A Bibliometric Analysis of Chrononutrition, Cardiometabolic Risk, and Public Health in International Research (1957–2025)
Abstract
1. Introduction
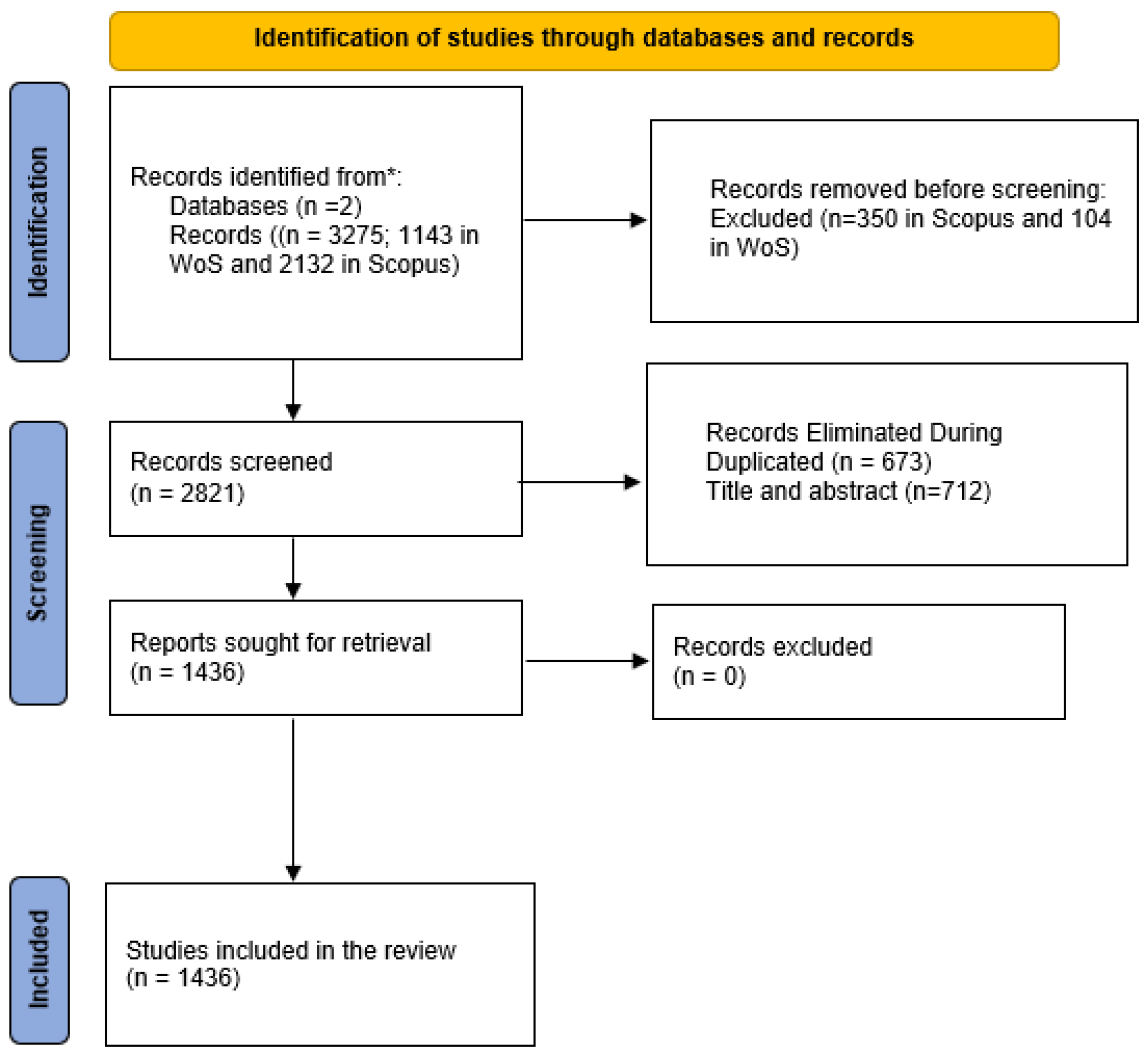
2. Methodology
2.1. Research Design and Search Protocol
- P (Population): Individuals of various ages (children, adolescents, adults, older adults) with daily dietary habits.
- I (Interest): Nutritional quality of breakfast (frequency, composition, omission) and its association with cardiovascular health markers (cholesterol, blood pressure, heart disease).
- Co (Context): Clinical, nutritional, or epidemiological studies conducted in population or institutional settings.
- How has international scientific research on breakfast evolved in relation to chrononutrition, cardiometabolic risk, and public health implications between 1957 and 2025?
- Q1: What are the dominant and emerging approaches in the literature on chrononutrition and its relationship with breakfast?
- Q2: Which cardiometabolic risk markers are most frequently associated with breakfast consumption in the literature, and what trends in their study can be identified?
- Q3: Which countries, institutions, and authors lead scientific production, and what collaboration networks have formed around breakfast and cardiometabolic health?
- Q4: What public health implications and recommendations related to breakfast consumption and cardiometabolic health are identified in the literature for school, community, or clinical contexts?
2.2. Data Sources and Search Strategy
2.3. Inclusion and Exclusion Criteria
- Studies published in any year were included.
- Documents in English and Spanish were accepted.
- Only original articles were considered, excluding systematic reviews, bibliometric analyses, conference papers, and editorials.
