Barriers to Immunosuppressant Medication Adherence in Thoracic Transplant Recipients: Initial Findings
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Primary Outcome: Basel Assessment of Adherence with Immunosuppressive Medication Scale (BAASIS)
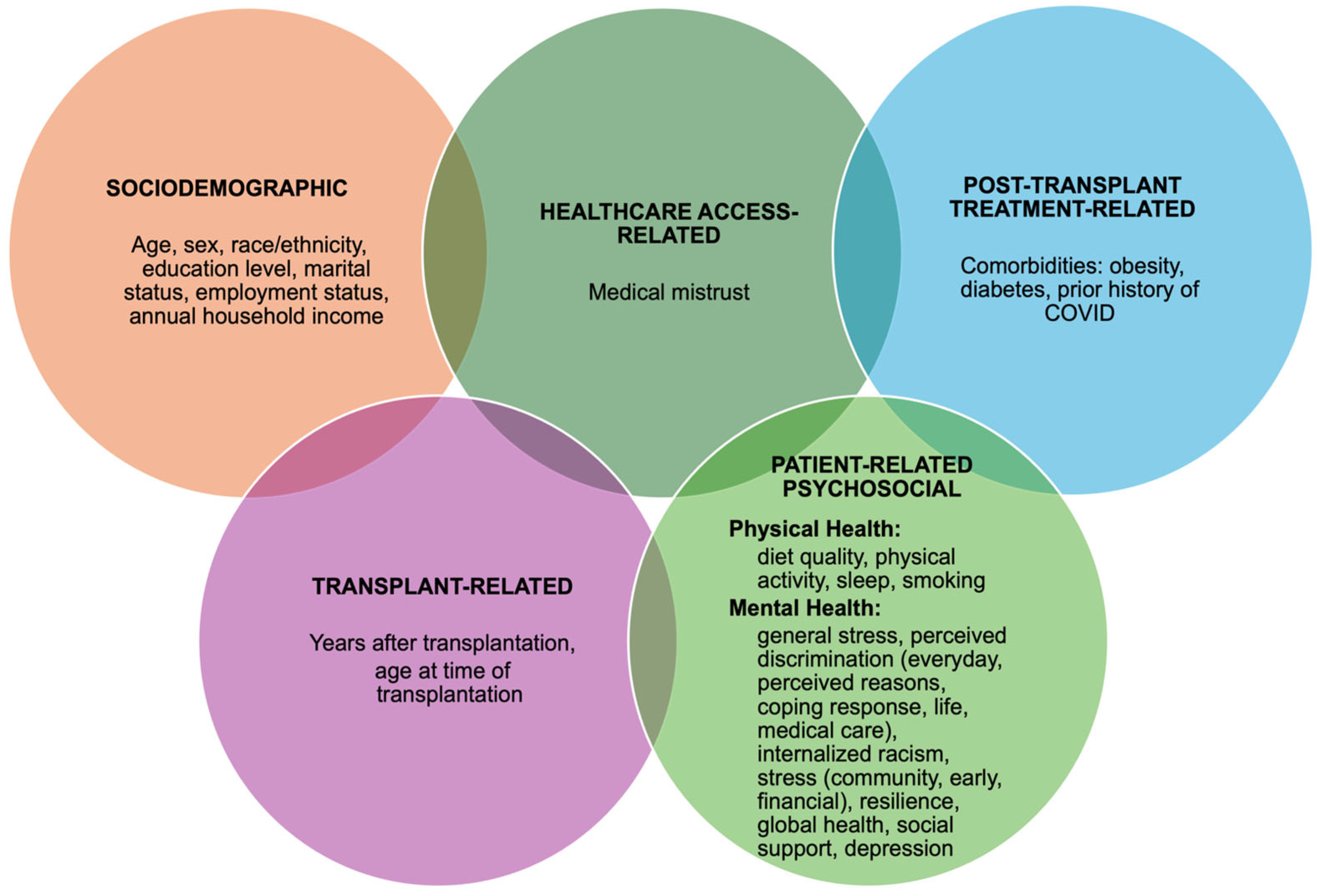
2.4. Exposure Measures: Five Dimensions of Adherence
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Overview
4.2. Key Findings
4.3. Limitations and Strengths
4.4. Public Health Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Adverse Childhood Experiences |
| BAASIS | Basel Assessment of Adherence with Immunosuppressive Medication Scale |
| BRS | Brief Resilience Scale |
| CDS | Coping with Discrimination |
| COVID-19 | Coronavirus disease 2019 |
| dd-cfDNA | Donor-derived cell-free DNA |
| EDS | Everyday Discrimination Scale |
| GRAfT | Genomic Research Alliance for Transplantation |
| GBMMS | Group-Based Medical Mistrust |
| IRS | Internalized Racism Scale |
| LUC | Loyola University Chicago |
| MED | Major Experiences of Discrimination |
| PHQ-2 | Patient Health Questionnaire-2 |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| PPS-4 | Perceived Stress Scale |
| SIMSS | Single Item Measure of Social Support |
| WELL | Wellness Living Laboratory |
Appendix A
| Dimension 1: Sociodemographic Factors | |
|---|---|
| Age | Self-reported birth date (e.g., mm/dd/yyyy). Open-ended. |
| Sex | Self-reported as male or female. |
| Race/ethnicity | Self-reported race/ethnicity. Response options were White, Black or African American, Hispanic or Latinx, and Other. |
| Education level | Self-reported highest level of education. Response options were less than a high school diploma, high school diploma or GED, some college or technical school, 4-year college degree, postgraduate degree. |
| Marital status | Self-reported marital status. Response options were (1 “single”, 2 “married”, 3 “living as married”, or 4 “widowed”). |
| Employment status | Self-reported employment status. Response options were employed full-time (FT), employed part-time (PT), self-employed, unemployed, retired, student, homemaker, or disabled. |
| Annual household income | Self-reported level of household income. Response options were Less than USD 35,000; USD 35,000 to 49,999; USD 50,000 to 74,999; and USD 75,000+. |
| Dimension 2: Transplant-Related Factors | |
| Years after transplantation | Calculated based on self-reported time of transplantation. Median and interquartile range were calculated. |
| Age at the time of transplantation | Measured based on self-reported age at transplantation. Mean and standard deviation were calculated. |
| Dimension 3: Healthcare Access-Related Factors | |
| Medical mistrust | The Group-Based Medical Mistrust (GBMMS) is a 12-item scale developed to measure race-based medical mistrust: the suspicion of mainstream healthcare systems and professionals and the treatment provided to individuals of the respondent’s ethnic or racial group [41]. While there is no existing literature that utilizes GBMMS among transplant recipients, the GBMMS has been used in other patient populations with chronic illness and has convergent validity demonstrated in studies that use GBMSS and other mistrust scales [85]. We asked participants six items related to GBMMS. Response options ranged from 1 to 5 (strongly disagree to strongly agree) and the mean of the responses was calculated to be the final score, which can range from 1–5. Higher scores indicate greater medical mistrust. Example: “People of my ethnic group cannot trust doctors and healthcare workers”. |
| Dimension 4: Post-Transplant Treatment-Related Factors | |
| Comorbidities | Self-reported weight and height, history of diabetes, and COVID-19 diagnosis. Weight and height data were used to calculate BMI and describe obesity (BMI ≥ 30). |
| Dimension 5: Patient-Related Psychosocial Factors | |
| Physical Health Outcomes | |
| Diet quality | Diet quality was measured by the WELL Diet Score, which has been correlated with the Alternative Healthy Eating Index-2010), a well-established measure of diet quality [42]. For the 12 diet-related items, the team of nutrition professionals working on the project agreed, by consensus, how to distribute points across the different frequencies of consumption. Participants were asked about their frequency of consumption. Example: “How often do you eat fish?”. Points were then combined to generate a total WELL diet quality score, which can range from 0 (indicating low quality diet) to 120 (indicating better diet quality). |
| Physical activity | Godin Leisure-Time Exercise Questionnaire has been employed across various populations, including healthy adults, individuals with chronic conditions, and cancer survivors to measure physical activity [43,86]. Participants were asked during a typical seven-day period (a week), how many times on average do you do the following kinds of exercise? Three items assessed the frequency per week of strenuous (heart beats rapidly), moderate (not exhausting) and mild (minimal effort) exercise for more than 15 min in addition to frequency of leisure and sedentary activity per week. One item assessed how often do you engage in regular activity long enough to work up a sweat. Response options were (1 “often”, 2 “sometimes”, and 3 “never/rarely”) [43]. Scores of 24 or above are considered a “high” level of physical activity, scores between 14 and 23 are considered “moderate” activity, and ones below 14 suggest a “low” level of physical activity. |
