Effects of a 16-Week Green Exercise Program on Body Composition, Sleep, and Nature Connection in Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
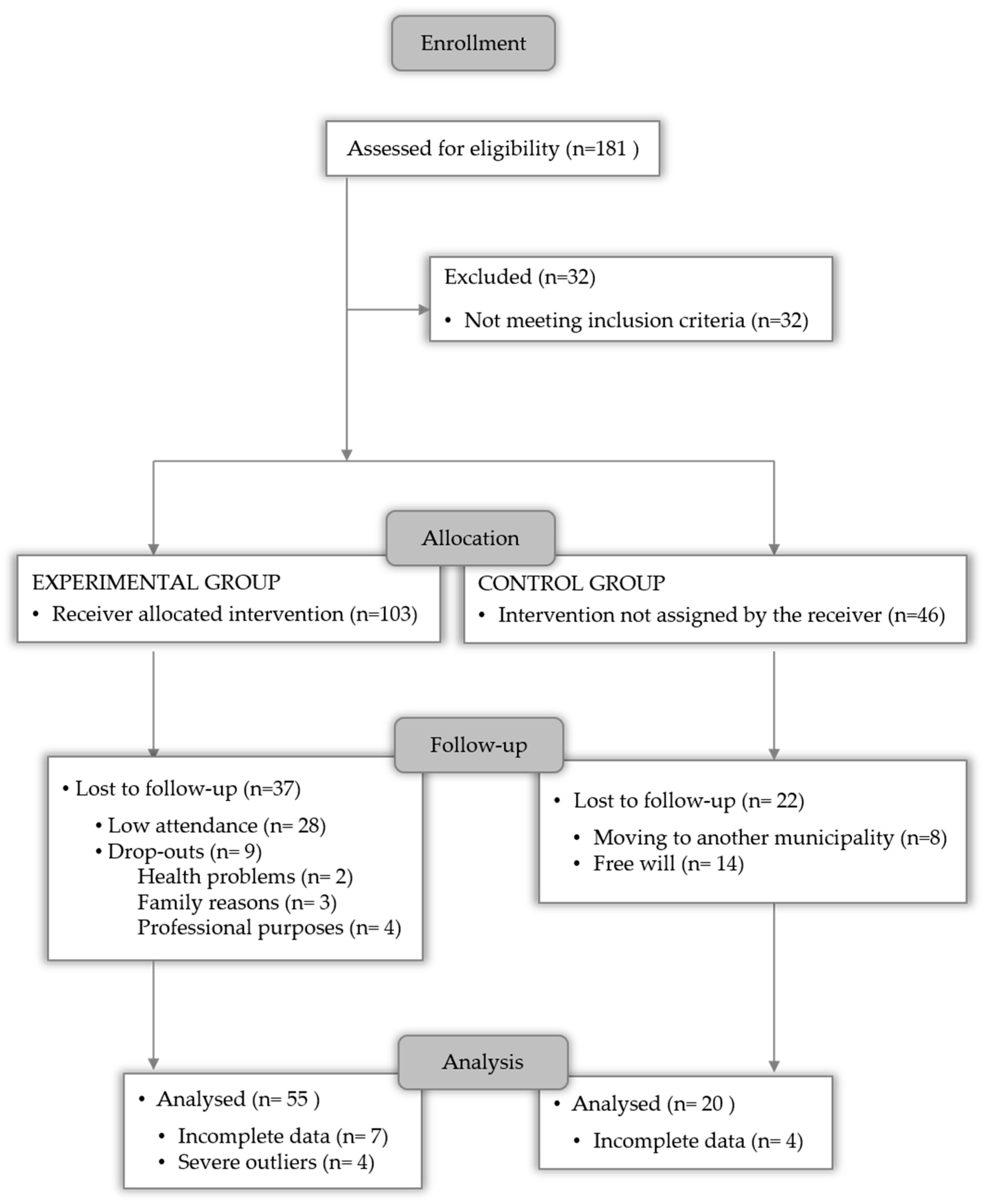
2.1. Study Design
2.2. Sample
2.3. Anthropometry/Body Composition
2.4. Sleep
2.5. Connection with Nature
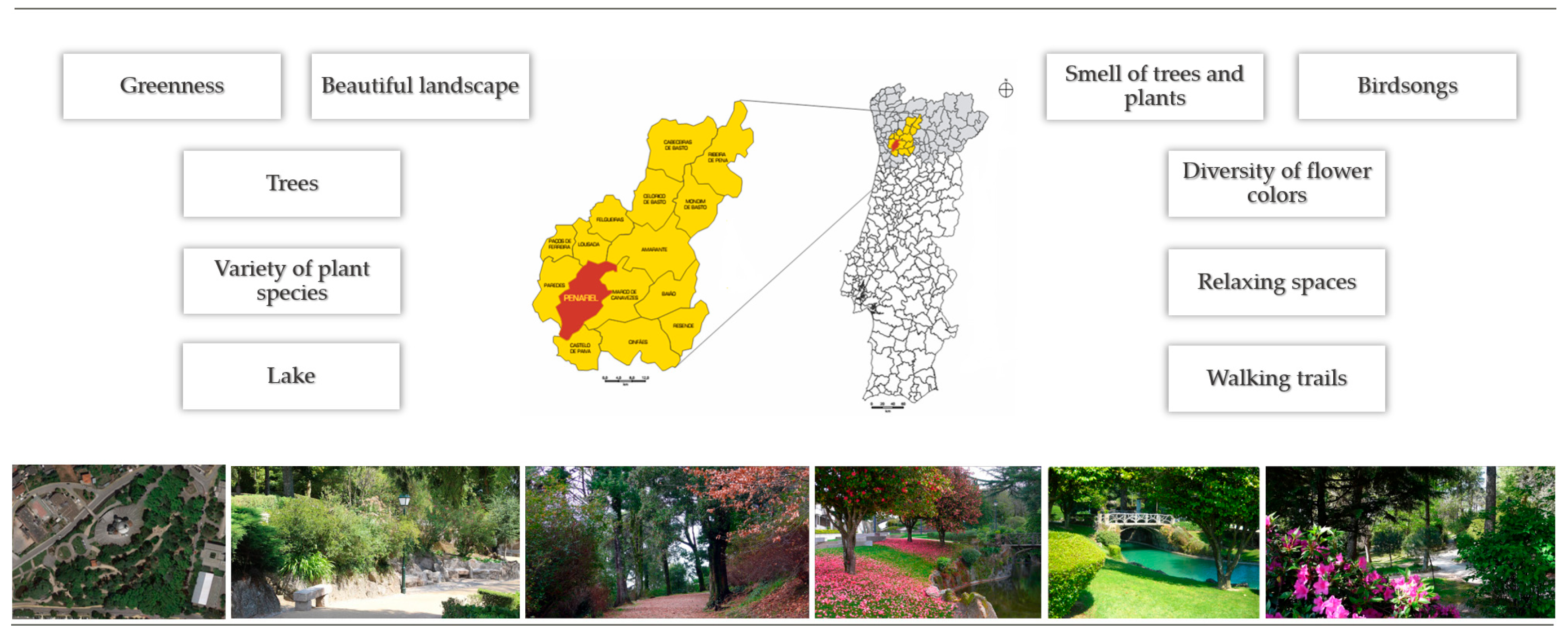
2.6. The Program Meno(s)Pausa+Movimento
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nisbet, E.; Zelenski, J.; Murphy, S. The Nature Relatedness Scale: Linking Individuals’ Connection with Nature to Environmental Concern and Behavior. Environ. Behav. 2008, 41, 715–740. [Google Scholar] [CrossRef]
- Martyn, P.; Brymer, E. The relationship between nature relatedness and anxiety. J. Health Psychol. 2016, 21, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Loewen, A.; Siemens, A.; Hanly, P. Sleep disruption in patients with sleep apnea and end-stage renal disease. J. Clin. Sleep Med. 2009, 5, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Beaunoyer, E.; Guitton, M.J.; Wang, J. Physical activity as a clinical tool against depression: Opportunities and challenges. J. Integr. Neurosci. 2022, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, J.; Li, P.; Liang, Y. Effects of mind-body exercise on perimenopausal and postmenopausal women: A systematic review and meta-analysis. Menopause 2024, 31, 457–467. [Google Scholar] [CrossRef]
- Abdel-Salam, D.M.; Mohamed, R.A.; Alruwaili, R.R.; Alhablani, F.S.; Aldaghmi, R.M.; ALghassab, R.E. Postmenopausal symptoms and their correlates among saudi women attending different primary health centers. Int. J. Environ. Res. Public Health 2021, 18, 6831. [Google Scholar] [CrossRef]
- Gujski, M.; Raczkiewicz, D.; Humeniuk, E.; Sarecka-Hujar, B.; Wdowiak, A.; Bojar, I. Depressive symptoms and healthy behavior frequency in polish postmenopausal women from urban and rural areas. Int. J. Environ. Res. Public Health 2021, 18, 2967. [Google Scholar] [CrossRef]
- Zimny, M.; Starczewska, M.; Szkup, M.; Karakiewicz-Krawczyk, K.; Grochans, E.; Sipak-Szmigiel, O. Analysis of the impact of type 2 diabetes on the psychosocial functioning and quality of life of perimenopausal women. Int. J. Environ. Res. Public Health 2020, 17, 4349. [Google Scholar] [CrossRef]