- Titles and abstracts were evaluated to confirm thematic relevance to breakfast habits and cardiovascular markers.
- Studies focusing exclusively on other meals were excluded.
2.4. Data Extraction and Cleaning
- readxl: For importing data from Web of Science.
- data.table: For managing large files extracted from Scopus.
- dplyr: For filtering, transforming, and merging records.
- openxlsx: For exporting processed files.
- ggplot2 and gridExtra: For generating productivity and citation graphs.
2.5. Network and Keyword Mapping
- Country Collaboration Networks: A co-authorship network at the country level was constructed, identifying key collaborating nations in studies on breakfast and cardiovascular health. Nodes represent countries, and the thickness of the links indicates the intensity of scientific collaborations. This analysis revealed clusters of international cooperation and potential regional leaders in the field.
- Author Keyword Co-occurrence Maps: This analysis highlighted the most frequently used terms by authors to describe their research content. A minimum occurrence threshold was applied to select significant terms, and clustering algorithms were used to form thematic clusters. The results underscore dominant conceptual themes, such as “breakfast quality,” “cardiometabolic risk,” “dietary patterns,” and “cardiovascular disease.”
- Index Keyword Co-occurrence Maps: A complementary analysis was conducted using keywords indexed by the databases (Scopus and WoS). This approach identifies terms controlled by thesauri and standardized vocabularies, offering a more standardized view of the field. The co-occurrence of these terms highlighted connections between clinical indicators (e.g., “cholesterol,” “blood pressure”) and dietary aspects (e.g., “meal skipping,” “nutrient intake”).