| Sleep quality | Self-reported hours of sleep per night. Participants were asked how many hours they slept per night during the past four weeks. Response options were (1 “5 h or less”, 2 “6 h”, 3 “7 h”, 4 “8 h”, 5 “9 h”, and 6 “10 or more hours”). Those with good sleep scores (7–9 h) are reported. |
| Smoking status | Self-reported smoking status. Do you smoke cigarettes now? (1 “yes”; 2 “no”). |
| Mental Health Outcomes | |
| General stress | The Perceived Stress Scale-4 (PSS-4) was used to measure the degree to which respondents find their lives unpredictable, uncontrollable, and overloaded [44]. The PSS-4 includes four items that measure self-appraised stress over the previous month. It has been found to be psychometrically valid in samples of African American women and has shown negative associations with overall health status and well-being. Response options ranged from “never” to “very often”. |
| Everyday Discrimination | Racial discrimination was assessed using five items from the Everyday Discrimination Scale (EDS) and was coded using two conventional approaches: (1) ‘situation-based coding’: number of different situations ever experienced; (2) ‘frequency-based coding’: sum of Likert scale responses ranging from never to almost every day; and (3) a new ‘chronicity-based coding’ approach: sum of responses, weighted to capture annual chronicity (e.g., ‘a few times a month’ = 3 × 12 = 36 ×/year) [49,53,87,88]. The EDS asks, “In your day-to-day life, how often do any of the following things happen to you?” and can include items such as, “You are treated with less courtesy or respect than other people”. Number of situations was reported, with response options ranged from never to almost every day. Higher scores indicate more types of situations where discrimination was experienced, greater frequency of discrimination, and greater chronicity. |
| Perceived Reasons for Discrimination | In the EDS, if participants answered “a few times a year” or more frequently to at least one everyday discrimination question, they were asked a follow-up question about the main reason for these experiences, with response options including race, gender, age, weight or height, education or income, physical disability, religion, or other. The frequency of the responses for each perceived reason were reported. |
| Coping Response to Unfair Treatment | Follow-up questions based on a version of the MED scale used in previous studies [46,47,48] asked participants eight items regarding their responses to unfair treatment. These response types included “tried to do something about it”, “accepted it as a fact of life”, “worked harder to prove them wrong”, “realized you brought it on yourself”, “talked to someone about how you were feeling”, “expressed anger or got mad”, and “prayed about the situation”. Response options were “yes” or “no”. The frequency of the responses for each coping response were reported. |
| Life Discrimination Events | Six items from the Major Experiences of Discrimination (Abbreviated Version) [53,87,88,89] were used to assess amount of lifetime discrimination events participants experienced. The MED asked participants, “Have any of the following ever happened to you?” Examples include “At any time in your life, have you ever been unfairly fired?” and “Have you ever been unfairly stopped, searched, questioned, physically threatened, or abused by the police?” Response options were “yes” or “no”. The frequencies of events were reported for each recipient. |
| Discrimination in Medical Care | A single item from the Major Experiences of Discrimination scale—based on an instrument used in the Coronary Artery Risk Development in Young Adults (CARDIA) study—asked participants: “Have you ever experienced discrimination, been prevented from doing something, or been hassled or made to feel inferior while getting medical care because of your race, ethnicity, or color?” Responses were “yes” or “no”. If yes, participants were asked how many times (once, two or three times, four or more times) [51]. |
| Internalized racism | The Internalized Racism Scale (originally with 36 items) assesses internalized racism, referring to the acceptance of societal beliefs about one’s group, which can negatively affect self-esteem, mental health, and behavior [52]. The scale was abbreviated to assess five items related to belief biases, alteration of physical appearance, internalized negative stereotypes, hair change, and African worldview and motifs. Response options ranged from 1 (strongly disagree) to 5 (strongly agree). An example item is “There are no institutions of higher learning in Africa”. Scores range from 1 to 5, with higher scores indicating greater levels of internalized racial oppression. |
| Community stress | Community stress was measured using a five-item subscale adapted from the Project on Human Development in Chicago Neighborhoods, focusing on violence occurring in the community [53]. Participants were asked, “In the past 6 months, has there been (1) a neighborhood fight involving a weapon; (2) a violent argument between neighbors; (3) gang fights; (4) sexual assault or rape; (5) robbery or mugging?” Response options ranged from 1 (never) to 5 (very often). Scores range from 0–5. Higher score corresponded to higher community stress. |
| Early stress | The Adverse Childhood Experiences scale (ACES) from Felitti et al.’s original scale was used to measure stress and trauma experienced in the first 18 years of life [54]. Higher scores have been causally linked to mental illnesses, addictions, chronic disease, multi-organ medical diseases, and worsened quality-of-life [55,90,91]. Participants responded to eight items, with response options “yes”, “no”, or “refuse/don’t know”. An example item is “Physical abuse: How often did a parent or adult in your home ever hit, beat, kick, or physically hurt you in any way?” |
