A Tandem MS Platform for Simultaneous Determination of Urinary Malondialdehyde and Diphenyl Phosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Standard Solutions
2.3. Sample Collection and Treatment
2.4. Instrumentation
2.5. Instrumental Setup
2.6. Statistical Analysis and Correlation Studies
3. Results and Discussion
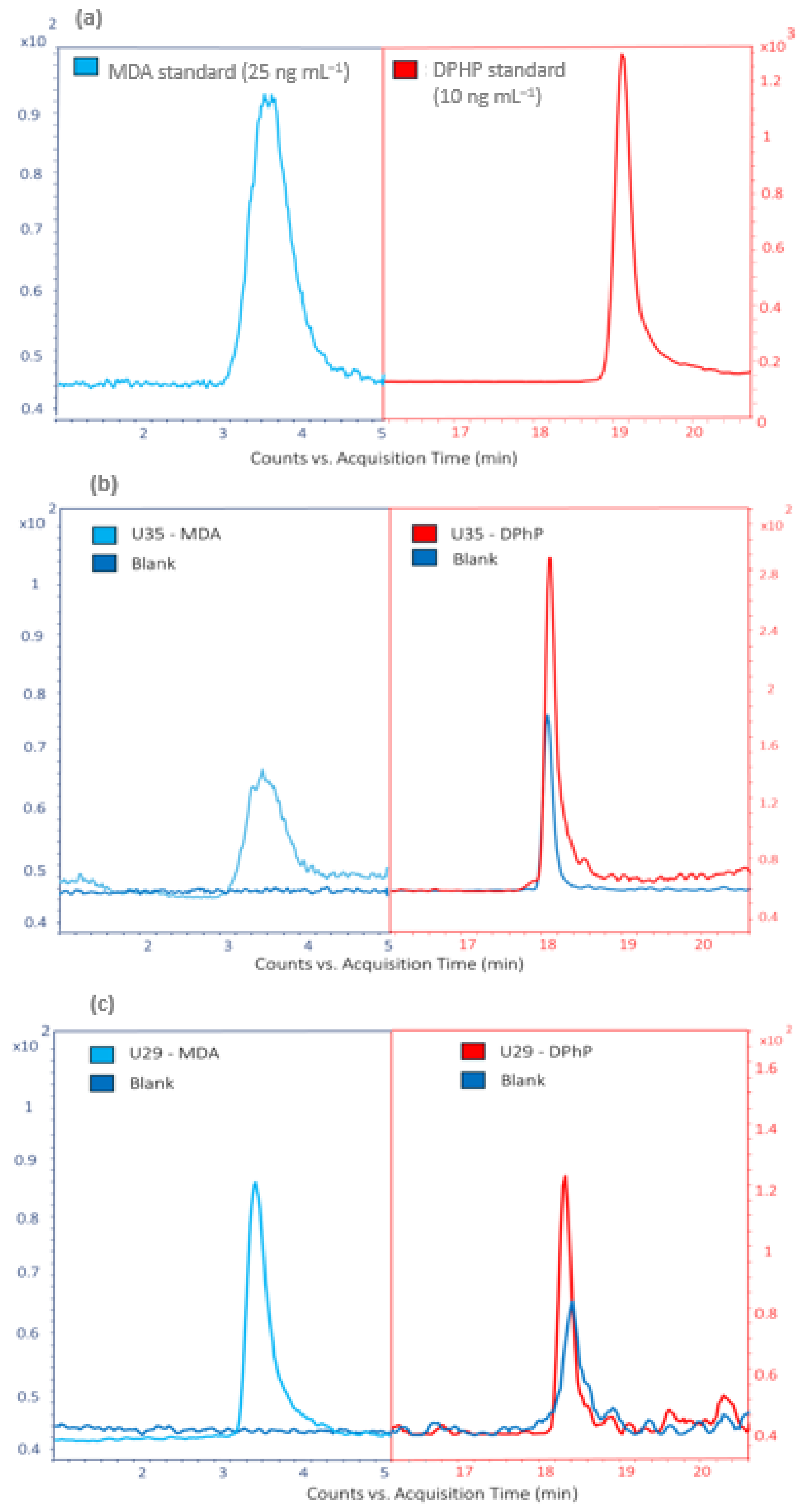
3.1. Analytical Performance
3.2. MDA and DPhP Concentrations
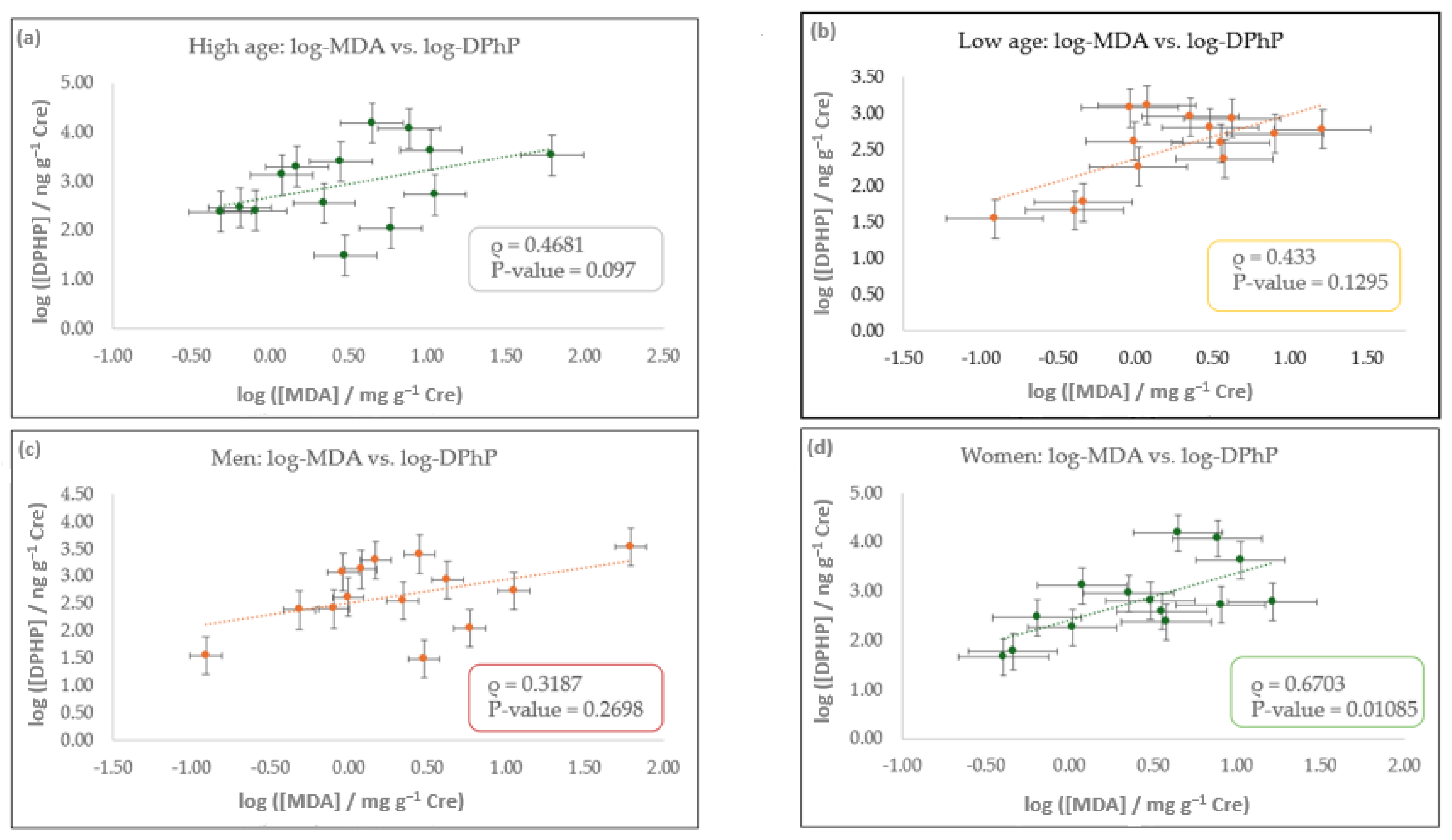
3.3. Correlation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toto, A.; Wild, P.; Graille, M.; Turcu, V.; Crézé, C.; Hemmendinger, M.; Sauvain, J.-J.; Bergamaschi, E.; Guseva Canu, I.; Hopf, N.B. Urinary Malondialdehyde (MDA) Concentrations in the General Population—A Systematic Literature Review and Meta-Analysis. Toxics 2022, 10, 160. [Google Scholar] [CrossRef]
- Tsikas, D.; Tsikas, S.A.; Mikuteit, M.; Ückert, S. Circulating and Urinary Concentrations of Malondialdehyde in Aging Humans in Health and Disease: Review and Discussion. Biomedicines 2023, 11, 2744. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Khosla, L.; Gong, S.; Weiss, J.P.; Birder, L.A. Oxidative Stress Biomarkers in Age-Related Lower Urinary Tract Disorders: A Systematic Review. Int. Neurourol. J. 2022, 26, 3–19. [Google Scholar] [CrossRef]
- Dator, R.P.; Solivio, M.J.; Villalta, P.W.; Balbo, S. Bioanalytical and Mass Spectrometric Methods for Aldehyde Profiling in Biological Fluids. Toxics 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Winnik, W.M.; Kitchin, K.T. Measurement of Oxidative Stress Parameters Using Liquid Chromatography–Tandem Mass Spectroscopy (LC–MS/MS). Toxicol. Appl. Pharmacol. 2008, 233, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Chango, G.; García-Gómez, D.; García Pinto, C.; Rodríguez-Gonzalo, E.; Pérez Pavón, J.L. Rapid and Reliable Quantification of Urinary Malondialdehyde by HILIC-MS/MS: A Derivatization-Free Breakthrough Approach. Anal. Chim. Acta 2024, 1311, 342737. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Gbadamosi, M.R.; Abdallah, M.A.-E.; Harrad, S. A Critical Review of Human Exposure to Organophosphate Esters with a Focus on Dietary Intake. Sci. Total Environ. 2021, 771, 144752. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Liao, C.; Jiang, G. Human Internal Exposure to Organophosphate Esters: A Short Review of Urinary Monitoring on the Basis of Biological Metabolism Research. J. Hazard. Mater. 2021, 418, 126279. [Google Scholar] [CrossRef]
- Hajeb, P.; Castaño, A.; Cequier, E.; Covaci, A.; López, M.E.; Antuña, A.G.; Haug, L.S.; Henríquez-Hernández, L.A.; Melymuk, L.; Pérez Luzardo, O.; et al. Critical Review of Analytical Methods for the Determination of Flame Retardants in Human Matrices. Anal. Chim. Acta 2022, 1193, 338828. [Google Scholar] [CrossRef]
- Jayatilaka, N.K.; Restrepo, P.; Williams, L.; Ospina, M.; Valentin-Blasini, L.; Calafat, A.M. Quantification of Three Chlorinated Dialkyl Phosphates, Diphenyl Phosphate, 2,3,4,5-Tetrabromobenzoic Acid, and Four Other Organophosphates in Human Urine by Solid Phase Extraction-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2017, 409, 1323–1332. [Google Scholar] [CrossRef]
- Van Den Eede, N.; Neels, H.; Jorens, P.G.; Covaci, A. Analysis of Organophosphate Flame Retardant Diester Metabolites in Human Urine by Liquid Chromatography Electrospray Ionisation Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1303, 48–53. [Google Scholar] [CrossRef]
- Cequier, E.; Marcé, R.M.; Becher, G.; Thomsen, C. A High-Throughput Method for Determination of Metabolites of Organophosphate Flame Retardants in Urine by Ultra Performance Liquid Chromatography–High Resolution Mass Spectrometry. Anal. Chim. Acta 2014, 845, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kosarac, I.; Kubwabo, C.; Foster, W.G. Quantitative Determination of Nine Urinary Metabolites of Organophosphate Flame Retardants Using Solid Phase Extraction and Ultra Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry (UPLC-MS/MS). J. Chromatogr. B 2016, 1014, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Schindler, B.K.; Förster, K.; Angerer, J. Quantification of Two Urinary Metabolites of Organophosphorus Flame Retardants by Solid-Phase Extraction and Gas Chromatography–Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2009, 395, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Petropoulou, S.-S.E.; Petreas, M.; Park, J.-S. Analytical Methodology Using Ion-Pair Liquid Chromatography–Tandem Mass Spectrometry for the Determination of Four Di-Ester Metabolites of Organophosphate Flame Retardants in California Human Urine. J. Chromatogr. A 2016, 1434, 70–80. [Google Scholar] [CrossRef]
- Schindler, B.K.; Förster, K.; Angerer, J. Determination of Human Urinary Organophosphate Flame Retardant Metabolites by Solid-Phase Extraction and Gas Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2009, 877, 375–381. [Google Scholar] [CrossRef]
- Chen, G.; Jin, Y.; Wu, Y.; Liu, L.; Fu, Z. Exposure of Male Mice to Two Kinds of Organophosphate Flame Retardants (OPFRs) Induced Oxidative Stress and Endocrine Disruption. Environ. Toxicol. Pharmacol. 2015, 40, 310–318. [Google Scholar] [CrossRef]
- Yao, Y.; Li, M.; Pan, L.; Duan, Y.; Duan, X.; Li, Y.; Sun, H. Exposure to Organophosphate Ester Flame Retardants and Plasticizers during Pregnancy: Thyroid Endocrine Disruption and Mediation Role of Oxidative Stress. Environ. Int. 2021, 146, 106215. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, M.; Liao, M.; Su, S.; Sun, W.; Gan, Z. Organophosphorus Flame Retardants and Their Metabolites in Paired Human Blood and Urine. Ecotoxicol. Environ. Saf. 2023, 268, 115696. [Google Scholar] [CrossRef]
- Chango, G.; Ballester-Caudet, A.; García-Gómez, D.; García Pinto, C.; Rodríguez-Gonzalo, E.; Pérez Pavón, J.L. Rapid Non-Separative Determination of Prevailing Organophosphate Flame Retardants Metabolites in Urine by Means of a Restricted Access Material Coupled to Tandem Mass Spectrometry. Microchem. J. 2025, 208, 112525. [Google Scholar] [CrossRef]