3. Results
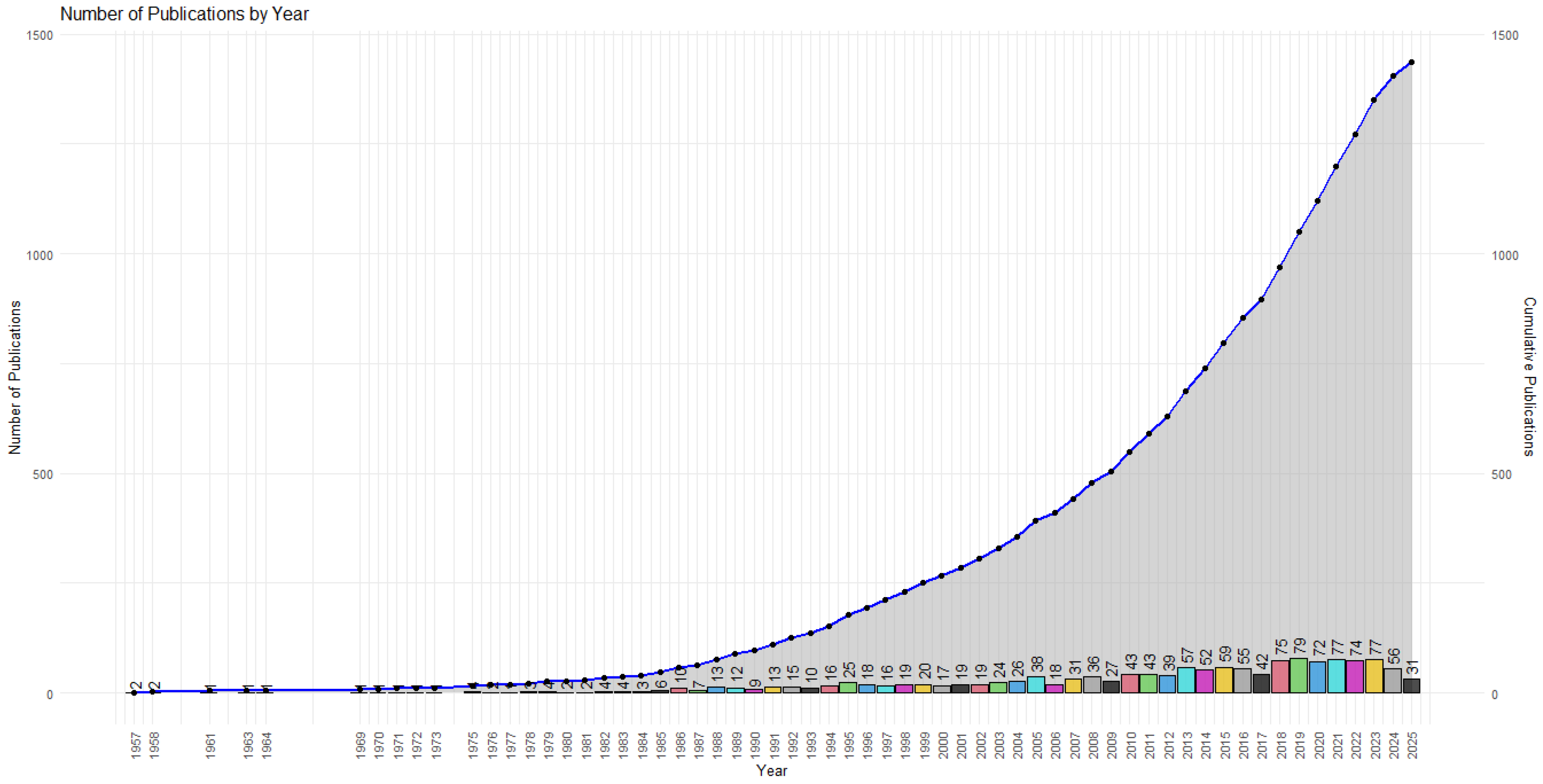
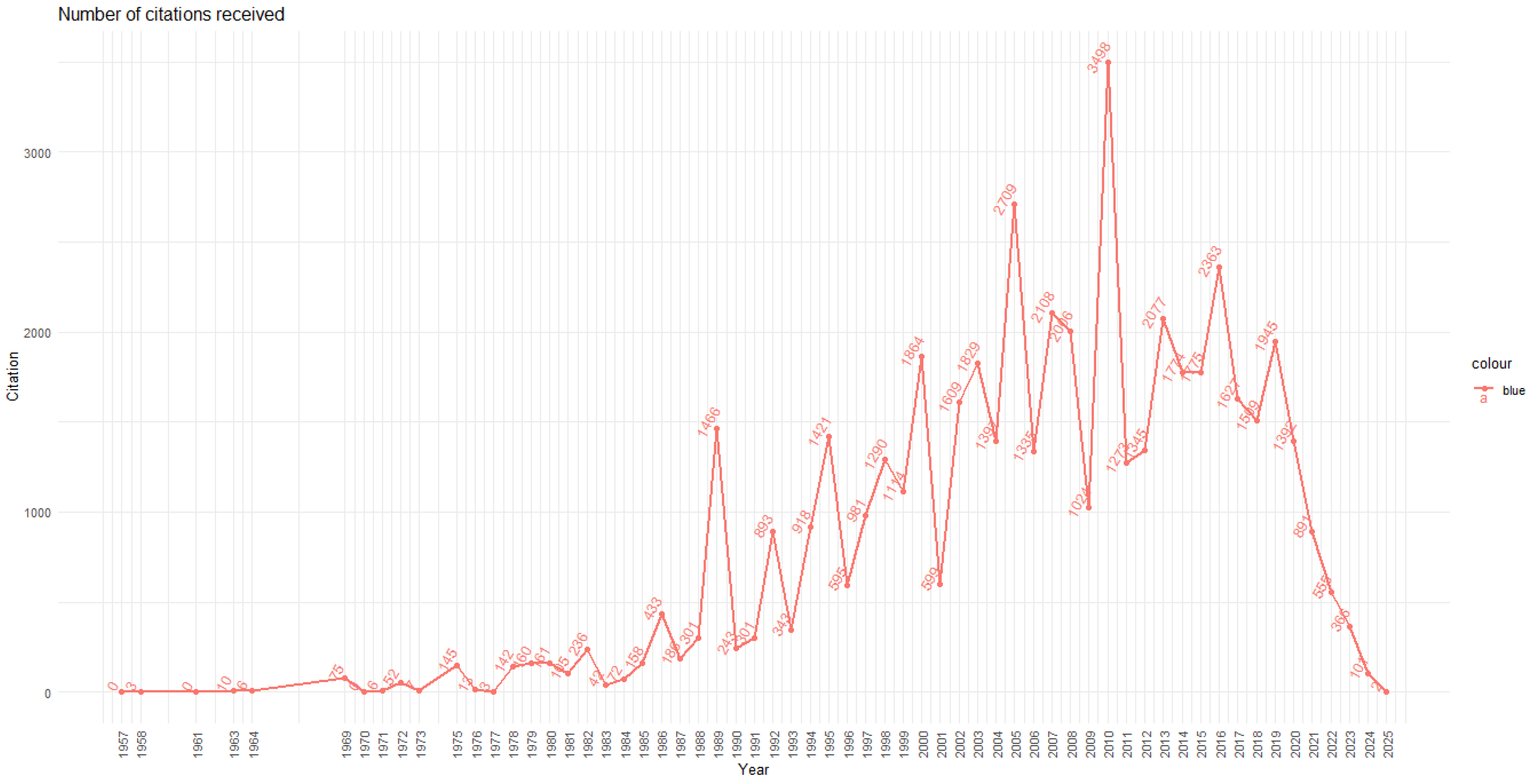
3.1. Productivity and Citations
- Initial Phase (1957–1985): Sporadic and low citations, corresponding to a marginal literature with limited visibility.
- Expansion Phase (1986–2004): Sustained citation growth, with notable peaks in 1989 (1466 citations) and 2000 (1864), suggesting the consolidation of seminal articles that provided a conceptual foundation for subsequent research.
- Maturity Phase (2005–2016): Period of greatest impact. The year 2010 recorded the highest citation count (3498), followed by 2005 (2709), 2007 (2108), and 2013 (2077). This pattern indicates that studies published in the 2000s remain highly referenced by the scientific community.
3.2. Most Influential Journals
- Wolever et al. [20] demonstrated, in a multicenter clinical trial with 367 subjects, that daily intake of 3 g of high-molecular-weight oat β-glucans significantly reduced LDL cholesterol (~5%) compared to wheat cereals. This study underscores that the physicochemical properties of breakfast components directly influence their hypocholesterolemic effect, providing a mechanistic basis for nutritional recommendations.
- Smith et al. [21], in a longitudinal study of over 2000 Australians, found that breakfast skipping in childhood and adulthood is associated with greater waist circumference, elevated basal insulin, and higher total and LDL cholesterol levels, even after adjusting for diet and lifestyle. This research provides robust evidence of the cumulative cardiometabolic implications of breakfast habits across the lifespan.