| Financial stress | Two routinely used questions on household financial situation and monthly financial strain were used to measure financial stress, which has been linked with psychological distress [56]. Participants were asked “How satisfied are you with your family’s financial situation?” with response options ranging from 1 (completely) to 5 (not at all), and “How difficult is it to meet monthly bill payments?” with response options ranging from 1 (not at all) to 5 (extremely). Scores range from 1–5. Higher scores correspond to greater financial stress. |
| Resilience | The Brief Resilience Scale (BRS), developed by Smith and colleagues in 2008, measures participants’ ability to bounce back from adversity [57]. Six items were used to assess the degree to which respondents agreed or disagreed with statements such as “I tend to bounce back quickly after hard times”. Response options ranged from “strongly agree” (1) to “strongly disagree” (5). Higher scores indicate greater resilience. |
| Global Health | Three items assessing global physical health and two items assessing global mental health derived from the Patient-Reported Outcomes Measurement Information System (PROMIS) Global Health (v 1.2) instrument were used to measure global health functioning. In kidney transplant recipients, the PROMIS-29 and PROMIS-57 (including the global health domain) showed strong internal consistency, structural validity, and test-retest reliability, supporting their use for assessing health-related quality of life in solid organ transplant recipients [92]. Participants were questioned regarding their physical health, ability to carry out daily physical activities, pain, mental health, and social satisfaction. Examples of questions include: “In general, how would you rate your physical health? In general, would you say your quality of life is: Excellent, Very Good, Good, Fair, Poor”. Pain was assessed with the question: “In the past 7 days, how would you rate your pain on average?” using a scale from 0 (no pain) to 10 (worst pain imaginable). Variations of the PROMIS Global Health scale have shown adequate reliability for group comparisons, and their associations with other indicators of health are similar to that of the original four-item scale [58,59]. |
| Social support | The Single Item Measure of Social Support (SIMSS) [60] asked participants “How many people do you have near you that you can readily count on for help in times of difficulty, such as watching over children or pets, giving rides to the hospital or store, or helping when you are sick?” Response options were 0, 1, 2–5, 6–10, or more than 10. Responses of 0 or 1 indicated low tangible assistance, while 2–5 indicated moderate, and 6–10 or more indicated high tangible assistance. Although extremely short, this instrument is a strong predictor of morbidity and has good psychometric properties [60,93]. Use of this short measure allowed us to avoid utilizing one of the much longer questionnaires on social support, thus rendering the assessment process less burdensome for participants. |
| Depression | The Patient Health Questionnaire (PHQ-2) [61] is routinely used to screen patients for depression and asks participants two items regarding anhedonia and low mood and frequency over two weeks. It has been recommended by the American Heart Association Science Advisory for routinely screening cardiac patients for depression, and has been validated among solid organ transplant recipients [62,63,94]. Participants were asked, “Over the last 2 weeks, how often have you been bothered by the following problems?” Item 1 was “Little interest or pleasure in doing things”, and item 2 was “Feeling down, depressed, or hopeless”. Scores can range from 0–6 with four possible answer choices, starting with “not at all” (scored as 0) to “nearly every day (scored as 3)”. The final score is the sum of responses. Scores greater than or equal to 2 suggest the likelihood of major depressive disorder [64]. |
References
- van der Mark, S.C.; Hoek, R.A.S.; Hellemons, M.E. Developments in lung transplantation over the past decade. Eur. Respir. Rev. 2020, 29, 190132. [Google Scholar] [CrossRef]
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2021 Annual Data Report; U.S. Department of Health and Human Services, Health Resources and Services Administration: Rockville, MD, USA, 2023. [Google Scholar]
- Chambers, D.C.; Perch, M.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report—2021; Focus on recipient characteristics. J. Heart Lung Transpl. 2021, 40, 1060–1072. [Google Scholar] [CrossRef]
- Khush, K.K.; Hsich, E.; Potena, L.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D., Jr.; Perch, M.; Sadavarte, A.; Toll, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult heart transplantation report—2021; Focus on recipient characteristics. J. Heart Lung Transpl. 2021, 40, 1035–1049. [Google Scholar] [CrossRef]
- Kvarnström, K.; Westerholm, A.; Airaksinen, M.; Liira, H. Factors Contributing to Medication Adherence in Patients with a Chronic Condition: A Scoping Review of Qualitative Research. Pharmaceutics 2021, 13, 1100. [Google Scholar] [CrossRef]
- De Geest, S.; Dobbels, F.; Fluri, C.; Paris, W.; Troosters, T. Adherence to the therapeutic regimen in heart, lung, and heart-lung transplant recipients. J. Cardiovasc. Nurs. 2005, 20, S88–S98. [Google Scholar] [CrossRef]
- Pinsky, B.W.; Takemoto, S.K.; Lentine, K.L.; Burroughs, T.E.; Schnitzler, M.A.; Salvalaggio, P.R. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am. J. Transpl. 2009, 9, 2597–2606. [Google Scholar] [CrossRef]