- Moreira, H.; Abrantes, C.; Teixeira, A.; Alves, E.; Silva, P.; Gabriel, R. Effects of green exercise program on body composition and connection with nature in postmenopausal women. Motricidade 2024, 20. [Google Scholar] [CrossRef]
- Irvine, K.N.; Fisher, D.; Currie, M.; Colley, K.; Warber, S.L. A nature-based intervention for promoting physical activity in older adults: A qualitative study using the COM-B model. Int. J. Environ. Res. Public Health 2024, 21, 843. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, B.; Huang, G.; Zhang, G.; Ding, Z.; Li, Z.; Sinclair, J.; Fan, Y. Sarcopenia: Body composition and gait analysis. Front. Aging Neurosci 2022, 14, 909551. [Google Scholar] [CrossRef] [PubMed]
- Gladwell, V.F.; Brown, D.K.; Wood, C.; Sandercock, G.R.; Barton, J.L. The great outdoors: How a green exercise environment can benefit all. Extrem. Physiol. Med. 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Lounassalo, I.; Salin, K.; Kankaanpää, A.; Hirvensalo, M.; Palomäki, S.; Tolvanen, A.; Yang, X.; Tammelin, T.H. Distinct trajectories of physical activity and related factors during the life course in the general population: A systematic review. BMC Public Health 2019, 19, 271. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Rodriguez-Manas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef]
- Chiu, T.-Y.; Yu, H.-W. Associations of multicomponent exercise and aspects of physical performance with frailty trajectory in older adults. BMC Geriatr. 2022, 22, 559. [Google Scholar] [CrossRef]
- Labata-Lezaun, N.; Gonzalez-Rueda, V.; Llurda-Almuzara, L.; Lopez-de-Celis, C.; Rodriguez-Sanz, J.; Bosch, J.; Vicente-Rodriguez, G.; Gorczakowska, D.; Araluze-Arizti, P.; Perez-Bellmunt, A. Effectiveness of multicomponent training on physical performance in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 104, 104838. [Google Scholar] [CrossRef]
- Linhares, D.G.; Borba-Pinheiro, C.J.; Castro, J.B.P.d.; Santos, A.O.B.d.; Santos, L.L.d.; Cordeiro, L.d.S.; Drigo, A.J.; Nunes, R.d.A.M.; Vale, R.G.d.S. Effects of multicomponent exercise training on the health of older women with osteoporosis: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 14195. [Google Scholar] [CrossRef]
- Oliveira Gonçalves, I.d.; Bandeira, A.N.; Coelho-Júnior, H.J.; Silva Aguiar, S.d.; Minucci Camargo, S.; Yukio Asano, R.; Batista Junior, M.L. Multicomponent exercise on physical function, cognition and hemodynamic parameters of community-dwelling older adults: A quasi-experimental study. Int. J. Environ. Res. Public Health 2019, 16, 2184. [Google Scholar] [CrossRef]