- Mekary et al. [22], in a cohort of over 29,000 U.S. men, found that breakfast skippers have a 21% higher risk of developing type 2 diabetes, even after adjusting for body mass index and diet quality. This finding supports the hypothesis that regular breakfast consumption may act as an independent protective factor against metabolic diseases.
- Farshchi et al. [23] showed that skipping breakfast for 14 days impairs insulin sensitivity and increases total and LDL cholesterol in healthy young women. This controlled clinical trial contributes to the experimental evidence on the immediate physiological effects of breakfast omission.
- Miller et al. [24] investigated whether egg consumption increases TMAO, a metabolite linked to atherosclerosis. They found that consuming ≥2 egg yolks significantly elevates plasma and urinary TMAO levels, though without altering inflammation or LDL oxidation markers, highlighting the need for further studies to assess cardiovascular risks from typical breakfast foods.
- Jamshed et al. [25] conducted the first randomized clinical trial evaluating early time-restricted feeding (eTRF), a form of intermittent fasting involving food consumption between 8 a.m. and 2 p.m. Compared to a standard schedule (8 a.m. to 8 p.m.), eTRF improved 24-h glycemic control, increased expression of longevity-related genes (SIRT1) and autophagy markers (LC3A), and favorably altered circadian rhythms and hormonal profiles without caloric reduction. This study positions breakfast timing as a potent metabolic regulator with anti-aging implications.
- Ballesteros et al. [26] assessed the effects of daily egg consumption versus an oatmeal breakfast in type 2 diabetes patients. Eggs did not adversely affect glucose, insulin, or lipid profiles and significantly reduced inflammatory markers like TNF-α and AST, suggesting a protective effect in individuals with mild chronic inflammation.
- Missimer et al. [27] compared two daily eggs versus oatmeal in young adults. Although egg consumption increased LDL and HDL cholesterol, the LDL/HDL ratio remained stable, posing no additional cardiovascular risk. Participants also reported greater satiety, correlated with reduced ghrelin levels, suggesting a beneficial effect on appetite control.
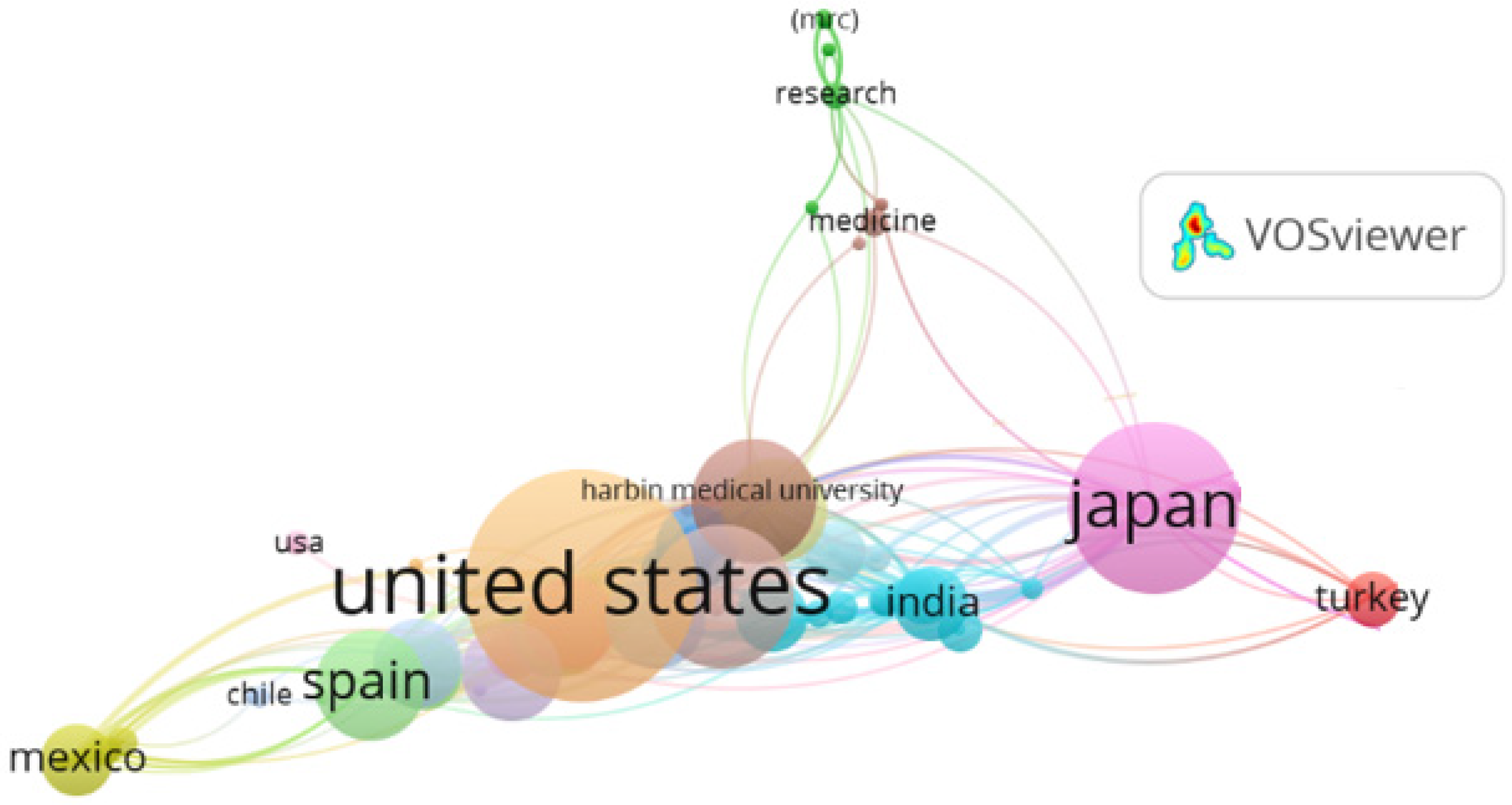
3.3. Leading Countries
- Australia: 62 publications, 3071 citations.