- Gaynor, J.J.; Ciancio, G.; Guerra, G.; Sageshima, J.; Hanson, L.; Roth, D.; Chen, L.; Kupin, W.; Mattiazzi, A.; Tueros, L.; et al. Graft Failure Due to Noncompliance Among 628 Kidney Transplant Recipients With Long-term Follow-up: A Single-Center Observational Study. Transplantation 2014, 97, 925–933. [Google Scholar] [CrossRef]
- Mellon, L.; Doyle, F.; Hickey, A.; Ward, K.D.; de Freitas, D.G.; McCormick, P.A.; O’Connell, O.; Conlon, P. Interventions for increasing immunosuppressant medication adherence in solid organ transplant recipients. Cochrane Database Syst. Rev. 2022, 9, Cd012854. [Google Scholar] [CrossRef]
- Dew, M.A.; Roth, L.H.; Thompson, M.E.; Kormos, R.L.; Griffith, B.P. Medical compliance and its predictors in the first year after heart transplantation. J. Heart Lung Transpl. 1996, 15, 631–645. [Google Scholar]
- Teichman, B.J.; Burker, E.J.; Weiner, M.; Egan, T.M. Factors associated with adherence to treatment regimens after lung transplantation. Prog. Transpl. 2000, 10, 113–121. [Google Scholar] [CrossRef]
- De Geest, S.; Burkhalter, H.; Bogert, L.; Berben, L.; Glass, T.R.; Denhaerynck, K. Describing the evolution of medication nonadherence from pretransplant until 3 years post-transplant and determining pretransplant medication nonadherence as risk factor for post-transplant nonadherence to immunosuppressives: The Swiss Transplant Cohort Study. Transpl. Int. 2014, 27, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Saint-Marcoux, F.; Woillard, J.-B.; Monchaud, C.; Friedl, J.; Bocquentin, F.; Essig, M.; Marquet, P. How to handle missed or delayed doses of tacrolimus in renal transplant recipients? A pharmacokinetic investigation. Pharmacol. Res. 2015, 100, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Castleberry, A.W.; Bishawi, M.; Worni, M.; Erhunmwunsee, L.; Speicher, P.J.; Osho, A.A.; Snyder, L.D.; Hartwig, M.G. Medication Nonadherence After Lung Transplantation in Adult Recipients. Ann. Thorac. Surg. 2017, 103, 274–280. [Google Scholar] [CrossRef]
- Dew, M.A.; Dimartini, A.F.; De Vito Dabbs, A.; Zomak, R.; De Geest, S.; Dobbels, F.; Myaskovsky, L.; Switzer, G.E.; Unruh, M.; Steel, J.L.; et al. Adherence to the medical regimen during the first two years after lung transplantation. Transplantation 2008, 85, 193–202. [Google Scholar] [CrossRef]
- Dew, M.A.; Kormos, R.L.; Roth, L.H.; Murali, S.; DiMartini, A.; Griffith, B.P. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J. Heart Lung Transplant. 1999, 18, 549–562. [Google Scholar] [CrossRef]
- Hussain, T.; Nassetta, K.; O’Dwyer, L.C.; Wilcox, J.E.; Badawy, S.M. Adherence to immunosuppression in adult heart transplant recipients: A systematic review. Transplant. Rev. 2021, 35, 100651. [Google Scholar] [CrossRef]
- Farmer, S.A.; Grady, K.L.; Wang, E.; McGee, E.C., Jr.; Cotts, W.G.; McCarthy, P.M. Demographic, psychosocial, and behavioral factors associated with survival after heart transplantation. Ann. Thorac. Surg. 2013, 95, 876–883. [Google Scholar] [CrossRef]
- Korb-Savoldelli, V.; Sabatier, B.; Gillaizeau, F.; Guillemain, R.; Prognon, P.; Bégué, D.; Durieux, P. Non-adherence with drug treatment after heart or lung transplantation in adults: A systematic review. Patient Educ. Couns. 2010, 81, 148–154. [Google Scholar] [CrossRef]
- Annette, L.; Annika, K.; Anna, F. Non-adherence to immunosuppressant after lung transplantation—A common risk behavior. Open Nurs. J. 2019, 13, 108–115. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, H.; Nelson, R.S.; Han, Y.; Wang, Y.; Xiang, H.; Cai, J.; Zhang, J.; Yuan, Y. Prevalence and risk factors of immunosuppressant nonadherence in heart transplant recipients: A single-center cross-sectional study. Patient Prefer. Adherence 2019, 13, 2185–2193. [Google Scholar] [CrossRef]
- Organization, W.H. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Brocks, Y.; Zittermann, A.; Grisse, D.; Schmid-Ott, G.; Stock-Gießendanner, S.; Schulz, U.; Brakhage, J.; Benkler, A.; Gummert, J.; Tigges-Limmer, K. Adherence of Heart Transplant Recipients to Prescribed Medication and Recommended Lifestyle Habits. Prog. Transpl. 2017, 27, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Denhaerynck, K.; Berben, L.; Dobbels, F.; Russell, C.L.; Crespo-Leiro, M.G.; Poncelet, A.J.; De Geest, S.; Team, B.S. Multilevel factors are associated with immunosuppressant nonadherence in heart transplant recipients: The international BRIGHT study. Am. J. Transplant. 2018, 18, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Kugler, C.; Gottlieb, J.; Dierich, M.; Haverich, A.; Strueber, M.; Welte, T.; Simon, A. Significance of patient self-monitoring for long-term outcomes after lung transplantation. Clin. Transpl. 2010, 24, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Marston, M.T.; Berben, L.; Dobbels, F.; Russell, C.L.; de Geest, S. Prevalence and Patient-Level Correlates of Intentional Non-Adherence to Immunosuppressive Medication After Heart-Transplantation—Findings From the International BRIGHT Study. Transpl. Int. 2023, 36, 11308. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Trulock, E.P.; Freedland, K.E.; Carney, R.M.; Davis, R.D.; Hoffman, B.M.; Palmer, S.M. Psychosocial Predictors of Mortality Following Lung Transplantation. Am. J. Transpl. 2016, 16, 271–277. [Google Scholar] [CrossRef]
- Jain, M.; Venkataraman, J.; Reddy, M.S.; Rela, M. Determinants of Medication Adherence in Liver Transplant Recipients. J. Clin. Exp. Hepatol. 2019, 9, 676–683. [Google Scholar] [CrossRef]