- Baena-García, L.; Flor-Alemany, M.; Marín-Jiménez, N.; Aranda, P.; Aparicio, V.A. A 16-week multicomponent exercise training program improves menopause-related symptoms in middle-aged women. The FLAMENCO project randomized control trial. Menopause 2022, 29, 537–544. [Google Scholar] [CrossRef]
- Ferreira, L.; Abrantes, C.; Alves, M.E.; Moreira, C.; Moreira, H. Effects of exercise programs on cardiorespiratory fitness and arterial stiffness on postmenopausal women: A systematic review study. Maturitas 2024, 181, 107917. [Google Scholar] [CrossRef]
- Paoletti, D.; DE Stasio, S.; Ragni, B.; Callea, A.; Rosati, N.; Berenguer, C.; Cinque, M.; Nisbet, E. Psychometric properties of the nature relatedness scale (NRS) in the italian context. Anthropol. Res. Stud. 2025, 28–42. [Google Scholar] [CrossRef]
- Lawton, E.; Brymer, E.; Clough, P.; Denovan, A. The relationship between the physical activity environment, nature relatedness, anxiety, and the psychological well-being benefits of regular exercisers. Front. Psychol 2017, 8, 1058. [Google Scholar] [CrossRef]
- Teixeira, A.; Gabriel, R.; Martinho, J.; Pinto, G.; Quaresma, L.; Faria, A.; Oliveira, I.; Moreira, H. Connectedness to nature does not explain the variation in physical activity and body composition in adults and older people. Int. J. Environ. Res. Public Health 2021, 18, 11951. [Google Scholar] [CrossRef] [PubMed]
- Zelenski, J.M.; Nisbet, E.K. Happiness and feeling connected: The distinct role of nature relatedness. Environ. Behav. 2014, 46, 3–23. [Google Scholar] [CrossRef]
- Mayer, F.; Frantz, C. The connectedness to nature scale: A measure of individuals’ feeling in community with nature. Environ. Psychol. 2004, 24, 503–515. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; De Villiers, T.J.; Group, S.C. Executive summary of the Stages of Reproductive Aging Workshop+ 10: Addressing the unfinished agenda of staging reproductive aging. Climacteric 2012, 15, 105–114. [Google Scholar] [CrossRef]
- Heyward, V.H.; Wagner, D.R. Applied Body Composition Assessment; 2004; Available online: https://archive.org/details/appliedbodycompo0002heyw (accessed on 19 June 2025).