- Canada: 58 publications, 3039 citations.
- Italy: 57 publications, 2529 citations.
- Sweden: 45 publications, 2277 citations.
- An Anglo-Saxon and Western cluster (United States, United Kingdom, Canada, Australia).
- An Asian cluster (Japan, India, China).
- A European/Ibero-American cluster, with Spain as a key connector to Mexico and Chile.
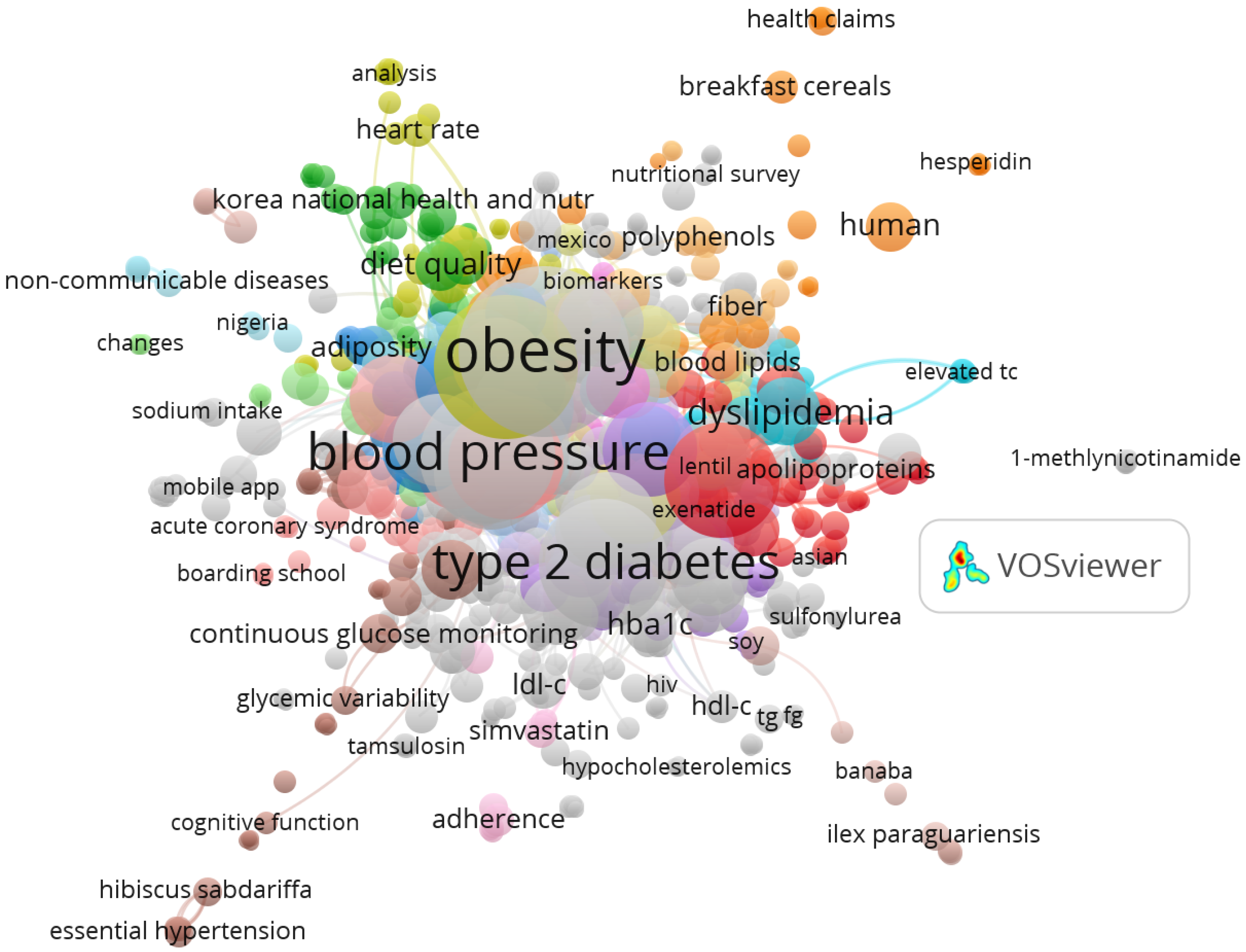
3.4. Emerging Research Themes
4. Discussion
4.1. Clinical Relevance of Breakfast in Cardiovascular Health
4.2. Historical Evolution and Field Maturation
4.3. Geographic Leadership and Collaborative Networks
4.4. Emerging Themes and Conceptual Innovation
4.5. Gaps and Methodological Challenges
4.6. Cardiometabolic Risk Markers and Breakfast
4.7. Public Health Implications and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Tian, X.Y.; Miyashita, M.; Sun, F.; Huang, W.Y.J.; Zheng, C.; Sum, M.K.; Wong, S.H.S. Effects of accumulated versus continuous individualized exercise on postprandial glycemia in young adults with obesity. Eur. J. Sport Sci. 2023, 23, 1446–1456. [Google Scholar] [CrossRef]
- Bano, R.; Ahmad, S.; Bahathig, A.A.; Fatima, W. A Study on the Effect of Breakfast Habits on Blood Pressure and Academic Performance among University Students in Saudi Arabia. Int. J. Child Health Nutr. 2025, 14, 24–31. [Google Scholar] [CrossRef]
- Fiore, G.; Scapaticci, S.; Neri, C.R.; Azaryah, H.; Escudero-Marín, M.; Pascuzzi, M.C.; La Mendola, A.; Mameli, C.; Chiarelli, F.; Campoy, C.; et al. Chrononutrition and metabolic health in children and adolescents: A systematic review and meta-Analysis. Nutr. Rev. 2024, 82, 1309–1354. [Google Scholar] [CrossRef]
- Giosuè, A.; Skantze, V.; Hjorth, T.; Hjort, A.; Brunius, C.; Giacco, R.; Costabile, G.; Vitale, M.; Wallman, M.; Jirstrand, M.; et al. Association of the glucose patterns after a single nonstandardized meal with the habitual diet composition and features of the daily glucose profile in individuals without diabetes. Am. J. Clin. Nutr. 2025, 121, 246–255. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Y.; Tang, J.; Ma, Y.; Liu, Y.; Ren, X. The association of energy or macronutrient intake in three meals with depression in adults with cardiovascular disease: The United States National Health and Nutrition Examination Survey, 2003–2018. BMC Psychiatry 2025, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Fiaidhi, J.; Kudadiya, R. Integrating a PICO Clinical Questioning to the QL4POMR Framework for Building Evidence-Based Clinical Case Reports. In Proceedings of the 2023 IEEE International Conference on Big Data (BigData), Sorrento, Italy, 15–18 December 2023; pp. 4940–4947. [Google Scholar] [CrossRef]
- Hosseini, M.-S.; Jahanshahlou, F.; Akbarzadeh, M.A.; Zarei, M.; Vaez-Gharamaleki, Y. Formulating research questions for evidence-based studies. J. Med. Surg. Public Health 2024, 2, 100046. [Google Scholar] [CrossRef]
- Singh, P.; Singh, V.K.; Piryani, R. Scholarly article retrieval from Web of Science, Scopus and Dimensions: A comparative analysis of retrieval quality. J. Inf. Sci. 2023, 01655515231191351. [Google Scholar] [CrossRef]
- Vasudevan, B.; Chatterjee, M.; Sharma, V.; Sahdev, R. Indexing of Journals and Indices of Publications. Indian J. Radiol. Imaging 2025, 35, S148–S154. [Google Scholar] [CrossRef]
- Wilkinson, C.F., Jr. Drugs other than anticoagulants in treatment of arteriosclerotic heart disease. J. Am. Med. Assoc. 1957, 163, 927–930. [Google Scholar] [CrossRef]
- Nicolaysen, R.; Westinnd, K. Plasma lipids in coronary heart disease. Scand. J. Clin. Lab. Investig. 1963, 15, 291–298. [Google Scholar] [CrossRef]