- Jaffe, M. About the Precipitate Which Picric Acid Produces in Normal Urine and about a New Reaction of Creatinine. J. Physiol. Chem. 1886, 10, 391–400. [Google Scholar]
- Van De Merbel, N.C. Quantitative Determination of Endogenous Compounds in Biological Samples Using Chromatographic Techniques. TrAC Trends Anal. Chem. 2008, 27, 924–933. [Google Scholar] [CrossRef]
- Boeniger, M.F.; Lowry, L.K.; Rosenberg, J. Interpretation of Urine Results Used to Assess Chemical Exposure with Emphasis on Creatinine Adjustments: A Review. Am. Ind. Hyg. Assoc. J. 1993, 54, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, Y.; Wang, Y.; Bastiaensen, M.; Covaci, A.; Sun, H. Organophosphate Ester Flame Retardants and Plasticizers in a Chinese Population: Significance of Hydroxylated Metabolites and Implication for Human Exposure. Environ. Pollut. 2020, 257, 113633. [Google Scholar] [CrossRef]
- Dodson, R.E.; Van Den Eede, N.; Covaci, A.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Urinary Biomonitoring of Phosphate Flame Retardants: Levels in California Adults and Recommendations for Future Studies. Environ. Sci. Technol. 2014, 48, 13625–13633. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Martínez-Moral, M.P.; Sun, H.; Kannan, K. Metabolites of Organophosphate Esters in Urine from the United States: Concentrations, Temporal Variability, and Exposure Assessment. Environ. Int. 2019, 122, 213–221. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, S.; Jin, Y.; Wu, Y.; Liu, L.; Qian, H.; Fu, Z. TPP and TCEP Induce Oxidative Stress and Alter Steroidogenesis in TM3 Leydig Cells. Reprod. Toxicol. 2015, 57, 100–110. [Google Scholar] [CrossRef]
- Lu, S.; Li, Y.; Zhang, T.; Cai, D.; Ruan, J.; Huang, M.; Wang, L.; Zhang, J.; Qiu, R. Effect of E-Waste Recycling on Urinary Metabolites of Organophosphate Flame Retardants and Plasticizers and Their Association with Oxidative Stress. Environ. Sci. Technol. 2017, 51, 2427–2437. [Google Scholar] [CrossRef]