- Sobral, F. Curso de Antropometria; Universidade Técnica de Lisboa: Lisboa, Portugal, 1985. [Google Scholar]
- Biospace. InBody 120 Manual de Usuario, 54th ed. 1996. Available online: http://inbodyargentina.com.ar/descargas/manual-inbody-120.pdf (accessed on 25 July 2025).
- Lohman, T.; Going, S. Assessment of body composition and energy balance. Perspect. Exerc. Sci. Sports Med. 1998, 11, 61–98. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cole, R.J.; Kripke, D.F.; Gruen, W.; Mullaney, D.J.; Gillin, J.C. Automatic sleep/wake identification from wrist activity. Sleep 1992, 15, 461–469. [Google Scholar] [CrossRef]
- Lacks, P.; Morin, C.M. Recent advances in the assessment and treatment of insomnia. J. Consult. Clin. Psychol 1992, 60, 586. [Google Scholar] [CrossRef]
- Conceição, A. Towards an Effective Change: A Reflection About the Past, Present and Future of Environmental Education. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 2021. [Google Scholar]
- Moreira, H.; Alves, M.; Abrantes, C. O programa de exercício no Meno(s)Pausa+ Movimento. In Meno(s)Pausa+Movimento: Uma Abordagem Multidisciplinar de Promoção do Exercício e da Saúde em Mulheres Pós-Menopáusicas; Moreira, H., Alves, M., Gabriel, R., Abrantes, C., Eds.; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2024; pp. 16–36. [Google Scholar]
- Quaresma, L.; Lourenço, J.; Santos, M.; Rodrigues, F.; Gabriel, R.; Alves, M.; Moreira, C.; Moreira, H. Avaliação e classificação de um percurso pedestre: “Trilho Sentir Penafiel—Entre a Cidade e o Cavalum”. In Meno(s)Pausa+Movimento: Uma Abordagem Multidisciplinar de Promoção do Exercício e da Saúde em Mulheres Pós-Menopáusicas; Moreira, H., Alves, M., Gabriel, R., Abrantes, C., Eds.; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2024; pp. 140–167. [Google Scholar]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Atakan, M.M.; Koşar, Ş.N.; Güzel, Y.; Tin, H.T.; Yan, X. The role of exercise, diet, and cytokines in preventing obesity and improving adipose tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Peacock, J.; Hine, R.; Pretty, J. The mental health benefits of green exercise activities and green care. Rep. MIND 2007, 1–18. Available online: https://www.piuvivi.com/docs/the-mental-health-benefits-of-green-exercise-activities-and-green-care.pdf (accessed on 19 June 2025).
