Feasibility and Impact of 6-Month Rowing on Arm Lymphedema, Flexibility, and Fatigue in Breast Cancer Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
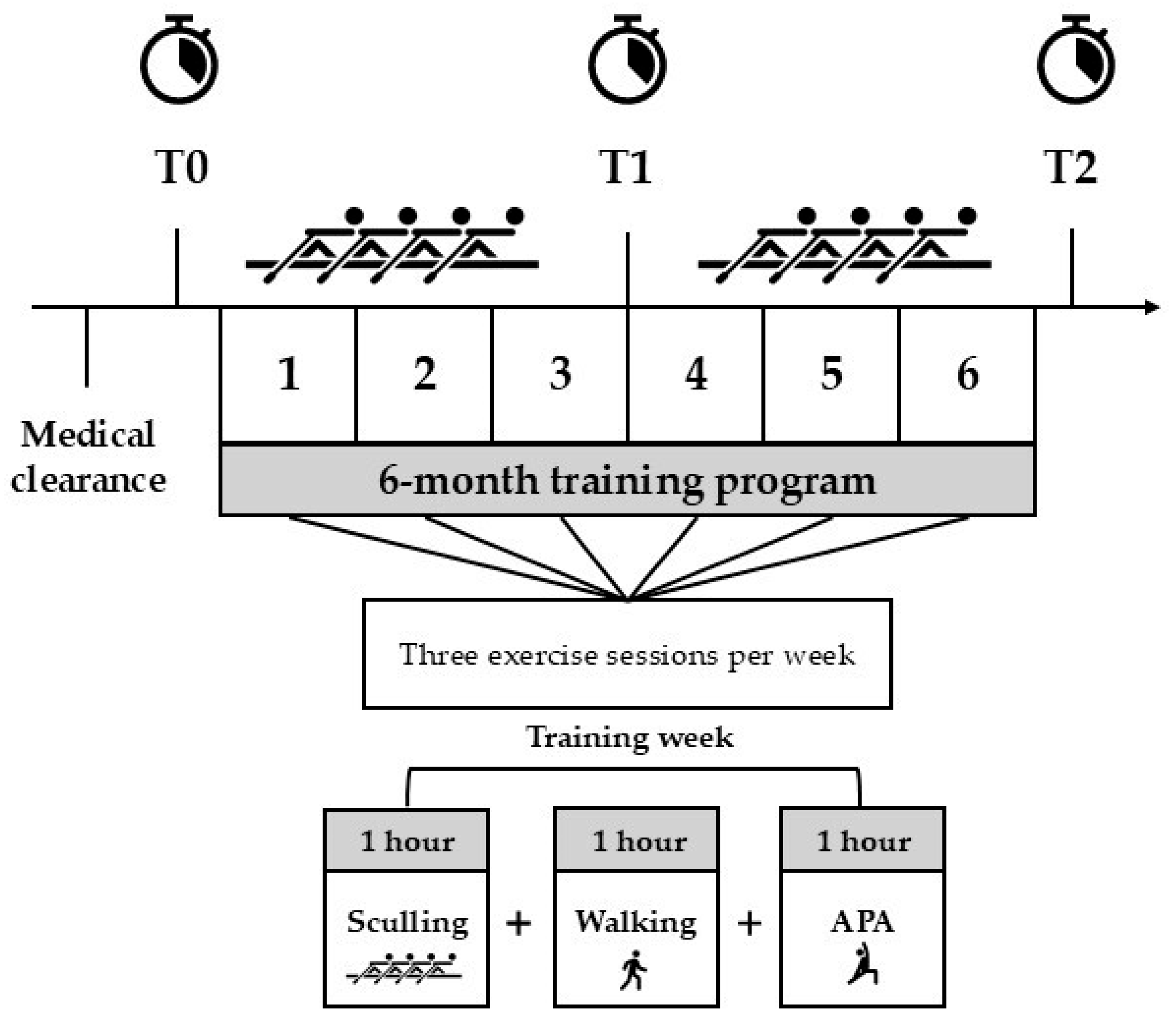
2.2. Study Design
2.3. Training Program
2.4. Measures
2.4.1. Anthropometry
2.4.2. Physical Activity Level
2.4.3. Lymphedema
2.4.4. Flexibility
2.4.5. Fatigue
2.5. Statistical Analysis
3. Results
3.1. Training Program
3.2. Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| QoL | Quality of life |
| DB | Dragon boat |
| PA | Physical activity |
| FSR | Fixed-seat rowing |
| SSR | Sliding-seat rowing |
| APA | Adapted physical activity |
| BMI | Body mass index |
| WC | Waist circumference |
| HC | Hip circumference |
| GPAQ | Global Physical Activity Questionnaire |
| SED | Sedentary behavior |
| MVPA | Moderate-to-vigorous physical activity |
| AVtot | Total arm volume |
| EV | Edema volume |
| RVC | Relative volume change |
| BS | Back scratch |
| EORTC QLQ-FA12 | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Fatigue |
| PFA | Physical fatigue |
| EFA | Emotional fatigue |
| CFA | Cognitive fatigue |
| IDL | Interference with daily life |
| SOC | Social sequelae |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- European Union. ECIS—European Cancer Information System. 2025. Available online: https://ecis.jrc.ec.europa.eu (accessed on 20 April 2025).
