Impact of prenatal exposure to cadmium on cognitive development at preschool age and the importance of selenium and iodine. Eur. J. Epidemiol. 2016, 31, 1123–1134. [Google Scholar] [CrossRef]
- Kou, X.; Millán, M.P.; Canals, J.; Moreno, V.R.; Renzetti, S.; Arija, V. Effects of prenatal exposure to multiple heavy metals on infant neurodevelopment: A multi-statistical approach. Environ. Pollut. 2025, 367, 125647. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.; Ha, E.; Hong, Y.-C.; Ha, M.; Park, H.; Kim, B.-N.; Lee, S.-J.; Lee, K.Y.; Kim, J.H.; et al. Stability of cognitive development during the first five years of life in relation to heavy metal concentrations in umbilical cord blood: Mothers’ and Children’s Environmental Health (MOCEH) birth cohort study. Sci. Total Environ. 2017, 609, 153–159. [Google Scholar] [CrossRef]
- Lee, M.-J.; Chou, M.-C.; Chou, W.-J.; Huang, C.-W.; Kuo, H.-C.; Lee, S.-Y.; Wang, L.-J. Heavy Metals’ Effect on Susceptibility to Attention-Deficit/Hyperactivity Disorder: Implication of Lead, Cadmium, and Antimony. Int. J. Environ. Res. Public Health 2018, 15, 1221. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, K.-N.; Ahn, Y.D.; Choi, Y.-J.; Cho, J.; Jang, Y.; Lim, Y.-H.; Kim, J.I.; Shin, C.H.; Lee, Y.A.; et al. Prenatal and postnatal exposures to four metals mixture and IQ in 6-year-old children: A prospective cohort study in South Korea. Environ. Int. 2021, 157, 106798. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chen, Y.-C.; Su, F.-C.; Lin, C.-M.; Liao, H.-F.; Hwang, Y.-H.; Hsieh, W.-S.; Jeng, S.-F.; Su, Y.-N.; Chen, P.-C. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ. Res. 2013, 123, 52–57. [Google Scholar] [CrossRef]
- Llop, S.; Guxens, M.; Murcia, M.; Lertxundi, A.; Ramon, R.; Riaño, I.; Rebagliato, M.; Ibarluzea, J.; Tardon, A.; Sunyer, J.; et al. Prenatal Exposure to Mercury and Infant Neurodevelopment in a Multicenter Cohort in Spain: Study of Potential Modifiers. Am. J. Epidemiol. 2012, 175, 451–465. [Google Scholar] [CrossRef]
- Llop, S.; Ballester, F.; Murcia, M.; Forns, J.; Tardon, A.; Andiarena, A.; Vioque, J.; Ibarluzea, J.; Fernández-Somoano, A.; Sunyer, J.; et al. Prenatal exposure to mercury and neuropsychological development in young children: The role of fish consumption. Int. J. Epidemiol. 2017, 46, 827–838. [Google Scholar] [CrossRef]
- Lu, A.-X.; Wang, S.-S.; Xu, X.; Wu, M.-Q.; Liu, J.-X.; Xu, M.; Cao, L.-L.; Wang, J.; Wu, W.; Li, H.; et al. Sex-specific associations between cord blood lead and neurodevelopment in early life: The mother-child cohort (Shanghai, China). Ecotoxicol. Environ. Saf. 2023, 249, 114337. [Google Scholar] [CrossRef]
- Ma, C.; Iwai-Shimada, M.; Nakayama, S.F.; Isobe, T.; Kobayashi, Y.; Tatsuta, N.; Taniguchi, Y.; Sekiyama, M.; Michikawa, T.; Yamazaki, S.; et al. Association of prenatal exposure to cadmium with neurodevelopment in children at 2 years of age: The Japan Environment and Children’s Study. Environ. Int. 2021, 156, 106762. [Google Scholar] [CrossRef]
- Merced-Nieves, F.M.; Chelonis, J.; Pantic, I.; Schnass, L.; Téllez-Rojo, M.M.; Braun, J.M.; Paule, M.G.; Wright, R.J.; Wright, R.O.; Curtin, P. Sexually dimorphic associations between prenatal blood lead exposure and performance on a behavioral testing battery in children. Neurotoxicology Teratol. 2022, 90, 107075. [Google Scholar] [CrossRef] [PubMed]
- Notario-Barandiaran, L.; Díaz-Coto, S.; Jimenez-Redondo, N.; Guxens, M.; Vrijheid, M.; Andiarena, A.; Irizar, A.; Riaño-Galan, I.; Fernández-Somoano, A.; Llop, S.; et al. Latent Childhood Exposure to Mixtures of Metals and Neurodevelopmental Outcomes in 4–5-Year-Old Children Living in Spain. Expo. Health 2024, 16, 1053–1066. [Google Scholar] [CrossRef]