- Zhao, F.; Wan, Y.; Zhao, H.; Hu, W.; Mu, D.; Webster, T.F.; Hu, J. Levels of Blood Organophosphorus Flame Retardants and Association with Changes in Human Sphingolipid Homeostasis. Environ. Sci. Technol. 2016, 50, 8896–8903. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Bastiaensen, M.; Araki, A.; Goudarzi, H.; Konno, S.; Ito, S.; Miyashita, C.; Yao, Y.; Covaci, A.; Kishi, R. Multiple Exposures to Organophosphate Flame Retardants Alter Urinary Oxidative Stress Biomarkers among Children: The Hokkaido Study. Environ. Int. 2019, 131, 105003. [Google Scholar] [CrossRef]
- Gao, D.; Yang, J.; Bekele, T.G.; Zhao, S.; Zhao, H.; Li, J.; Wang, M.; Zhao, H. Organophosphate Esters in Human Serum in Bohai Bay, North China. Environ. Sci. Pollut. Res. 2020, 27, 2721–2729. [Google Scholar] [CrossRef]
- Li, J.; Dong, Z.; Wang, Y.; Bao, J.; Yan, Y.; Jin, J. Different Organophosphate Flame Retardant and Metabolite Concentrations in Urine from Male and Female University Students in Beijing and an Assessment of Exposure via Indoor Dust. Environ. Toxicol. Chem. 2019, 38, 760–768. [Google Scholar] [CrossRef]


| Sample | Sex | Age | [Cre] mg·dL−1 | [MDA] ng·mL−1 | [MDA] mg·g−1 Cre | [DPhP] ng·mL−1 | [DPhP] ng·g−1 Cre |
|---|---|---|---|---|---|---|---|
| U1 | Woman | 19 | 145 | 3600 ± 300 | 2.5 ± 0.2 | 0.9 ± 0.7 | 600 ± 400 |
| U2 | Man | 55 | 53 | 5900 ± 200 | 11.0 ± 0.4 | 0.3 ± 0.2 | 500 ± 300 |
| U3 | Woman | 36 | 48 | 1500 ± 200 | 3.0 ± 0.3 | 0.3 ± 0.1 | 700 ± 200 |
| U4 | Man | 37 | 20 | 340 ± 90 | 1.7 ± 0.5 | <LOD | <LOD |
| U5 | Woman | 78 | 11 | 550 ± 80 | 5.1 ± 0.8 | <LOD | <LOD |
| U6 | Man | 87 | 49 | 700 ± 100 | 1.3 ± 0.3 | <LOD | <LOD |
| U7 | Man | 52 | 285 | 8600 ± 600 | 3.0 ± 0.2 | 0.09 ± 0.03 | 31 ± 9 |
| U8 | Woman | 22 | 13 | 360 ± 80 | 2.9 ± 0.6 | <LOD | <LOD |
| U9 | Man | 86 | 80 | 650 ± 50 | 0.82 ± 0.06 | <LOD | <LOD |
| U10 | Woman | 81 | 15 | 630 ± 30 | 4.1 ± 0.2 | <LOD | <LOD |
| U11 | Man | 54 | 104 | 1540 ± 90 | 1.48 ± 0.09 | 2.1 ± 0.3 | 2000 ± 300 |