- Zhou, W.; Wang, Q.; Li, R.; Kadier, A.; Wang, W.; Zhou, F.; Ling, L. Combined effects of heatwaves and air pollution, green space and blue space on the incidence of hypertension: A national cohort study. Sci. Total Environ. 2023, 867, 161560. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.D.; Wohlfart, T. Walking, hiking and running in parks: A multidisciplinary assessment of health and well-being benefits. Landsc. Urban Plan 2014, 130, 89–103. [Google Scholar] [CrossRef]
- Melton III, L.J.; Khosla, S.; Crowson, C.S.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Epidemiology of sarcopenia. J. Am. Geriatr. Soc. 2000, 48, 625–630. [Google Scholar] [CrossRef]
- Gallagher, D.; Visser, M.; De Meersman, R.E.; Sepúlveda, D.; Baumgartner, R.N.; Pierson, R.N.; Harris, T.; Heymsfield, S.B. Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J. Appl. Physiol. 1997, 83, 229–239. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Izquierdo, M.; Ibañez, J.; Gorostiaga, E.; Garrues, M.; Zúñiga, A.; Antón, A.; Larrión, J.L.; Häkkinen, K. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta. Physiol. Scand. 1999, 167, 57–68. [Google Scholar] [CrossRef]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and menopause: The role of estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef] [PubMed]
- Wright, V.J.; Schwartzman, J.D.; Itinoche, R.; Wittstein, J. The musculoskeletal syndrome of menopause. Climacteric 2024, 27, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.M.; Perry Iii, H.M.; Patrick, P.; Banks, W.A.; Morley, J.E. Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J. Gerontol. A. Biol. Sci. Med. Sci. 2007, 62, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose–response relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Cho, H.H.; Kim, M.-R. Sleep disorders and menopause. J. Menopausal Med. 2019, 25, 83–87. [Google Scholar] [CrossRef]
- Resta, O.; Pannacciulli, N.; Di Gioia, G.; Stefano, A.; Barbaro, M.F.; De Pergola, G. High prevalence of previously unknown subclinical hypothyroidism in obese patients referred to a sleep clinic for sleep disordered breathing. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 248–253. [Google Scholar] [CrossRef]
- Brown, A.M.; Gervais, N.J. Role of ovarian hormones in the modulation of sleep in females across the adult lifespan. Endocrinology 2020, 161, bqaa128. [Google Scholar] [CrossRef]
- Tandon, V.R.; Sharma, S.; Mahajan, A.; Mahajan, A.; Tandon, A. Menopause and sleep disorders. J. Mid-Life Health 2022, 13, 26–33. [Google Scholar] [CrossRef]
- Buxton, O.M.; Pavlova, M.; Reid, E.W.; Wang, W.; Simonson, D.C.; Adler, G.K. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010, 59, 2126–2133. [Google Scholar] [CrossRef]
- Freeman, E.W.; Sammel, M.D.; Gross, S.A.; Pien, G.W. Poor sleep in relation to natural menopause: A population-based 14-year follow-up of midlife women. Menopause 2015, 22, 719–726. [Google Scholar] [CrossRef]
- Driver, H.S.; Taylor, S.R. Exercise and sleep. Sleep Med. Rev. 2000, 4, 387–402. [Google Scholar] [CrossRef]