- Orenstein, S.T.C.; Thurston, S.W.; Bellinger, D.C.; Schwartz, J.D.; Amarasiriwardena, C.J.; Altshul, L.M.; Korrick, S.A. Prenatal Organochlorine and Methylmercury Exposure and Memory and Learning in School-Age Children in Communities Near the New Bedford Harbor Superfund Site, Massachusetts. Environ. Health Perspect. 2014, 122, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, R.P.; Fujiwara, T.; Umezaki, M.; Watanabe, C. Association of cord blood levels of lead, arsenic, and zinc with neurodevelopmental indicators in newborns: A birth cohort study in Chitwan Valley, Nepal. Environ. Res. 2013, 121, 45–51. [Google Scholar] [CrossRef]
- Parajuli, R.P.; Fujiwara, T.; Umezaki, M.; Furusawa, H.; Watanabe, C. Home environment and prenatal exposure to lead, arsenic and zinc on the neurodevelopment of six-month-old infants living in Chitwan Valley, Nepal. Neurotoxicology Teratol. 2014, 41, 89–95. [Google Scholar] [CrossRef]
- Parajuli, R.P.; Umezaki, M.; Fujiwara, T.; Watanabe, C. Association of Cord Blood Levels of Lead, Arsenic, and Zinc and Home Environment with Children Neurodevelopment at 36 Months Living in Chitwan Valley, Nepal. PLoS ONE 2015, 10, e0120992. [Google Scholar] [CrossRef]
- Polanska, K.; Hanke, W.; Pawlas, N.; Wesolowska, E.; Jankowska, A.; Jagodic, M.; Mazej, D.; Dominowska, J.; Grzesiak, M.; Mirabella, F.; et al. Sex-Dependent Impact of Low-Level Lead Exposure during Prenatal Period on Child Psychomotor Functions. Int. J. Environ. Res. Public Health 2018, 15, 2263. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Y.; Gan, M.; Wang, W.; Jiang, T.; Jiang, Y.; Lv, H.; Lu, Q.; Qin, R.; Tao, S.; et al. Association of prenatal multiple metal exposures with child neurodevelopment at 3 years of age: A prospective birth cohort study. Sci. Total Environ. 2024, 942, 173812. [Google Scholar] [CrossRef]
- Rosa, M.J.; Foppa Pedretti, N.; Goldson, B.; Mathews, N.; Merced-Nieves, F.; Xhani, N.; Enlow, M.B.; Gershon, R.; Ho, E.; Huddleston, K.; et al. Integrating Data Across Multiple Sites in the Northeastern United States to Examine Associations Between a Prenatal Metal Mixture and Child Cognition. Am. J. Epidemiol. 2024, 193, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.E.; Yu, X.; Liu, J.; Biasini, F.J.; Hong, C.; Jiang, X.; Nong, Y.; Cheng, Y.; Korrick, S.A. Maternal methylmercury exposure through rice ingestion and offspring neurodevelopment: A prospective cohort study. Int. J. Hyg. Environ. Health 2016, 219, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castell, M.; Paco, P.; Barbieri, F.-L.; Duprey, J.-L.; Forns, J.; Carsin, A.-E.; Freydier, R.; Casiot, C.; Sunyer, J.; Gardon, J. Child neurodevelopment in a Bolivian mining city. Environ. Res. 2012, 112, 147–154. [Google Scholar] [CrossRef]
- Shah-Kulkarni, S.; Lee, S.; Jeong, K.S.; Hong, Y.-C.; Park, H.; Ha, M.; Kim, Y.; Ha, E.-H. Prenatal exposure to mixtures of heavy metals and neurodevelopment in infants at 6 months. Environ. Res. 2020, 182, 109122. [Google Scholar] [CrossRef]
- Shekhawat, D.S.; Janu, V.C.; Singh, P.; Sharma, P.; Singh, K. Association of newborn blood lead concentration with neurodevelopment outcome in early infancy. J. Trace Elem. Med. Biol. 2021, 68, 126853. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Nakai, K.; Murata, K.; Suzuki, K.; Iwai-Shimada, M.; Kurokawa, N.; Hosokawa, T.; Satoh, H. Impacts of prenatal exposures to polychlorinated biphenyls, methylmercury, and lead on intellectual ability of 42-month-old children in Japan. Environ. Res. 2014, 133, 321–326. [Google Scholar] [CrossRef]