| U12 | Woman | 48 | 29 | 150 ± 40 | 0.5 ± 0.1 | <LOD | <LOD |
| U13 | Woman | 32 | 101 | 3800 ± 200 | 3.8 ± 0.2 | 0.24 ± 0.04 | 240 ± 40 |
| U14 | Woman | 35 | 30 | 4900 ± 300 | 16 ± 1 | 0.18 ± 0.03 | 600 ± 100 |
| U15 | Woman | 52 | 77 | 1900 ± 100 | 2.4 ± 0.2 | <LOD | <LOD |
| U16 | Man | 39 | 149 | 8800 ± 300 | 5.9 ± 0.2 | 0.17 ± 0.03 | 110 ± 20 |
| U17 | Man | 53 | 69 | 1600 ± 100 | 2.3 ± 0.2 | <LOD | <LOD |
| U18 | Woman | 39 | 15 | 1720 ± 70 | 11.3 ± 0.5 | <LOD | <LOD |
| U19 | Man | 47 | 42 | 210 ± 70 | 0.5 ± 0.1 | <LOD | <LOD |
| U20 | Man | 58 | 90 | 1200 ± 300 | 1.3 ± 0.3 | <LOD | <LOD |
| U21 | Woman | 56 | 23 | 60 ± 40 | 0.3 ± 0.2 | <LOD | <LOD |
| U22 | Woman | 28 | 103 | 50 ± 30 | 0.05 ± 0.03 | <LOD | <LOD |
| U23 | Man | 32 | 263 | 110 ± 50 | 0.04 ± 0.02 | <LOD | <LOD |
| U24 | Woman | 24 | 115 | 60 ± 40 | 0.06 ± 0.04 | <LOD | <LOD |
| U25 | Man | 38 | 123 | 220 ± 80 | 0.18 ± 0.06 | <LOD | <LOD |
| U26 | Man | 51 | 99 | 1100 ± 200 | 1.1 ± 0.2 | <LOD | <LOD |
| U27 | Woman | 31 | 14 | 7000 ± 1000 | 49 ± 9 | <LOD | <LOD |
| U28 | Woman | 39 | 198 | 1600 ± 100 | 0.79 ± 0.05 | <LOD | <LOD |
| U29 | Man | 66 | 52 | 14,000 ± 2000 | 27 ± 3 | <LOD | <LOD |
| U30 | Man | 39 | 64 | 290 ± 60 | 0.45 ± 0.09 | <LOD | <LOD |
| U31 | Man | 64 | 33 | 20,000 ± 2000 | 62 ± 5 | 1.13 ± 0.06 | 3400 ± 200 |
| U32 | Woman | 43 | 14 | 1500 ± 100 | 10.5 ± 0.9 | 0.6 ± 0.1 | 4300 ± 700 |
| U33 | Woman | 50 | 57 | 360 ± 60 | 0.60 ± 0.10 | 0.17 ± 0.07 | 300 ± 100 |
| U34 | Man | 45 | 12 | 340 ± 50 | 2.8 ± 0.4 | 0.31 ± 0.01 | 2500 ± 100 |
| U35 | Woman | 64 | 15 | 670 ± 50 | 4.5 ± 0.3 | 2.3 ± 0.3 | 15,000 ± 2000 |
| U36 | Woman | 9 | 13 | 290 ± 40 | 2.3 ± 0.3 | 0.12 ± 0.05 | 900 ± 400 |
| U37 | Woman | 41 | 8 | 650 ± 20 | 7.7 ± 0.3 | 1.0 ± 0.2 | 12,000 ± 200 |
| U38 | Woman | 35 | 10 | 340 ± 40 | 3.6 ± 0.4 | 0.04 ± 0.02 | 400 ± 200 |
| U39 | Woman | 72 | 4 | 136 ± 6 | 3.4 ± 0.1 | <LOD | <LOD |
| U40 | Man | 37 | 53 | 310 ± 50 | 0.59 ± 0.09 | <LOD | <LOD |