- Dal Maso, L.; Toffolutti, F.; De Paoli, A.; Giudici, F.; Francisci, S.; Bucchi, L.; Zorzi, M.; Fusco, M.; Caldarella, A.; Rossi, S.; et al. Cure Indicators and Prevalence by Stage at Diagnosis for Breast and Colorectal Cancer Patients: A Population-Based Study in Italy. Int. J. Cancer 2024, 155, 270–281. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Shien, T.; Iwata, H. Adjuvant and Neoadjuvant Therapy for Breast Cancer. Jpn. J. Clin. Oncol. 2020, 50, 225–229. [Google Scholar] [CrossRef]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers 2021, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Hiensch, A.E.; Depenbusch, J.; Schmidt, M.E.; Monninkhof, E.M.; Pelaez, M.; Clauss, D.; Gunasekara, N.; Zimmer, P.; Belloso, J.; Trevaskis, M.; et al. Supervised, Structured and Individualized Exercise in Metastatic Breast Cancer: A Randomized Controlled Trial. Nat. Med. 2024, 30, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Avancini, A.; Borsati, A.; Toniolo, L.; Ciurnelli, C.; Belluomini, L.; Budolfsen, T.; Lillelund, C.; Milella, M.; Quist, M.; Pilotto, S. Physical Activity Guidelines in Oncology: A Systematic Review of the Current Recommendations. Crit. Rev. Oncol. Hematol. 2025, 210, 104718. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Tao, W.W.; Jiang, H.; Tao, X.M.; Jiang, P.; Sha, L.Y.; Sun, X.C. Effects of Acupuncture, Tuina, Tai Chi, Qigong, and Traditional Chinese Medicine Five-Element Music Therapy on Symptom Management and Quality of Life for Cancer Patients: A Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 728–747. [Google Scholar] [CrossRef]
- Ng, A.V.; Cybulski, A.N.; Engel, A.A.; Papanek, P.E.; Sheffer, M.A.; Waltke, L.J.; Tjoe, J.A. Triathlon Training for Women Breast Cancer Survivors: Feasibility and Initial Efficacy. Support. Care Cancer 2017, 25, 1465–1473. [Google Scholar] [CrossRef]
- Sánchez-Lastra, M.A.; Torres, J.; Martínez-Lemos, I.; Ayán, C. Nordic Walking for Women with Breast Cancer: A Systematic Review. Eur. J. Cancer Care 2019, 28, e13130. [Google Scholar] [CrossRef]
- Bloomquist, K.; Krustrup, P.; Fristrup, B.; Sørensen, V.; Helge, J.W.; Helge, E.W.; Soelberg Vadstrup, E.; Rørth, M.; Hayes, S.C.; Uth, J. Effects of Football Fitness Training on Lymphedema and Upper-Extremity Function in Women after Treatment for Breast Cancer: A Randomized Trial. Acta Oncol. 2021, 60, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Omorou, A.Y.; Peiffert, D.; Rotonda, C.; Van Hoye, A.; Allado, E.; Hily, O.; Temperelli, M.; Chenuel, B.; Hornus-Dragne, D.; Poussel, M. Adapted Fencing for Patients With Invasive Breast Cancer: The RIPOSTE Pilot Randomized Controlled Trial. Front. Sports Act. Living 2022, 4, 786852. [Google Scholar] [CrossRef]
- Cuvelier, S.; Goetgheluck-Villaron, C.; Cohen, M.; Tallet, A.; Berline, M.; Boher, J.M.; Jowett, S.; Justafré, S.; Dantin, P.; Viens, P.; et al. Aqua Polo: Preliminary Feasibility and Efficacy Study of a Programme of Adapted, Supervised Water Polo to Reduce Fatigue and Improve Women’s Psychological and Social Recovery after Breast Cancer Treatment: A Mixed-Methods Design. Contemp. Clin. Trials Commun. 2023, 33, 101120. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Facchini, B.A.; Garozzo, D.; Necci, V.; Taibi, R.; Torrisi, C.; Ficarra, G.; Bitto, A. Adapted Physical Activity for Breast Cancer Patients: Shared Considerations with Two Olympic and World Italian Sports Champions. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5393–5398. [Google Scholar]
- Herrero-Zapirain, I.; Álvarez-Pardo, S.; Castañeda-Babarro, A.; Moreno-Villanueva, A.; Mielgo-Ayuso, J.F. The Effect of Dragon Boating on the Quality of Life for Breast Cancer Survivors: A Systematic Review. Healthcare 2024, 12, 1290. [Google Scholar] [CrossRef]