- Mann, J.I.; Truswell, A.S. Effect of controlled breakfast on serum cholesterol and triglycerides. Am. J. Clin. Nutr. 1971, 24, 1300. [Google Scholar] [CrossRef]
- Schilling, F.J.; Hashim, S.A.; Leonardy, J.G. Effect of Controlled Breakfast on Serum Cholesterol and Triglycerides. Am. J. Clin. Nutr. 1964, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.C.; Berenson, G.S.; Webber, L.S. Dietary studies and the relationship of diet to cardiovascular disease risk factor variables in 10 year old children. The Bogalusa Heart study. Am. J. Clin. Nutr. 1978, 31, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Brydon, W.G.; Tadesse, K.; Wenham, P.; Walls, A.; Eastwood, M.A. The effect of raw carrot on serum lipids and colon function. Am. J. Clin. Nutr. 1979, 32, 1889–1892. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; De Vries, J.H.M.; Van Leeuwen, S.D.; Mengelers, M.J.B.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef]
- Pereira, M.A.; Jacobs, D.R., Jr.; Pins, J.J.; Raatz, S.K.; Gross, M.D.; Slavin, J.L.; Seaquist, E.R. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am. J. Clin. Nutr. 2002, 75, 848–855. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K.; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the childhood determinants of adult health study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef]
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; Van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189. [Google Scholar] [CrossRef]
- Farshchi, H.R.; Taylor, M.A.; Macdonald, I.A. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am. J. Clin. Nutr. 2005, 81, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Corbin, K.D.; Da Costa, K.-A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.N.; Valenzuela, F.; Robles, A.E.; Artalejo, E.; Aguilar, D.; Andersen, C.J.; Valdez, H.; Fernandez, M.L. One egg per day improves inflammation when compared to an oatmeal-based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Nutrients 2015, 7, 3449–3463. [Google Scholar] [CrossRef]
- Missimer, A.; DiMarco, D.M.; Andersen, C.J.; Murillo, A.G.; Vergara-Jimenez, M.; Fernandez, M.L. Consuming Two Eggs per Day, as Compared to an Oatmeal Breakfast, Decreases Plasma Ghrelin while Maintaining the LDL/HDL Ratio. Nutrients 2017, 9, 89. [Google Scholar] [CrossRef]
- Shimizu, H.; Hanzawa, F.; Kim, D.; Sun, S.; Laurent, T.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Delayed first active-phase meal, a breakfast-skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE 2018, 13, e0206669. [Google Scholar] [CrossRef]
- Phillips, D.L.; Pirkle, J.L.; Burse, V.W.; Bernert, J.T., Jr.; Henderson, L.O.; Needham, L.L. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch. Environ. Contam. Toxicol. 1989, 18, 495–500. [Google Scholar] [CrossRef]
- Plotnick, G.D.; Corretti, M.C.; Vogel, R.A. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA 1997, 278, 1682–1686. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Cahill, L.E.; Chiuve, S.E.; Mekary, R.A.; Jensen, M.K.; Flint, A.J.; Hu, F.B.; Rimm, E.B. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 2013, 128, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Watanabe, H.; Goto, N.; Onizawa, K.; Taguchi, H.; Matsuo, N.; Yasukawa, T.; Tsushima, R.; Shimasaki, H.; Itakura, H. Dietary diacylglycerol suppresses accumulation of body fat compared to triacylglycerol in men in a double-blind controlled trial. J. Nutr. 2000, 130, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef] [PubMed]