| U41 | Man | 44 | 13 | 3100 ± 80 | 23.4 ± 0.6 | <LOD | <LOD |
| U42 | Woman | 43 | 6 | 200 ± 20 | 3.5 ± 0.3 | <LOD | <LOD |
| U43 | Man | 3 | 35 | 1480 ± 70 | 4.2 ± 0.2 | 0.30 ± 0.05 | 900 ± 100 |
| U44 | Man | 38 | 15 | 340 ± 30 | 2.2 ± 0.2 | 0.05 ± 0.02 | 400 ± 100 |
| U45 | Man | 38 | 21 | 250 ± 90 | 1.2 ± 0.7 | 0.28 ± 0.08 | 1300 ± 400 |
| U46 | Man | 51 | 18 | 340 ± 40 | 1.9 ± 0.2 | <LOD | <LOD |
| U47 | Man | 15 | 26 | 240 ± 50 | 0.9 ± 0.2 | 0.32 ± 0.04 | 1200 ± 200 |
| U48 | Woman | 14 | 26 | 310 ± 40 | 1.2 ± 0.2 | 0.34 ± 0.04 | 1300 ± 200 |
| U49 | Man | 17 | 38 | 380 ± 30 | 0.99 ± 0.06 | 0.16 ± 0.05 | 400 ± 100 |
| U50 | Man | 49 | 26 | 1300 ± 100 | 5.1 ± 0.4 | 0.42 ± 0.08 | 250 ± 50 |
| U51 | Woman | 53 | 38 | 70 ± 30 | 0.17 ± 0.09 | <LOD | <LOD |
| U52 | Man | 39 | 84 | 360 ± 50 | 0.43 ± 0.06 | <LOD | <LOD |
| U53 | Woman | 24 | 166 | 290 ± 40 | 0.17 ± 0.02 | <LOD | <LOD |
| U54 | Woman | 34 | 86 | 700 ± 50 | 0.81 ± 0.06 | <LOD | <LOD |
| U55 | Man | 55 | 84 | 1060 ± 40 | 1.26 ± 0.05 | 0.5 ± 0.1 | 240 ± 50 |
| U56 | Woman | 25 | 76 | 1520 ± 90 | 2.0 ± 0.1 | 0.20 ± 0.05 | 60 ± 10 |
| U57 | Man | 31 | 101 | 290 ± 10 | 0.29 ± 0.01 | 0.08 ± 0.03 | 25 ± 9 |
| U58 | Woman | 29 | 218 | 450 ± 60 | 0.21 ± 0.03 | <LOD | <LOD |
| U59 | Woman | 29 | 330 | 110 ± 40 | 0.03 ± 0.01 | <LOD | <LOD |
| U60 | Woman | 28 | 235 | 570 ± 70 | 0.24 ± 0.03 | 0.06 ± 0.01 | 130 ± 10 |
| U61 | Woman | 27 | 111 | 1310 ± 30 | 1.18 ± 0.03 | 0.23 ± 0.03 | 160 ± 20 |
| U2204 | NA | NA | 146 | 870 ± 90 | 0.6 ± 0.1 | 0.03 ± 0.02 | 21 ± 9 |
| U2206 | NA | NA | 119 | 490 ± 50 | 0.41 ± 0.06 | 0.01 ± 0.01 | 13 ± 9 |
| U2106 | NA | NA | 93 | 310 ± 40 | 0.34 ± 0.06 | 0.03 ± 0.01 | 30 ± 10 |
| U2205 | NA | NA | 143 | 450 ± 60 | 0.31 ± 0.01 | 0.04 ± 0.02 | 30 ± 10 |
| U2303 | NA | NA | 137 | 650 ± 50 | 0.48 ± 0.05 | 0.03 ± 0.02 | 19 ± 8 |
| U2203 | NA | NA | 131 | 490 ± 30 | 0.38 ± 0.01 | 0.01 ± 0.01 | 6 ± 5 |
| U2104 | NA | NA | 119 | 730 ± 50 | 0.61 ± 0.09 | 0.08 ± 0.02 | 60 ± 20 |