- Belaiche, S.; Décaudin, B.; Dharancy, S.; Noel, C.; Odou, P.; Hazzan, M. Factors relevant to medication non-adherence in kidney transplant: A systematic review. Int. J. Clin. Pharm. 2017, 39, 582–593. [Google Scholar] [CrossRef]
- Laederach-Hofmann, K.; Bunzel, B. Noncompliance in organ transplant recipients: A literature review. Gen. Hosp. Psychiatry 2000, 22, 412–424. [Google Scholar] [CrossRef]
- Drick, N.; Seeliger, B.; Fuge, J.; Tudorache, I.; Greer, M.; Welte, T.; Haverich, A.; Gottlieb, J. Self-reported non-adherence to immunosuppressive medication in adult lung transplant recipients—A single-center cross-sectional study. Clin. Transplant. 2018, 32, e13214. [Google Scholar] [CrossRef]
- Khush, K.K.; Valantine, H.A. The Time to Act Is Now: Racial Disparities After Heart Transplantation. Circulation 2023, 148, 207–209. [Google Scholar] [CrossRef]
- Chan, N.W.; Moya-Mendez, M.; Henson, J.B.; Zaribafzadeh, H.; Sendak, M.P.; Bhavsar, N.A.; Balu, S.; Kirk, A.D.; McElroy, L.M. Social determinants of health data in solid organ transplantation: National data sources and future directions. Am. J. Transpl. 2022, 22, 2293–2301. [Google Scholar] [CrossRef]
- Breathett, K.; Yee, E.; Pool, N.; Hebdon, M.; Crist, J.D.; Yee, R.H.; Knapp, S.M.; Solola, S.; Luy, L.; Herrera-Theut, K.; et al. Association of Gender and Race With Allocation of Advanced Heart Failure Therapies. JAMA Netw. Open 2020, 3, e2011044. [Google Scholar] [CrossRef] [PubMed]
- Bui, Y.T.; Hathcock, M.A.; Benzo, R.P.; Budev, M.M.; Chandrashekaran, S.; Erasmus, D.B.; Lease, E.D.; Levine, D.J.; Thompson, K.L.; Johnson, B.K.; et al. Evaluating resilience as a predictor of outcomes in lung transplant candidates. Clin. Transpl. 2020, 34, e14056. [Google Scholar] [CrossRef] [PubMed]
- Agbor-Enoh, S.; Shah, P.; Tunc, I.; Hsu, S.; Russell, S.; Feller, E.; Shah, K.; Rodrigo, M.E.; Najjar, S.S.; Kong, H.; et al. Cell-Free DNA to Detect Heart Allograft Acute Rejection. Circulation 2021, 143, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, M.T.; Midtvedt, K.; Lønning, K.; Jacobsen, T.; Reisæter, A.V.; De Geest, S.; Andersen, M.H.; Hartmann, A.; Åsberg, A. Evaluation of tools for annual capture of adherence to immunosuppressive medications after renal transplantation–a single-centre open prospective trial. Transpl. Int. 2019, 32, 614–625. [Google Scholar] [CrossRef]
- Dobbels, F.; Berben, L.; De Geest, S.; Drent, G.; Lennerling, A.; Whittaker, C.; Kugler, C. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: A systematic review. Transplantation 2010, 90, 205–219. [Google Scholar] [CrossRef]
- Shemesh, Y.; Peles-Bortz, A.; Peled, Y.; HarZahav, Y.; Lavee, J.; Freimark, D.; Melnikov, S. Feelings of indebtedness and guilt toward donor and immunosuppressive medication adherence among heart transplant (HT x) patients, as assessed in a cross-sectional study with the Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS). Clin. Transplant. 2017, 31, e13053. [Google Scholar] [CrossRef]
- Denhaerynck, K.; Dobbels, F.; Košťálová, B.; De Geest, S.; consortium, B. Psychometric properties of the BAASIS: A meta-analysis of individual participant data. Transplantation 2023, 107, 1795–1809. [Google Scholar] [CrossRef]
- Thompson, H.S.; Valdimarsdottir, H.B.; Winkel, G.; Jandorf, L.; Redd, W. The Group-Based Medical Mistrust Scale: Psychometric properties and association with breast cancer screening. Prev. Med. 2004, 38, 209–218. [Google Scholar] [CrossRef]
- Springfield, S.; Cunanan, K.; Heaney, C.; Peng, K.; Gardner, C. The WELL diet score correlates with the alternative healthy eating index-2010. Food Sci. Nutr. 2020, 8, 2710–2718. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G. The Godin-Shephard leisure-time physical activity questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R. Measuring discrimination resource. Psychology 1997, 2, 335–351. [Google Scholar]
- Krieger, N. Racial Gend. Discrim: Risk Factors High. Blood Press ? Soc. Sci. Med. 1990, 30, 1273–1281. [Google Scholar] [CrossRef]
- Krieger, N.; Sidney, S. Racial discrimination and blood pressure: The CARDIA Study of young black and white adults. Am. J. Public. Health 1996, 86, 1370–1378. [Google Scholar] [CrossRef]
- McNeilly, M.D.; Anderson, N.B.; Armstead, C.A.; Clark, R.; Corbett, M.; Robinson, E.L.; Pieper, C.F.; Lepisto, E.M. The perceived racism scale: A multidimensional assessment of the experience of white racism among African Americans. Ethn. Dis. 1996, 6, 154–166. [Google Scholar]
- Michaels, E.; Thomas, M.; Reeves, A.; Price, M.; Hasson, R.; Chae, D.; Allen, A. Coding the Everyday Discrimination Scale: Implications for exposure assessment and associations with hypertension and depression among a cross section of mid-life African American women. J. Epidemiol. Community Health 2019, 73, 577–584. [Google Scholar] [CrossRef]
- Williams, D.R.; John, D.A.; Oyserman, D.; Sonnega, J.; Mohammed, S.A.; Jackson, J.S. Research on discrimination and health: An exploratory study of unresolved conceptual and measurement issues. Am. J. Public. Health 2012, 102, 975–978. [Google Scholar] [CrossRef]
- Krieger, N.; Smith, K.; Naishadham, D.; Hartman, C.; Barbeau, E.M. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Soc. Sci. Med. 2005, 61, 1576–1596. [Google Scholar] [CrossRef]
- Bailey, T.-K.M.; Chung, Y.B.; Williams, W.S.; Singh, A.A.; Terrell, H.K. Development and validation of the Internalized Racial Oppression Scale for Black individuals. J. Couns. Psychol. 2011, 58, 481. [Google Scholar] [CrossRef]