- del Rosario Asensio-García, M.; Tomás-Rodríguez, M.I.; Palazón-Bru, A.; Hernández-Sánchez, S.; Nouni-García, R.; Romero-Aledo, A.L.; Gil-Guillén, V.F. Effect of Rowing on Mobility, Functionality, and Quality of Life in Women with and without Breast Cancer: A 4-Month Intervention. Support. Care Cancer 2021, 29, 2639–2644. [Google Scholar] [CrossRef] [PubMed]
- Gavala-González, J.; Torres-Pérez, A.; Fernández-García, J.C. Impact of Rowing Training on Quality of Life and Physical Activity Levels in Female Breast Cancer Survivors. Int. J. Environ. Res. Public Health 2021, 18, 7188. [Google Scholar] [CrossRef]
- Gavala-González, J.; Gálvez-Fernández, I.; Mercadé-Melé, P.; Fernández-García, J.C. Rowing Training in Breast Cancer Survivors: A Longitudinal Study of Physical Fitness. Int. J. Environ. Res. Public Health 2020, 17, 4938. [Google Scholar] [CrossRef]
- Gavala-González, J.; Real-Pérez, M.; Benítez-García, L.; Fernández-García, J.C. Fixed-Seat Rowing versus Sliding-Seat Rowing: Effects on Physical Fitness in Breast Cancer Survivors. Cancers 2024, 16, 2207. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Jassem, J. Cardiovascular Effects of Systemic Cancer Treatment. Cancer Treat. Rev. 2011, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki. Int. J. Pharm. Med. 2000, 14, 279–281. [CrossRef]
- ACSM—American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Liguori, G., Ed.; Wolters Kluwer: Philadelphia, PA, USA, 2021; ISBN 9781975150211. [Google Scholar]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Peros, G.; Cataliotti, L.; Vlastos, G. Lymphedema Following Axillary Lymph Node Dissection for Breast Cancer. Surg. Oncol. 2006, 15, 153–165. [Google Scholar] [CrossRef]
- Petrek, J.A.; Senie, R.T.; Peters, M.; Peterrosen, P. Lymphedema in a Cohort of Breast Carcinoma Survivors 20 Years after Diagnosis. Cancer 2001, 92, 1368–1377. [Google Scholar] [CrossRef]
- Breast Cancer—Arm Volume Calculator. Available online: https://riskcalc.org/BreastCancerArmLymphedemaArmVolume/ (accessed on 27 April 2025).
- Bevilacqua, J.L.B.; Kattan, M.W.; Yu, C.; Koifman, S.; Mattos, I.E.; Koifman, R.J.; Bergmann, A. Nomograms for Predicting the Risk of Arm Lymphedema after Axillary Dissection in Breast Cancer. Ann. Surg. Oncol. 2012, 19, 2580–2589. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The Diagnosis and Treatment of Peripheral Lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Vargo, M.; Aldrich, M.; Donahue, P.; Iker, E.; Koelmeyer, L.; Crescenzi, R.; Cheville, A. Current Diagnostic and Quantitative Techniques in the Field of Lymphedema Management: A Critical Review. Med. Oncol. 2024, 41, 241. [Google Scholar] [CrossRef]
- Ancukiewicz, M.; Miller, C.L.; Skolny, M.N.; O’Toole, J.; Warren, L.E.; Jammallo, L.S.; Specht, M.C.; Taghian, A.G. Comparison of Relative versus Absolute Arm Size Change as Criteria for Quantifying Breast Cancer-Related Lymphedema: The Flaws in Current Studies and Need for Universal Methodology. Breast Cancer Res. Treat. 2012, 135, 145–152. [Google Scholar] [CrossRef]
- Weis, J.; Tomaszewski, K.A.; Hammerlid, E.; Arraras, J.I.; Conroy, T.; Lanceley, A.; Schmidt, H.; Wirtz, M.; Singer, S.; Pinto, M.; et al. International Psychometric Validation of an EORTC Quality of Life Module Measuring Cancer Related Fatigue (EORTC QLQ-FA12). J. Natl. Cancer Inst. 2017, 109, djw273. [Google Scholar] [CrossRef] [PubMed]
- EORTC QLQ-FA12 Scoring Manual. Available online: https://www.eortc.be/qol/ScoringInstructions/FA12%20Summary.pdf (accessed on 9 June 2025).