| U2302 | NA | NA | 118 | 580 ± 30 | 0.49 ± 0.09 | 0.01 ± 0.01 | 7 ± 6 |
| U2301 | NA | NA | 113 | 1830 ± 30 | 1.62 ± 0.07 | 0.02 ± 0.01 | 20 ± 10 |
| U2202 | NA | NA | 93 | 460 ± 80 | 0.50 ± 0.06 | 0.02 ± 0.01 | 20 ± 10 |
| U2201 | NA | NA | 79 | 520 ± 30 | 0.66 ± 0.06 | 0.01 ± 0.01 | 18 ± 9 |
| Category | MDA | DPhP | |||||
|---|---|---|---|---|---|---|---|
| N a | GM | 95% CI | N a | GM | 95% CI | ||
| Age b | High | 36 | 1.41 | 0.89–2.22 | 14 | 19.14 | 7.44–49.23 |
| Low | 25 | 0.95 | 0.46–1.95 | 14 | 12.65 | 6.85–23.34 | |
| Sex | Woman | 32 | 1.15 | 0.62–2.12 | 14 | 17.23 | 7.32–40.58 |
| Man | 29 | 1.25 | 0.72–2.18 | 14 | 14.04 | 6.33–31.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chango, G.; García-Gómez, D.; García Pinto, C.; Rodríguez-Gonzalo, E.; Pérez Pavón, J.L. A Tandem MS Platform for Simultaneous Determination of Urinary Malondialdehyde and Diphenyl Phosphate. Int. J. Environ. Res. Public Health 2025, 22, 1130. https://doi.org/10.3390/ijerph22071130
Chango G, García-Gómez D, García Pinto C, Rodríguez-Gonzalo E, Pérez Pavón JL. A Tandem MS Platform for Simultaneous Determination of Urinary Malondialdehyde and Diphenyl Phosphate. International Journal of Environmental Research and Public Health. 2025; 22(7):1130. https://doi.org/10.3390/ijerph22071130
Chicago/Turabian StyleChango, Gabriela, Diego García-Gómez, Carmelo García Pinto, Encarnación Rodríguez-Gonzalo, and José Luis Pérez Pavón. 2025. "A Tandem MS Platform for Simultaneous Determination of Urinary Malondialdehyde and Diphenyl Phosphate" International Journal of Environmental Research and Public Health 22, no. 7: 1130. https://doi.org/10.3390/ijerph22071130
APA StyleChango, G., García-Gómez, D., García Pinto, C., Rodríguez-Gonzalo, E., & Pérez Pavón, J. L. (2025). A Tandem MS Platform for Simultaneous Determination of Urinary Malondialdehyde and Diphenyl Phosphate. International Journal of Environmental Research and Public Health, 22(7), 1130. https://doi.org/10.3390/ijerph22071130