- AbuMweis, S.S.; Vanstone, C.A.; Ebine, N.; Kassis, A.; Ausman, L.M.; Jones, P.J.H.; Lichtenstein, A.H. Intake of a single morning dose of standard and novel plant sterol preparations for 4 weeks does not dramatically affect plasma lipid concentrations in humans. J. Nutr. 2006, 136, 1012–1016. [Google Scholar] [CrossRef]
- Vitale, M.; Costabile, G.; Bergia, R.E.; Hjorth, T.; Campbell, W.W.; Landberg, R.; Riccardi, G.; Giacco, R. The effects of Mediterranean diets with low or high glycemic index on plasma glucose and insulin profiles are different in adult men and women: Data from MEDGI-Carb randomized clinical trial. Clin. Nutr. 2023, 42, 2022–2028. [Google Scholar] [CrossRef]
- Ma, Y.; Bertone, E.R.; Stanek, E.J., III; Reed, G.W.; Hebert, J.R.; Cohen, N.L.; Merriam, P.A.; Ockene, I.S. Association between eating patterns and obesity in a free-living US adult population. Am. J. Epidemiol. 2003, 158, 85–92. [Google Scholar] [CrossRef]
- Rynarzewski, J.; Dicks, L.; Zimmermann, B.F.; Stoffel-Wagner, B.; Ludwig, N.; Helfrich, H.-P.; Ellinger, S. Impact of a Usual Serving Size of Flavanol-Rich Cocoa Powder Ingested with a Diabetic-Suitable Meal on Postprandial Cardiometabolic Parameters in Type 2 Diabetics—A Randomized, Placebo-Controlled, Double-Blind Crossover Study. Nutrients 2019, 11, 417. [Google Scholar] [CrossRef]
- Hale, P.J.; Horrocks, P.M.; Wright, A.D.; Fitzgerald, M.G.; Nattrass, M.; Bailey, C.J. Xiaoke Tea, a Chinese Herbal Treatment for Diabetes Mellitus. Diabet. Med. 1989, 6, 675–676. [Google Scholar] [CrossRef]
- Basu, A.; Betts, N.M.; Leyva, M.J.; Fu, D.; Aston, C.E.; Lyons, T.J. Acute Cocoa Supplementation Increases Postprandial HDL Cholesterol and Insulin in Obese Adults with Type 2 Diabetes after Consumption of a High-Fat Breakfast. J. Nutr. 2015, 145, 2325–2332. [Google Scholar] [CrossRef]
- Anoshirike, C.O.; Eme, B.N.; Ibeanu, V.N.; Makuochi, V.U. Prevalence of overweight and obesity among the staff (25–70 years) of the University of Nigeria, Nsukka Campus. Niger. J. Nutr. Sci. 2019, 40, 79–83. [Google Scholar]
- Arenaza, L.; Muñoz-Hernández, V.; Medrano, M.; Oses, M.; Amasene, M.; Merchán-Ramírez, E.; Cadenas-Sanchez, C.; Ortega, F.B.; Ruiz, J.R.; Labayen, I. Association of breakfast quality and energy density with cardiometabolic risk factors in overweight/obese children: Role of physical activity. Nutrients 2018, 10, 1066. [Google Scholar] [CrossRef]
- Kitaoka, K.; Takenouchi, A.; Minato-Inokawa, S.; Takeuchi, M.; Tsuboi, A.; Kurata, M.; Fukuo, K.; Kazumi, T. Association of ABC (HbA1c, blood pressure and LDL-cholesterol) goal achievement with visit-to-visit ABC variability and postprandial dysmetabolism in type 2 diabetic patients. Asia Pac. J. Clin. Nutr. 2020, 29, 476–482. [Google Scholar] [CrossRef]
- Hayashi, T.; Ai, M.; Goto, S.; Nakamura, M.; Nagaike, H.; Suzuki, R.; Abe, Y.; Ohta, M.; Ito, Y.; Hirano, T. Circadian Rhythm of Subspecies of Low-Density Lipoprotein-Cholesterol and High-Density Lipoprotein-Cholesterol in Healthy Subjects and Patients with Type 2 Diabetes. J. Atheroscler. Thromb. 2023, 30, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Lin, Q.; Xu, J.; Zhu, L.; Guo, L.; Xie, Y.; Du, X.; Zhang, S.; Wen, T.; Liu, L. Non-fasting Changes in Blood Lipids After Three Daily Meals Within a Day in Chinese Inpatients with Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 799300. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.-I.; An, S.H.; Bang, J.S.; Kim, K.J. Comparative Effectiveness of Wearable Devices and Built-In Step Counters in Reducing Metabolic Syndrome Risk in South Korea: Population-Based Cohort Study. JMIR Mhealth Uhealth 2025, 13, e64527. [Google Scholar] [CrossRef] [PubMed]