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Moulton, S.T. Encyclopedia of Research Design; Salkind, N.J., Ed.; Mauchly Test; SAGE Publications: Thousand Oaks, CA, USA, 2010; pp. 777–778. ISBN 978-1-4129-6127-1. [Google Scholar]
- Blanca, M.J.; Arnau, J.; García-Castro, F.J.; Alarcón, R.; Bono, R. Repeated Measures ANOVA and Adjusted F-Tests When Sphericity Is Violated: Which Procedure Is Best? Front. Psychol. 2023, 14, 1192453. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Eribaum Associates: Hillsdale, NJ, USA, 1988; ISBN 0-8058-0283-5. [Google Scholar]
- Moro, T.; Casolo, A.; Bordignon, V.; Sampieri, A.; Schiavinotto, G.; Vigo, L.; Ghisi, M.; Paoli, A.; Cerea, S. Keep Calm and Keep Rowing: The Psychophysical Effects of Dragon Boat Program in Breast Cancer Survivors. Support. Care Cancer 2024, 32, 218. [Google Scholar] [CrossRef] [PubMed]
- Real-Pérez, M.; Fernández-García, J.C.; Gavala-González, J. Effects of a 6-Month Rowing Training Program in Breast Cancer Survivors. PLoS ONE 2025, 20, e0317118. [Google Scholar] [CrossRef]
- Troeschel, A.N.; Leach, C.R.; Shuval, K.; Stein, K.D.; Patel, A.V. Physical Activity in Cancer Survivors during “Re-Entry” Following Cancer Treatment. Prev. Chronic Dis. 2018, 15, E65. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Desmond, K.A.; Bernaards, C.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Fatigue in Long-Term Breast Carcinoma Survivors: A Longitudinal Investigation. Cancer 2006, 106, 751–758. [Google Scholar] [CrossRef]
- De Luca, V.; Minganti, C.; Borrione, P.; Grazioli, E.; Cerulli, C.; Guerra, E.; Bonifacino, A.; Parisi, A. Effects of Concurrent Aerobic and Strength Training on Breast Cancer Survivors: A Pilot Study. Public Health 2016, 136, 126–132. [Google Scholar] [CrossRef]