- Taylor, C.M.; Emond, A.M.; Lingam, R.; Golding, J. Prenatal lead, cadmium and mercury exposure and associations with motor skills at age 7 years in a UK observational birth cohort. Environ. Int. 2018, 117, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Liang, C.; Wu, X.; Huang, K.; Zhu, B.; Gao, H.; Zhu, Y.; Li, Z.; Qi, J.; Han, Y.; et al. Prenatal serum thallium exposure and cognitive development among preschool-aged children: A prospective cohort study in China. Environ. Pollut. 2022, 293, 118545. [Google Scholar] [CrossRef]
- Tong, J.; Liang, C.; Tao, S.; Geng, M.; Gan, H.; Yan, S.; Cao, H.; Xie, L.; Huang, K.; Tao, F.; et al. Association of maternal and cord blood barium exposure with preschoolers’ intellectual function: Evidence from the Ma’anshan Birth Cohort (MABC) study. Sci. Total Environ. 2023, 858, 160029. [Google Scholar] [CrossRef]
- Valent, F.; Mariuz, M.; Bin, M.; Little, D.; Mazej, D.; Tognin, V.; Tratnik, J.; McAfee, A.J.; Mulhern, M.S.; Parpinel, M.; et al. Associations of Prenatal Mercury Exposure From Maternal Fish Consumption and Polyunsaturated Fatty Acids With Child Neurodevelopment: A Prospective Cohort Study in Italy. J. Epidemiol. 2013, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Vejrup, K.; Brandlistuen, R.E.; Brantsæter, A.L.; Knutsen, H.K.; Caspersen, I.H.; Alexander, J.; Lundh, T.; Meltzer, H.M.; Magnus, P.; Haugen, M. Prenatal mercury exposure, maternal seafood consumption and associations with child language at five years. Environ. Int. 2018, 110, 71–79. [Google Scholar] [CrossRef]
- Xue, Q.; Zhou, Y.; Gu, H.; Xie, X.; Hou, F.; Liu, Q.; Wu, H.; Zhu, K.; Wan, Z.; Song, R. Urine metals concentrations and dyslexia among children in China. Environ. Int. 2020, 139, 105707. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.P.; Portella, A.K.; Goldani, M.Z.; Barbieri, M.A. Developmental origins of health and disease (DOHaD). J. Pediatr. (Rio J) 2007, 83, 494–504. [Google Scholar] [CrossRef]
- Margolis, E.T.; Gabard-Durnam, L.J. Prenatal influences on postnatal neuroplasticity: Integrating DOHaD and sensitive/critical period frameworks to understand biological embedding in early development. Infancy 2025, 30, e12588. [Google Scholar] [CrossRef]
- de Water, E.; Curtin, P.; Gennings, C.; Chelonis, J.J.; Paule, M.; Bixby, M.; McRae, N.; Svensson, K.; Schnaas, L.; Pantic, I.; et al. Prenatal metal mixture concentrations and reward motivation in children. NeuroToxicology 2022, 88, 124–133. [Google Scholar] [CrossRef]
- Heng, Y.Y.; Asad, I.; Coleman, B.; Menard, L.; Benki-Nugent, S.; Hussein Were, F.; Karr, C.J.; McHenry, M.S.; Lehmann, D.M. Heavy metals and neurodevelopment of children in low and middle-income countries: A systematic review. PLoS ONE 2022, 17, e0265536. [Google Scholar] [CrossRef]
- Desye, B.; Tesfaye, A.H.; Berihun, G.; Ademas, A.; Sewunet, B. A systematic review of the health effects of lead exposure from electronic waste in children. Front. Public Health 2023, 11, 1113561. [Google Scholar] [CrossRef]
- Rosenauer, V.; Schwarz, M.I.; Vlasak, T.; Barth, A. Childhood lead exposure increases the risk of attention-deficit-hyperactivity disorder: A meta-analysis. Sci. Total Environ. 2024, 951, 175574. [Google Scholar] [CrossRef] [PubMed]
- Anchidin-Norocel, L.; Iatcu, O.C.; Lobiuc, A.; Covasa, M. Heavy Metal–Gut Microbiota Interactions: Probiotics Modulation and Biosensors Detection. Biosensors 2025, 15, 188. [Google Scholar] [CrossRef]