- Sternthal, M.J.; Slopen, N.; Williams, D.R. Racial Disparities in Health: How Much Does Stress Really Matter? 1. Du Bois Rev. Soc. Sci. Res. Race 2011, 8, 95–113. [Google Scholar]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Zarse, E.M.; Neff, M.R.; Yoder, R.; Hulvershorn, L.; Chambers, J.E.; Chambers, R.A. The adverse childhood experiences questionnaire: Two decades of research on childhood trauma as a primary cause of adult mental illness, addiction, and medical diseases. Cogent Med. 2019, 6, 1581447. [Google Scholar] [CrossRef]
- Ryu, S.; Fan, L. The Relationship Between Financial Worries and Psychological Distress Among U. S. Adults. J. Fam. Econ. Issues 2023, 44, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Schalet, B.D.; Spritzer, K.L.; Cella, D. Two-item PROMIS® global physical and mental health scales. J. Patient-Rep. Outcomes 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Flynn, K.E.; Dew, M.A.; Lin, L.; Fawzy, M.; Graham, F.L.; Hahn, E.A.; Hays, R.D.; Kormos, R.L.; Liu, H.; McNulty, M. Reliability and construct validity of PROMIS® measures for patients with heart failure who undergo heart transplant. Qual. Life Res. 2015, 24, 2591–2599. [Google Scholar] [CrossRef]
- Blake, R.L., Jr.; McKay, D.A. A single-item measure of social supports as a predictor of morbidity. J. Fam. Pract. 1986, 22, 82–84. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med. Care 2003, 41, 1284–1292. [Google Scholar] [CrossRef]
- Rollman, B.L.; Belnap, B.H.; Mazumdar, S.; Houck, P.R.; He, F.; Alvarez, R.J.; Schulberg, H.C.; Reynolds III, C.F.; McNamara, D.M. A Positive PHQ-2 Depression Screen Among Hospitalized Heart Failure Patients Is Associated with Elevated 12-Month Mortality. J. Card. Fail. 2012, 18, 238. [Google Scholar] [CrossRef]
- Levis, B.; Sun, Y.; He, C.; Wu, Y.; Krishnan, A.; Bhandari, P.M.; Neupane, D.; Imran, M.; Brehaut, E.; Negeri, Z. Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: Systematic review and meta-analysis. Jama 2020, 323, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Dano, S.; Lan, H.H.; Macanovic, S.; Bartlett, S.; Howell, D.; Li, M.; Hanmer, J.; Peipert, J.D.; Novak, M.; Mucsi, I. Two-step screening for depressive symptoms in patients treated with kidney replacement therapies: A cross-sectional analysis. Nephrol. Dial. Transplant. 2022, 38, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Bulbuloglu, S.; Sayim, H.I. Investigation of immunosuppressive treatment compliance, dyspnea, anxiety, and depression levels in lung transplant recipients: Online interview. Front. Psychol. 2024, 15, 1378594. [Google Scholar] [CrossRef] [PubMed]
- Dobbels, F.; De Bleser, L.; Berben, L.; Kristanto, P.; Dupont, L.; Nevens, F.; Vanhaecke, J.; Verleden, G.; De Geest, S. Efficacy of a medication adherence enhancing intervention in transplantation: The MAESTRO-Tx trial. J. Heart Lung Transpl. 2017, 36, 499–508. [Google Scholar] [CrossRef]
- Axon, D.R.; Jang, A.; Son, L.; Pham, T. Determining the association of perceived health status among united states older adults with self-reported pain. Aging Health Res. 2022, 2, 100051. [Google Scholar] [CrossRef]
- Ko, Y.; Boo, S. Self-perceived health versus actual cardiovascular disease risks. Jpn. J. Nurs. Sci. 2016, 13, 65–74. [Google Scholar] [CrossRef]
- Pradipta, I.S.; Aprilio, K.; Ningsih, Y.F.; Pratama, M.A.A.; Alfian, S.D.; Abdulah, R. Treatment Nonadherence among Multimorbid Chronic Disease Patients: Evidence from 3515 Subjects in Indonesia. Medicina 2024, 60, 634. [Google Scholar] [CrossRef]
- Ali, M.U.; Sherifali, D.; Fitzpatrick-Lewis, D.; Kenny, M.; Lamarche, L.; Raina, P.; Mangin, D. Interventions to address polypharmacy in older adults living with multimorbidity: Review of reviews. Can. Fam. Physician 2022, 68, e215–e226. [Google Scholar] [CrossRef]
- Joo, J.; Williamson, S.A.; Vazquez, A.I.; Fernandez, J.R.; Bray, M.S. The influence of 15-week exercise training on dietary patterns among young adults. Int. J. Obes. 2019, 43, 1681–1690. [Google Scholar] [CrossRef]
- Hooker, S.; Punjabi, A.; Justesen, K.; Boyle, L.; Sherman, M.D. Encouraging Health Behavior Change: Eight Evidence-Based Strategies. Fam. Pract. Manag. 2018, 25, 31–36. [Google Scholar]
- Assari, S. Hostility, Anger, and Cardiovascular Mortality Among Blacks and Whites. Res. Cardiovasc. Med. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Zilioli, S.; Gómez, J.M.; Jiang, Y.; Rodriguez-Stanley, J. Childhood Socioeconomic Status and Cardiometabolic Health: A Test of the John Henryism Hypothesis in African American Older Adults. J. Gerontol. Ser. A 2021, 77, e56–e64. [Google Scholar] [CrossRef]
- Allen, J.O.; Watkins, D.C.; Mezuk, B.; Chatters, L.; Johnson-Lawrence, V. Mechanisms of Racial Health Disparities: Relationships between Coping and Psychological and Physiological Stress Responses. Ethn. Dis. 2020, 30, 563–574. [Google Scholar] [CrossRef]
- Glanz, K.; Schwartz, M.D. Stress, coping, and health behavior. Health behavior and health education: Theory, research, and practice 2008, 4, 211–236. [Google Scholar]
- Nayak, A.; Cole, R.T.; Morris, A.A. Race/Ethnic Disparities in Cardiac Transplantation. Curr. Cardiovasc. Risk Rep. 2019, 13, 33. [Google Scholar] [CrossRef]
- Mellon, L.; Doyle, F.; Hickey, A.; Ward, K.D.; de Freitas, D.G.; McCormick, P.A.; O’Connell, O.; Conlon, P. Interventions for improving medication adherence in solid organ transplant recipients. Cochrane Database Syst. Rev. 2017, 2017, CD012854. [Google Scholar] [CrossRef]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef]
- Forsyth, J.; Schoenthaler, A.; Chaplin, W.F.; Ogedegbe, G.; Ravenell, J. Perceived Discrimination and Medication Adherence in Black Hypertensive Patients: The Role of Stress and Depression. Psychosom. Med. 2014, 76, 229–236. [Google Scholar] [CrossRef]
- Al Meslamani, A.Z. Policy solutions for medication non-adherence: What can governments do? Expert Rev. Pharmacoecon.Outcomes Res. 2024, 24, 777–781. [Google Scholar] [CrossRef]