- Dolezal, B.A.; Neufeld, E.V.; Boland, D.M.; Martin, J.L.; Cooper, C.B. Interrelationship between sleep and exercise: A systematic review. Adv. Prev. Med. 2017, 2017, 1364387. [Google Scholar]
- Shin, J.C.; Parab, K.V.; An, R.; Grigsby-Toussaint, D.S. Greenspace exposure and sleep: A systematic review. Environ. Res. 2020, 182, 109081. [Google Scholar] [CrossRef]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3 (Suppl. S5), S7–S10. [Google Scholar] [CrossRef]
- Glass, J.; Lanctôt, K.L.; Herrmann, N.; Sproule, B.A.; Busto, U.E. Sedative hypnotics in older people with insomnia: Meta-analysis of risks and benefits. Bmj 2005, 331, 1169. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, A.M.; Crowther, R.; Lotter, A.; Cheng, C.; King, D. Meta-analysis of benzodiazepine use in the treatment of insomnia. Cmaj 2000, 162, 225–233. [Google Scholar] [PubMed]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.H.; Shanahan, D.F.; Bush, R.; Gaston, K.J.; Lin, B.B.; Barber, E.; Franco, L.; Fuller, R.A. Is nature relatedness associated with better mental and physical health? Int. J. Environ. Res. Public Health 2018, 15, 1371. [Google Scholar] [CrossRef]
- Sachs, A.L.; Kolster, A.; Wrigley, J.; Papon, V.; Opacin, N.; Hill, N.; Howarth, M.; Rochau, U.; Hidalgo, L.; Casajuana, C. Connecting through nature: A systematic review of the effectiveness of nature-based social prescribing practices to combat loneliness. Landsc. Urban Plan. 2024, 248, 105071. [Google Scholar] [CrossRef]
- Hoga, L.; Rodolpho, J.; Gonçalves, B.; Quirino, B. Women’s experience of menopause: A systematic review of qualitative evidence. JBI Evid. Synth. 2015, 13, 250–337. [Google Scholar] [CrossRef][Green Version]
- McNeil, D.G.; Singh, A.; Chambers, T. Exploring nature-and social-connectedness as mediators of the relationship between nature-based exercise and subjective wellbeing. Ecopsychology 2022, 14, 226–234. [Google Scholar] [CrossRef]
- Sheffield, D.; Butler, C.W.; Richardson, M. Improving nature connectedness in adults: A meta-analysis, review and agenda. Sustainability 2022, 14, 12494. [Google Scholar] [CrossRef]

| Variables | Experimental Group (n = 55) | Control Group (n = 20) | ||||
|---|---|---|---|---|---|---|
| Baseline Mean ± SD | 16 Weeks Mean ± SD | Δ % | Baseline Mean ± SD | 16 Weeks Mean ± SD | Δ % | |
| Body Composition | ||||||
| Body mass (BM, kg) | 69.07 ± 11.10 | 67.14 ± 10.03 | −2.53 ± 4.52 | 71.10 ± 11.60 (a) | 71.67 ± 12.70 | 0.62 ± 2.98 ** (b) |
| Height (H, m) | 1.57 ± 0.05 | 1.57 ± 0.05 | −0.11 ± 0.97 | 1.58 ± 0.05 (a) | 1.58 ± 0.05 | 0.24 ± 0.58 (b) |
| Fat mass (FM, kg) | 28.18 ± 8.84 | 25.48 ± 8.20 | −9.26 ± 9.89 | 29.44 ± 8.79 (b) | 29.23 ± 9.39 | −1.21 ± 6.21 ** (b) |
| Fat mass (FM, %) | 39.97 ± 6.81 | 37.15 ± 7.06 | −7.16 ± 7.00 | 40.69 ± 6.38 (a) | 39.98 ± 6.74 | −1.87 ± 3.80 ** (b) |
| Visceral fat level (VFL, points) | 13.60 ± 4.42 | 11.80 ± 4.37 | −13.46 ± 11.62 | 14.20 ± 4.64 (b) | 13.75 ± 4.82 | −3.80 ± 6.91 ** (b) |
| Trunk skeletal muscle mass (TSMM, kg) | 18.85 ± 2.11 | 18.76 ± 1.96 | −0.36 ± 3.07 | 19.26 ± 2.17 (b) | 19.37 ± 2.37 | 0.52 ± 2.83 (a) |
| Appendicular skeletal muscle mass (ASMM, kg) | 16.14 ± 2.01 | 16.55 ± 1.98 | 2.64 ± 3.71 | 16.51 ± 2.37 (a) | 17.0 ± 2.62 | 3.22 ± 3.87 (a) |