- Ghosh, S.; Nukavarapu, S.P.; Jala, V.R. Effects of heavy metals on gut barrier integrity and gut microbiota. Microbiota Host 2023, 2, e230015. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Chamorro, F.; Otero, P.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Seyyedi-Mansour, S.; Cassani, L.; Prieto, M.A. Health Benefits of Oily Fish: Illustrated with Blue Shark (Prionace glauca), Shortfin Mako Shark (Isurus oxyrinchus), and Swordfish (Xiphias gladius). Nutrients 2023, 15, 4919. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Spiller, P.; Brenna, J.T.; Golding, J.; Holub, B.J.; Harris, W.S.; Kris-Etherton, P.; Lands, B.; Connor, S.L.; Myers, G.; et al. Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: Two systematic reviews. Prostaglandins Leukot. Essent. Fat. Acids 2019, 151, 14–36. [Google Scholar] [CrossRef]
- Lyall, K.; Westlake, M.; Musci, R.J.; Gachigi, K.; Barrett, E.S.; Bastain, T.M.; Bush, N.R.; Buss, C.; Camargo, C.A.; Croen, L.A.; et al. Association of maternal fish consumption and ω-3 supplement use during pregnancy with child autism-related outcomes: Results from a cohort consortium analysis. Am. J. Clin. Nutr. 2024, 120, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Hernández, A.; Alda, J.A.; Farran-Codina, A.; Ferreira-García, E.; Izquierdo-Pulido, M. The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics 2017, 139, e20162027. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Spill, M.K.; Saha, S.; Balalian, A.; Davis, J.S.; MacFarlane, A.J. Seafood and Neurocognitive Development in Children: A Systematic Review. Adv. Nutr. 2025, 16, 100391. [Google Scholar] [CrossRef]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee on Nutrition; Daniels, S.; Corkins, M.; Golden, N.H.; Kim, J.H.; Lindsey, C.W.; Maggeet, S.N. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [PubMed]
| Country | N Studies | N Sample |
|---|---|---|
| Japan | 5 | 181,145 |
| China | 12 | 8485 |
| Spain | 5 | 4479 |
| Norway | 2 | 4375 |
| South Korea | 6 | 2863 |
| Saudi Arabia | 3 | 1970 |
| United Kingdom | 1 | 1558 |
| Mexico | 4 | 1481 |
| Brazil | 4 | 1167 |
| USA | 4 | 1057 |
| India | 2 | 992 |
| Taiwan | 5 | 762 |
| Italy | 2 | 760 |
| Canada | 2 | 727 |
| Seychelles | 1 | 643 |
| Greece | 1 | 575 |
| Bangladesh | 1 | 525 |
| Tanzania | 1 | 439 |
| Poland | 1 | 402 |
| Nepal | 3 | 300 |
| Bolivia | 1 | 246 |
| Malaysia | 1 | 155 |
| Democratic Republic of Congo | 1 | 89 |
| Total | 68 | 215,195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.S.; Santos, R.M.S.; De Marco, P.G.; Rezende, V.H.M.; Martins, T.C.; Silva, J.R.; Romano-Silva, M.A.; Miranda, D.M.d. Neurodevelopmental Outcomes Associated with Early-Life Exposure to Heavy Metals: A Systematic Review. Int. J. Environ. Res. Public Health 2025, 22, 1308. https://doi.org/10.3390/ijerph22081308
da Silva AS, Santos RMS, De Marco PG, Rezende VHM, Martins TC, Silva JR, Romano-Silva MA, Miranda DMd. Neurodevelopmental Outcomes Associated with Early-Life Exposure to Heavy Metals: A Systematic Review. International Journal of Environmental Research and Public Health. 2025; 22(8):1308. https://doi.org/10.3390/ijerph22081308
Chicago/Turabian Styleda Silva, André Soares, Renata Maria Silva Santos, Patricia Gazire De Marco, Victhor Hugo Martins Rezende, Tamires Coelho Martins, Joyce Romano Silva, Marco Aurélio Romano-Silva, and Débora Marques de Miranda. 2025. "Neurodevelopmental Outcomes Associated with Early-Life Exposure to Heavy Metals: A Systematic Review" International Journal of Environmental Research and Public Health 22, no. 8: 1308. https://doi.org/10.3390/ijerph22081308
APA Styleda Silva, A. S., Santos, R. M. S., De Marco, P. G., Rezende, V. H. M., Martins, T. C., Silva, J. R., Romano-Silva, M. A., & Miranda, D. M. d. (2025). Neurodevelopmental Outcomes Associated with Early-Life Exposure to Heavy Metals: A Systematic Review. International Journal of Environmental Research and Public Health, 22(8), 1308. https://doi.org/10.3390/ijerph22081308