- Choudhry, N.K.; Krumme, A.A.; Ercole, P.M.; Girdish, C.; Tong, A.Y.; Khan, N.F.; Brennan, T.A.; Matlin, O.S.; Shrank, W.H.; Franklin, J.M. Effect of reminder devices on medication adherence: The REMIND randomized clinical trial. JAMA Intern. Med. 2017, 177, 624–631. [Google Scholar] [CrossRef]
- Gackowski, M.; Jasińska-Stroschein, M.; Osmałek, T.; Waszyk-Nowaczyk, M. Innovative approaches to enhance and measure medication adherence in chronic disease management: A review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e944605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.B.; Goh, W.W.; Balakrishnan, S. Smart medicine reminder device for the elderly. In Proceedings of the 2018 Fourth International Conference on Advances in Computing, Communication & Automation (ICACCA), Subang Jaya, Malaysia, 26–28 October 2018; pp. 1–6. [Google Scholar]
- Hall, M.J.; Park, C.Y.; Ruth, K.J.; Kelly, P.J.A.; Singley, K.; Luck, C.C.; Chertock, Y.; Bauerle Bass, S. Prevalence and Predictors of Medical Mistrust Among Socioeconomically and Racially Diverse Cancer Patients in Philadelphia. Cancers 2025, 17, 649. [Google Scholar] [CrossRef] [PubMed]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: A systematic review. BMC Med. Res. Methodol. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Cozier, Y.; Palmer, J.R.; Horton, N.J.; Fredman, L.; Wise, L.A.; Rosenberg, L. Racial discrimination and the incidence of hypertension in US black women. Ann. Epidemiol. 2006, 16, 681–687. [Google Scholar] [CrossRef]
- Jones, C.P. Levels of racism: A theoretic framework and a gardener’s tale. Am. J. Public Health 2000, 90, 1212. [Google Scholar]
- Williams, D.R.; Gonzalez, H.M.; Williams, S.; Mohammed, S.A.; Moomal, H.; Stein, D.J. Perceived discrimination, race and health in South Africa. Soc. Sci. Med. 2008, 67, 441–452. [Google Scholar] [CrossRef]
- Cronholm, P.F.; Forke, C.M.; Wade, R.; Bair-Merritt, M.H.; Davis, M.; Harkins-Schwarz, M.; Pachter, L.M.; Fein, J.A. Adverse childhood experiences: Expanding the concept of adversity. Am. J. Prev. Med. 2015, 49, 354–361. [Google Scholar] [CrossRef]
- Barile, J.P.; Edwards, V.J.; Dhingra, S.S.; Thompson, W.W. Associations among county-level social determinants of health, child maltreatment, and emotional support on health-related quality of life in adulthood. Psychol. Violence 2015, 5, 183–191. [Google Scholar] [CrossRef]
- Tang, E.; Ekundayo, O.; Peipert, J.D.; Edwards, N.; Bansal, A.; Richardson, C.; Bartlett, S.J.; Howell, D.; Li, M.; Cella, D.; et al. Validation of the Patient-Reported Outcomes Measurement Information System (PROMIS)-57 and -29 item short forms among kidney transplant recipients. Qual. Life Res. 2019, 28, 815–827. [Google Scholar] [CrossRef]
- Laganà, L.; Maria, L. Bratly, and Ioakim Boutakidis. The validation of a new measure quantifying the social quality of life of ethnically diverse older women: Two cross-sectional studies. BMC Geriatr. 2011, 11, 1–13. [Google Scholar] [CrossRef]
- Müller, E.; Härter, M.; Higgen, S.; Barten, M.J.; Eickhoff, D.; Grahammer, F.; Reinsberg, N.; Sterneck, M.R.; Buchholz, A. The development and psychometric evaluation of specific problem lists reflecting psychosocial distress of patients before and after solid organ transplantation. Front. Psychol. 2025, 16, 1481641. [Google Scholar] [CrossRef]


| Dimension | BAASIS Items | Response | N (%) |
|---|---|---|---|
| Taking adherence | Do you remember missing a dose of your anti-rejection medications in the last four weeks? How often? | Never | 52 (80.0) |
| Once | 11 (16.9) | ||
| Twice | 2 (3.1) | ||
| Three times | - | ||
| Four times | - | ||
| More than four times | - | ||
| Drug holiday | Do you remember having skipped two or more doses of your anti-rejection medications in a row in the last four weeks? How often? | Never | 65 (100.0) |
| Once | - | ||
| Twice | - | ||
| Three times | - | ||
| Four times | - | ||
| More than four times | - | ||
| Timing adherence | Do you remember having taken your anti-rejection medication more than 2 h before or after the recommended dosing time in the last four weeks? How often? | Never | 39 (60.0) |
| Once | 13 (20.0) | ||
| Two to three times | 6 (9.2) | ||
| About once weekly | 2 (3.1) | ||
| A couple times per week | 4 (6.2) | ||
| Almost every day | 1 (5.4) | ||
| Dose alteration | Have you altered the prescription amount (e.g., taken more or fewer pills or changed your dose) of your anti-rejection medication during the last four weeks without your doctor telling you to do so? Have you stopped taking your anti-rejection medications completely within the last year without your doctor telling you to do so? | No | 64 (98.5) |
| Altered amount | - | ||
| Stopped completely | 1 (1.5) | ||
| Overall level of nonadherence to immunosuppressant medication | 32 (49.2%) | ||
| Characteristics | All Recipients N = 65 | Adherent N = 33, 50.8% | Nonadherent N = 32, 49.2% | p |
|---|---|---|---|---|
| Sociodemographic factors | ||||
| Age, mean (SD) | 62 (9) | 63 (8) | 62 (10) | 0.74 |
| Female, n (%) | 22 (33.8) | 12 (36.4) | 10 (31.3) | 0.66 |
| Race/ethnicity, n (%) | ||||
| White | 47 (72.3) | 24 (72.7) | 23 (71.9) | 0.82 |
| Black | 14 (21.5) | 8 (24.2) | 6 (18.8) | |
| Hispanic | 1 (1.5) | 0 (0.0) | 1 (3.1) | |
| Other | 3 (4.6) | 1 (3.0) | 2 (6.3) | |
| Education, n (%) | ||||
| Highschool graduate | 8 (12.3) | 5 (15.2) | 3 (9.4) | 0.53 |
| Some college | 21 (32.3) | 11 (33.3) | 10 (31.3) | |
| 4-year college degree | 19 (29.2) | 7 (21.2) | 12 (37.5) | |
| Graduate school | 17 (26.2) | 10 (30.3) | 7 (21.9) | |