- Rothmund, M.; Pilz, M.J.; Egeter, N.; Lidington, E.; Piccinin, C.; Arraras, J.I.; Groenvold, M.; Holzner, B.; van Leeuwen, M.; Petersen, M.A.; et al. Comparing the Contents of Patient-Reported Outcome Measures for Fatigue: EORTC CAT Core, EORTC QLQ-C30, EORTC QLQ-FA12, FACIT, PRO-CTCAE, PROMIS, Brief Fatigue Inventory, Multidimensional Fatigue Inventory, and Piper Fatigue Scale. Health Qual. Life Outcomes 2024, 22, 104. [Google Scholar] [CrossRef]
- Mao, H.; Bao, T.; Shen, X.; Li, Q.; Seluzicki, C.; Im, E.O.; Mao, J.J. Prevalence and risk factors for fatigue among breast cancer survivors on aromatase inhibitors. Eur. J. Cancer 2018, 101, 47–54. [Google Scholar] [CrossRef]
- Ackah, M.; Barakou, I.; Abonie, U.S.; Hettinga, F.J. Adherence to Exercise in Breast Cancer Survivors during and after Active Treatment: A Systematic Review and Meta-Analysis. JSAMS Plus 2024, 4, 100071. [Google Scholar] [CrossRef]

| Variables | Classification | Sample (n = 20) |
|---|---|---|
| Age (years) | 55.8 ± 6.1 | |
| Weight (kg) | 64.6 ± 9.0 | |
| Height (m) | 1.62 ± 0.05 | |
| Time since surgery (months) | 51.2 ± 62.6 | |
| Stage of cancer (%) | ||
| Stage 0 | 10 | |
| Stage 1 | 50 | |
| Stage 2 | 20 | |
| Stage 3 | 15 | |
| Unknown | 5 | |
| Type of surgery (%) | ||
| Preservation | 55 | |
| Mastectomy | 45 | |
| Cancer side (%) | ||
| Left | 65 | |
| Right | 35 | |
| Type of treatment (%) | ||
| Chemotherapy | 65 | |
| Radiotherapy | 70 | |
| Hormone therapy | 55 |
| Variables | T0 | T1 | T2 | ANOVA-Time | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | F | p-Value | η2p | |
| BMI (kg/m2) | 24.6 ± 3.3 | 24.4 ± 3.3 | 24.5 ± 3.1 | 0.35 † | 0.642 | - |
| WC (cm) | 83.4 ± 9.4 | 84.8 ± 10.1 | 84.8 ± 10.1 | 3.40 | 0.044 | 0.15 |
| HC (cm) | 101.8 ± 8.1 | 100.7 ± 7.3 | 100.3 ± 7.8 | 2.79 † | 0.09 | - |
| MVPA (min/week) | 297 ± 351 | 359 ± 275 | 354 ± 262 | 0.45 † | 0.542 | - |
| SED (min/week) | 279 ± 153 | 270 ± 121 | 240 ± 143 | 0.90 † | 0.338 | - |
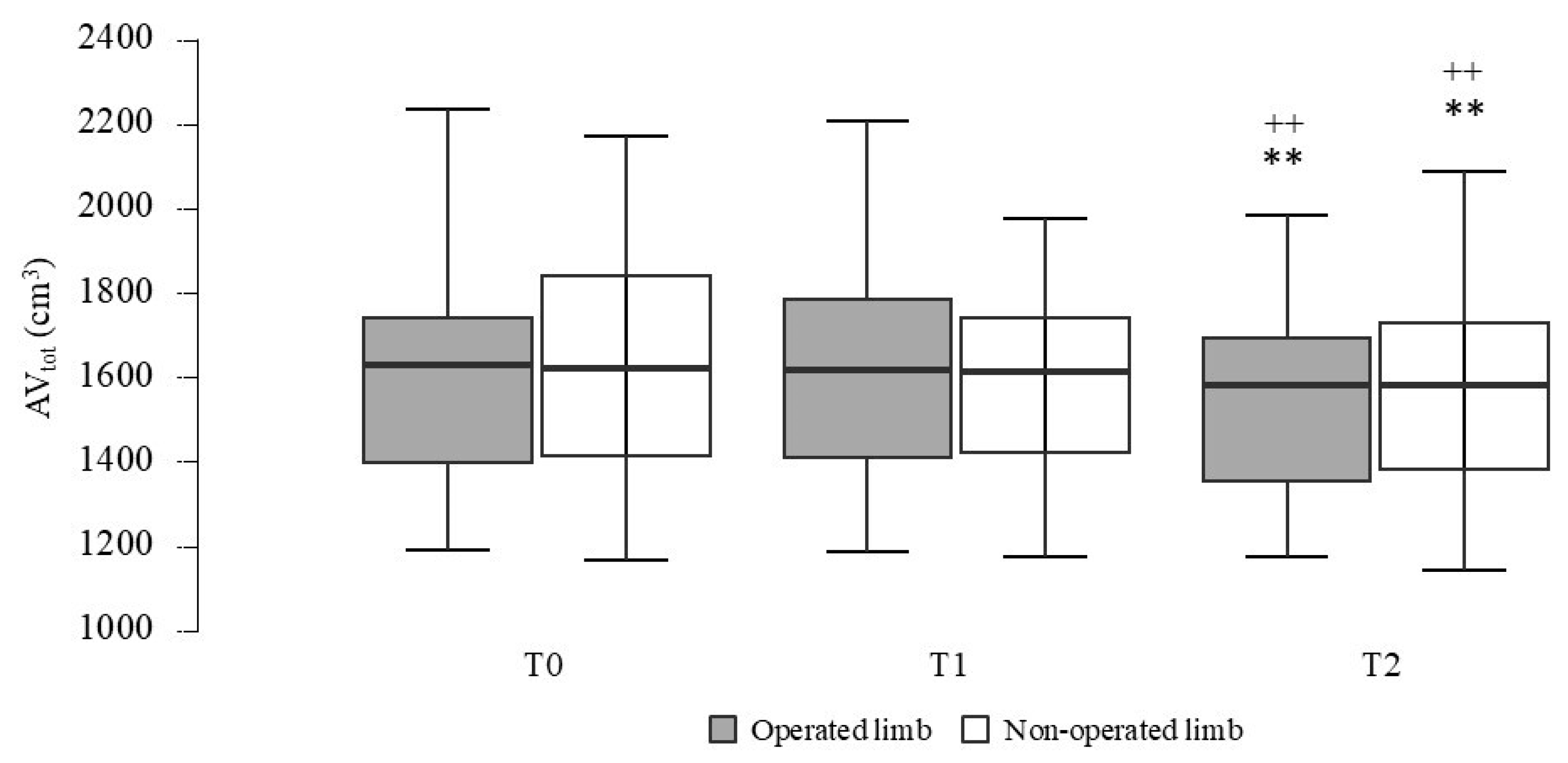
| AVtot—operated limb (cm3) | 1616 ± 274 | 1607 ± 259 | 1537 ± 218 | 10.91 | <0.001 | 0.37 |
| AVtot—non-operated limb (cm3) | 1617 ± 266 | 1612 ± 263 | 1553 ± 244 | 9.10 | <0.001 | 0.32 |