| Appendicular skeletal muscle mass index (ASMMI kg/m2) | 6.49 ± 0.64 | 6.68 ± 0.61 | 3.01 ± 4.17 | 6.61 ± 0.68 (a) | 6.80 ± 0.81 | 2.73 ± 3.67 (b) |
| Sleep | ||||||
| Total sleep time (TST, Hrs/night) | 7.46 ± 1.01 | 6.81 ± 0.82 | −7.23 ± 16.17 | 7.57 ± 1.05 (a) | 6.60 ± 1.02 | −11.86 ± 14.63 (b) |
| Sleep efficiency (SE, %) | 90.84 ± 4.14 | 87.47 ± 5.48 | −3.56 ± 6.81 | 89.48 ± 2.94 (a) | 87.10 ± 6.66 | −2.59 ± 7.61 (b) |
| Nocturnal awakenings (NA, No.) | 14.48 ± 5.88 | 15.56 ± 5.08 | 25.10 ± 86.87 | 16.70 ± 4.44 (b) | 17.78 ± 9.50 | 7.54 ± 45.35 (b) |
| Minutes of awakenings (MA, Min) | 2.89 ± 0.78 | 3.51 ± 1.21 | 28.62 ± 53.28 | 3.13 ± 0.78 (a) | 3.91 ± 2.66 | 28.24 ± 86.20 (b) |
| Sleep fragmentation index (SFI, No.) | 3.10 ± 1.01 | 3.66 ± 1.20 | 25.45 ± 44.73 | 3.56 ± 0.69 (a) | 3.64 ± 1.48 | 7.39 ± 55.35 * (b) |
| Nature Relatedness | ||||||
| NR-Self (points) | 4.35 ± 0.37 | 4.51 ± 0.26 | 4.20 ± 9.27 | 4.34 ± 0.32 (a) | 4.41 ± 0.32 | 1.93 ± 9.90 (b) |
| NR-Perspective (points) | 2.73 ± 0.39 | 2.75 ± 0.38 | 2.33 ± 18.43 | 2.91 ± 0.44 (a) | 2.88 ± 0.54 | −1.32 ± 10.62 (b) |
| NR-Experience (points) | 3.28 ± 0.41 | 3.35 ± 0.43 | 3.03 ± 15.23 | 3.37 ± 0.47 (b) | 3.36 ± 0.39 | 1.59 ± 17.94 (a) |
| NR-Scale (points) | 3.50 ± 0.23 | 3.59 ± 0.24 | 2.61 ± 7.11 | 3.59 ± 0.26 (a) | 3.60 ± 0.27 | 0.54 ± 7.55 (a) |
| Variables | Experimental Group (n = 55) | Control Group (n = 20) | ||
| n (%) | n (%) | |||
| Nature of menopause | ||||
| Natural | 49 (89.1) | 20 (100.0) | ||
| Induced | 6 (10.9) | 0 (0.0) | ||
| Hormone therapy | ||||
| No | 44 (80.0) | 17 (85.0) | ||
| Yes | 11 (20.0) | 3 (15.0) | ||
| Stage of menopause | ||||
| Early postmenopause | 27 (49.1) | 9 (45.0) | ||
| Late postmenopause | 28 (50.9) | 11 (55.0) | ||
| Variables | Experimental Group (n = 55) | Control Group (n = 20) | ||
| Baseline n (%) | 16 weeks n (%) | Baseline n (%) | 16 weeks n (%) | |
| Obesity | ||||
| No | 13 (23.6) | 19 (34.5) | 5 (25.0) | 5 (25.0) |
| Yes | 42 (76.4) | 35 (65.5) | 15 (75.0) | 15 (75.0) |
| Central obesity | ||||
| No | 11 (20.0) | 17 (30.9) | 2 (10.0) | 6 (30.0) |
| Yes | 41 (80.0) | 38 (69.1) | 18 (90.0) | 14 (70.0) |
| Muscle condition | ||||
| Normal | 51 (92.7) | 53 (96.4) | 20 (100.0) | 19 (95.0) |
| Low | 4 (7.3) | 2 (3.6) | 0 (0.0) | 1 (5.0) |
| Sleep duration | ||||
| Sufficient sleep | 42 (76.4) | 27 (49.1) | 15 (75.0) | 7 (35.0) |
| Insufficient sleep | 13 (23.6) | 28 (50.9) | 5 (25.0) | 13 (65.0) |
| Sleep efficiency | ||||
| Recommended | 50 (90.9) | 41 (74.5) | 20 (100.0) | 15 (75.0) |
| Not recommended | 5 (9.1) | 14 (25.5) | 0 (0.0) | 5 (25.0) |
| Sleep fragmentation index | ||||
| Normal | 53 (96.4) | 44 (80) | 20 (100) | 18 (9) |
| Possible sleep disturbance | 2 (3.6) | 11 (20) | 0 (0) | 2 (10) |
| Dependent Variables | Two-Way Mixed ANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Time × Group | Time × Group × Medication | ||||||||||
| F | p | Partial η2 | Observed Power | F | p | Partial η2 | Observed Power | F | p | Partial η2 | Observed Power | |
| Body Composition | ||||||||||||