- Belobrajdic, D.P.; Regina, A.; Klingner, B.; Zajac, I.; Chapron, S.; Berbezy, P.; Bird, A.R. High-Amylose Wheat Lowers the Postprandial Glycemic Response to Bread in Healthy Adults: A Randomized Controlled Crossover Trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef]
- Snyder, S.M.; Reber, J.D.; Freeman, B.L.; Orgad, K.; Eggett, D.L.; Parker, T.L. Controlling for sugar and ascorbic acid, a mixture of flavonoids matching navel oranges significantly increases human postprandial serum antioxidant capacity. Nutr. Res. 2011, 31, 519–526. [Google Scholar] [CrossRef]
- Zunft, H.J.F.; Lüder, W.; Harde, A.; Haber, B.; Graubaum, H.-J.; Gruenwald, J. Carob pulp preparation for treatment of hypercholesterolemia. Adv. Ther. 2001, 18, 230–236. [Google Scholar] [CrossRef]
- Cavalot, F.; Petrelli, A.; Traversa, M.; Bonomo, K.; Fiora, E.; Conti, M.; Anfossi, G.; Costa, G.; Trovati, M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga diabetes study. J. Clin. Endocrinol. Metab. 2006, 91, 813–819. [Google Scholar] [CrossRef]

| Source Title | Documents | Citations |
|---|---|---|
| American Journal of Clinical Nutrition | 53 | 6264 |
| Nutrients | 52 | 1267 |
| British Journal of Nutrition | 31 | 1350 |
| European Journal of Clinical Nutrition | 26 | 1660 |
| Diabetes Care | 22 | 1703 |
| Journal of Nutrition | 22 | 1406 |
| Nutrition Research | 18 | 380 |
| Journal of the American College of Nutrition | 17 | 1294 |
| PLoS ONE | 16 | 541 |
| Appetite | 14 | 550 |
| Country | Documents | Citations | Total Link Strength |
|---|---|---|---|
| United states | 307 | 17,976 | 154 |
| Spain | 70 | 1958 | 80 |
| Japan | 166 | 3901 | 74 |
| United Kingdom | 117 | 5775 | 74 |
| Germany | 48 | 1821 | 73 |
| Italy | 57 | 2529 | 69 |
| China | 89 | 1161 | 63 |
| Belgium | 17 | 445 | 58 |
| Sweden | 45 | 2277 | 53 |
| Australia | 62 | 3071 | 48 |
| Greece | 22 | 512 | 47 |
| Canada | 58 | 3039 | 42 |
| India | 33 | 402 | 33 |
| France | 33 | 1678 | 32 |
| Hungary | 6 | 104 | 30 |
| Poland | 18 | 171 | 28 |
| United Arab Emirates | 7 | 72 | 28 |
| Austria | 8 | 277 | 27 |
| Switzerland | 21 | 1498 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos-García, E.G.; Mederos-Mollineda, K.; Burgos-Angulo, D.J.; Morales-Neira, D.J.; Peralta-Gamboa, D.A. A Bibliometric Analysis of Chrononutrition, Cardiometabolic Risk, and Public Health in International Research (1957–2025). Int. J. Environ. Res. Public Health 2025, 22, 1205. https://doi.org/10.3390/ijerph22081205
Burgos-García EG, Mederos-Mollineda K, Burgos-Angulo DJ, Morales-Neira DJ, Peralta-Gamboa DA. A Bibliometric Analysis of Chrononutrition, Cardiometabolic Risk, and Public Health in International Research (1957–2025). International Journal of Environmental Research and Public Health. 2025; 22(8):1205. https://doi.org/10.3390/ijerph22081205
Chicago/Turabian StyleBurgos-García, Emily Gabriela, Katiuska Mederos-Mollineda, Darley Jhosue Burgos-Angulo, David Job Morales-Neira, and Dennis Alfredo Peralta-Gamboa. 2025. "A Bibliometric Analysis of Chrononutrition, Cardiometabolic Risk, and Public Health in International Research (1957–2025)" International Journal of Environmental Research and Public Health 22, no. 8: 1205. https://doi.org/10.3390/ijerph22081205
APA StyleBurgos-García, E. G., Mederos-Mollineda, K., Burgos-Angulo, D. J., Morales-Neira, D. J., & Peralta-Gamboa, D. A. (2025). A Bibliometric Analysis of Chrononutrition, Cardiometabolic Risk, and Public Health in International Research (1957–2025). International Journal of Environmental Research and Public Health, 22(8), 1205. https://doi.org/10.3390/ijerph22081205