| EV (%) | 0.1 ± 4.6 | −0.2 ± 3.4 | −0.8 ± 4.2 | 0.29 | 0.752 | - |
| BS test—operated limb (cm) | 0.2 ± 9.5 | 1.4 ± 9.9 | 2.9 ± 7.9 | 4.71 † | 0.030 | 0.20 |
| BS test—non-operated limb (cm) | 0.2 ± 9.1 | 2.2 ± 8.0 | 3.5 ± 7.6 | 14.15 | <0.001 | 0.43 |
| Variables | T0 | T1 | T2 | ANOVA-Time | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | F | p-Value | η2p | |
| PFA | 24.0 ± 18.8 | 18.7 ± 12.7 | 23.3 ± 22.0 | 0.63 | 0.540 | - |
| EFA | 22.2 ± 24.7 | 12.8 ± 15.0 | 12.8 ± 17.8 | 2.36 † | 0.125 | - |
| CFA | 14.2 ± 17.3 | 16.7 ± 15.3 | 11.7 ± 21.0 | 0.84 | 0.441 | - |
| IDL | 21.7 ± 22.4 | 20.0 ± 22.7 | 20.0 ± 25.1 | 0.04 † | 0.916 | - |
| SOC | 25.0 ± 21.3 | 13.3 ± 19.9 | 15.0 ± 27.5 | 2.75 | 0.077 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommasini, E.; Bruseghini, P.; Rovera, F.A.; Grande, A.M.; Galvani, C. Feasibility and Impact of 6-Month Rowing on Arm Lymphedema, Flexibility, and Fatigue in Breast Cancer Survivors. Int. J. Environ. Res. Public Health 2025, 22, 987. https://doi.org/10.3390/ijerph22070987
Tommasini E, Bruseghini P, Rovera FA, Grande AM, Galvani C. Feasibility and Impact of 6-Month Rowing on Arm Lymphedema, Flexibility, and Fatigue in Breast Cancer Survivors. International Journal of Environmental Research and Public Health. 2025; 22(7):987. https://doi.org/10.3390/ijerph22070987
Chicago/Turabian StyleTommasini, Ester, Paolo Bruseghini, Francesca Angela Rovera, Anna Maria Grande, and Christel Galvani. 2025. "Feasibility and Impact of 6-Month Rowing on Arm Lymphedema, Flexibility, and Fatigue in Breast Cancer Survivors" International Journal of Environmental Research and Public Health 22, no. 7: 987. https://doi.org/10.3390/ijerph22070987
APA StyleTommasini, E., Bruseghini, P., Rovera, F. A., Grande, A. M., & Galvani, C. (2025). Feasibility and Impact of 6-Month Rowing on Arm Lymphedema, Flexibility, and Fatigue in Breast Cancer Survivors. International Journal of Environmental Research and Public Health, 22(7), 987. https://doi.org/10.3390/ijerph22070987