| Fat mass (FM, %) | 31.672 | <0.001 | 0.303 | 1.000 | 11.410 | 0.001 | 0.135 | 0.915 | ||||
| Visceral fat level (VFL, points) µ | 31.931 | <0.001 | 0.304 | 1.000 | 11.495 | 0.001 | 0.136 | 0.917 | ||||
| Trunk skeletal muscle mass (TSMM, kg) | 0.018 | 0.893 | <0.001 | 0.052 | 1.648 | 0.203 | 0.022 | 0.245 | ||||
| Appendicular skeletal muscle mass (ASMM, kg) β | 33.575 | <0.001 | 0.315 | 1.000 | 11.641 | 0.001 | 0.138 | 0.920 | ||||
| Appendicular skeletal muscle mass index (ASMMI kg/m2) | 35.185 | <0.001 | 0.325 | 1.000 | 0.725 | 0.397 | 0.010 | 0.134 | ||||
| Sleep | ||||||||||||
| Total sleep time (TST, Hrs/night) | 25.542 | <0.001 | 0.265 | 0.999 | 1.939 | 0.169 | 0.026 | 0.278 | 1.048 | 0.310 | 0.015 | 0.172 |
| Sleep efficiency (SE, %) α | 17.976 | <0.001 | 0.202 | 0.987 | 0.186 | 0.668 | 0.003 | 0.071 | 4.697 | 0.034 | 0.062 | 0.571 |
| Nocturnal awakenings (NA, No) γ | 1.599 | 0.210 | 0.022 | 0.239 | 0.871 | 0.354 | 0.012 | 0.151 | 0.046 | 0.830 | 0.001 | 0.055 |
| Minutes of awakenings (MA, Min) χ | 7.004 | 0.010 | 0.090 | 0.742 | 0.300 | 0.586 | 0.004 | 0.084 | 0.102 | 0.750 | 0.001 | 0.062 |
| Sleep fragmentation index (SFI, No) | 9.010 | 0.004 | 0.113 | 0.842 | 0.063 | 0.802 | 0.001 | 0.057 | 3.189 | 0.078 | 0.043 | 0.422 |
| Nature Relatedness | ||||||||||||
| NR-Self (points) α | 4.246 | 0.043 | 0.055 | 0.529 | 0.744 | 0.391 | 0.010 | 0.136 | ||||
| NR-Perspective (points) µ | 0.025 | 0.874 | <0.001 | 0.053 | 0.240 | 0.625 | 0.003 | 0.077 | ||||
| NR-Experience (points) ε | 0.119 | 0.731 | 0.002 | 0.063 | 0.371 | 0.544 | 0.005 | 0.092 | ||||
| NR-Scale (points) | 2.103 | 0.151 | 0.028 | 0.299 | 1.265 | 0.264 | 0.017 | 0.199 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, H.; Tuccella, C.; Alves, E.; Teixeira, A.; Moreira, C.; Oliveira, I.; Bonavolontà, V.; Abrantes, C. Effects of a 16-Week Green Exercise Program on Body Composition, Sleep, and Nature Connection in Postmenopausal Women. Int. J. Environ. Res. Public Health 2025, 22, 1216. https://doi.org/10.3390/ijerph22081216
Moreira H, Tuccella C, Alves E, Teixeira A, Moreira C, Oliveira I, Bonavolontà V, Abrantes C. Effects of a 16-Week Green Exercise Program on Body Composition, Sleep, and Nature Connection in Postmenopausal Women. International Journal of Environmental Research and Public Health. 2025; 22(8):1216. https://doi.org/10.3390/ijerph22081216
Chicago/Turabian StyleMoreira, Helena, Chiara Tuccella, Emília Alves, Andreia Teixeira, Carlos Moreira, Irene Oliveira, Valerio Bonavolontà, and Catarina Abrantes. 2025. "Effects of a 16-Week Green Exercise Program on Body Composition, Sleep, and Nature Connection in Postmenopausal Women" International Journal of Environmental Research and Public Health 22, no. 8: 1216. https://doi.org/10.3390/ijerph22081216
APA StyleMoreira, H., Tuccella, C., Alves, E., Teixeira, A., Moreira, C., Oliveira, I., Bonavolontà, V., & Abrantes, C. (2025). Effects of a 16-Week Green Exercise Program on Body Composition, Sleep, and Nature Connection in Postmenopausal Women. International Journal of Environmental Research and Public Health, 22(8), 1216. https://doi.org/10.3390/ijerph22081216