| Married, n (%) | 56 (86.2) | 29 (87.9) | 27 (84.4) | 0.73 |
| Employment, n (%) | ||||
| Full-time | 17 (26.2) | 8 (24.2) | 9 (28.1) | 0.78 |
| Part-time | 2 (3.1) | 1 (3.0) | 1 (3.1) | 0.99 |
| Self-employed | 3 (4.6) | 2 (6.1) | 1 (3.1) | 0.99 |
| Unemployed | 2 (3.1) | 2 (6.1) | 0 (0.0) | 0.49 |
| Retired | 35 (53.8) | 18 (54.5) | 17 (53.1) | 0.99 |
| Student | 1 (1.5) | 0 (0.0) | 1 (3.1) | 0.49 |
| Homemaker | 5 (7.7) | 2 (6.1) | 3 (9.4) | 0.67 |
| Disabled | 11 (16.9) | 7 (21.2) | 4 (12.5) | 0.51 |
| Annual income, n (%) | ||||
| <USD 35,000 | 5 (8.1) | 3 (9.7) | 2 (6.5) | 0.99 |
| USD 35,000–49,999 | 4 (6.5) | 2 (6.5) | 2 (6.5) | |
| USD 50,000–74,999 | 6 (9.7) | 3 (9.7) | 3 (9.7) | |
| ≥USD 75,000 | 47 (75.8) | 23 (74.2) | 24 (77.4) | |
| Transplant-related factors | ||||
| Years after transplantation median (IQR) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.99 |
| Age at the time of transplantation in years, mean (SD) | 59 (9) | 59 (8) | 58 (10) | 0.61 |
| Healthcare system access factors | ||||
| Medical mistrust, median (IQR) Higher score indicates greater medical mistrust (1–5) | 1.5 (1.0–2.0) | 1.3 (1.0–1.8) | 1.8 (1.0–2.0) | 0.15 |
| Post-transplant treatment-related factors | ||||
| Comorbidities | ||||
| Obesity BMI ≥ 30, n (%) [n = 64] | 13 (20.3) | 10 (30.3) | 3 (9.7) | 0.04 * |
| Diabetes, n (%) | 30 (46.2) | 20 (60.6) | 10 (31.3) | 0.02 * |
| Ever diagnosed with COVID-19 n (%) | 13 (20.0) | 9 (27.3) | 4 (12.5) | 0.14 |
| Patient-related psychosocial factors | ||||
| Physical Health | ||||
| Physical activity, median (IQR) ≥24: high 14–23: moderate <14: low | 36 (21–48) | 30 (16–41) | 42 (26–52) | 0.08 |
| WELL Diet score, median (IQR) Range: 0–120 Higher score indicates better diet quality | 73 (63–82) | 77 (69–84) | 70 (59–77) | 0.04 * |
| Adequate sleep (7–9 h), n (%) | 41 (63.1) | 22 (66.7) | 19 (59.4) | 0.54 |
| Smoking status, n (%) | 0 | 0 | 0 | 0 |
| Mental Health | ||||
| Perceived stress, median (IQR) Range: 0–16 A higher score indicated greater perceived stress | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 3.0 (1.0–5.5) | 0.46 |
| Everyday discrimination, median (IQR) | ||||
| Situation Range: 0–5 Higher scores reflect greater median number of types of discrimination events experienced | 2 (0–3) | 2 (1–3) | 2 (0–3) | 0.95 |
| Frequency Range: 5–30 Higher scores reflect higher median number of discrimination events | 7 (5–10) | 7 (6–9) | 7 (5–10) | 0.89 |
| Reason for Discrimination, n (%) [n = 31] Higher score corresponds to more participants | ||||
| Age | 4 (12.9) | 2 (14.3) | 2 (11.8) | 0.99 |
| Gender | 2 (6.5) | 2 (14.3) | 0 (0.0) | 0.20 |
| Race/skin color | 6 (19.4) | 2 (14.3) | 4 (23.5) | 0.66 |
| Weight or height | 3 (9.7) | 1 (7.1) | 2 (11.8) | 0.99 |
| Education or income | 1 (3.2) | 0 (0.0) | 1 (5.9) | 0.99 |
| Physical disability | 4 (12.9) | 2 (14.3) | 2 (11.8) | 0.99 |
| Religion | 1 (3.2) | 0 (0.0) | 1 (5.9) | 0.99 |
| Other | 10 (32.3) | 5 (35.7) | 5 (29.4) | 0.99 |
| Coping with Discrimination, n (%) Higher score corresponds to more participants | ||||
| Tried to do something about it | 25 (38.5) | 13 (39.4) | 12 (37.5) | 0.99 |
| Accepted it as a fact of life | 44 (67.7) | 22 (66.7) | 22 (68.8) | 0.99 |
| Worked harder to prove them wrong | 24 (36.9) | 8 (24.2) | 16 (50.0) | 0.03 * |
| Realized you brought it on yourself | 10 (15.4) | 3 (9.1) | 7 (21.9) | 0.15 |
| Talked to someone about how you were feeling | 28 (43.1) | 11 (33.3) | 17 (53.1) | 0.11 |
| Expressed anger or got mad | 23 (35.4) | 7 (21.2) | 16 (50.0) | 0.02 * |
| Prayed about the situation | 25 (38.5) | 11 (33.3) | 14 (43.8) | 0.39 |
| Life discrimination events median (IQR) | 1 (0–2) | 1 (0–2) | 0 (0–1) | 0.14 |
| Discrimination in medical care, n (%) | 5 (7.7) | 1 (3.0) | 4 (12.5) | 0.20 |
| Internalized racism, median (IQR) [n = 63] | 2.2 (1.6–2.6) | 2.0 (1.6–2.6) | 2.2 (1.6–2.6) | 0.67 |
| Community stress, median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0.15 |
| ACES, median (IQR) | 0 (0–2) | 0 (0–1) | 0 (0–2) | 0.67 |
| Financial stress, median (IQR) Range = 1–5 Higher score reflects more financial stress | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 0.82 |
| Global health ratings (PROMIS) | ||||
| Pain in past week, median (IQR) Range = 0–10 Higher score reflects higher pain level | 2 (0–5) | 2 (1–5) | 2 (0–5) | 0.34 |
| Physical health, n (%) | ||||
| Poor/fair | 13 (20.0) | 7 (21.2) | 6 (18.8) | 0.83 |
| Good | 28 (43.1) | 13 (39.4) | 15 (46.9) | |
| Very good/excellent | 24 (36.9) | 13 (39.4) | 11 (34.4) | |
| Mostly/completely able to carry out daily physical activities, n (%) | 57 (87.7) | 29 (87.9) | 28 (87.5) | 0.99 |
| Very good/excellent mental health, n (%) | 48 (73.8) | 22 (66.7) | 26 (81.3) | 0.18 |
| Very good/excellent satisfaction with social activities and relationships, n (%) [n = 64] | 45 (70.3) | 20 (60.6) | 25 (80.6) | 0.08 |
| Social support level, n (%) [n = 64] | ||||
| Low | 3 (4.7) | 1 (3.0) | 2 (6.5) | 0.45 |
| Moderate | 42 (65.6) | 20 (60.6) | 22 (71.0) | |
| High | 19 (29.7) | 12 (36.4) | 7 (22.6) | |
| Depression (PHQ-2), n (%) Range = 0–6 Scores of 2 or more indicate high likelihood of major depressive disorder | ||||
| 0–1 | 53 (81.5) | 28 (84.8) | 25 (78.1) | 0.80 |
| 2 | 8 (12.3) | 3 (9.1) | 5 (15.6) | |
| 3–4 | 4 (6.2) | 2 (6.1) | 2 (6.3) | |
| Characteristics | Overall N = 32 | Adherent N = 17, 53.1% | Nonadherent N = 15, 46.9% |
|---|---|---|---|
| Sociodemographic factors | |||
| Age, mean (SD) | 64 (8) | 65 (6) | 62 (9) |
| Female, n (%) | 7 (21.9) | 3 (17.6) | 4 (26.7) |
| Race/ethnicity, n (%) | |||
| White | 20 (62.5) | 10 (58.8) | 10 (66.7) |
| Black | 11 (34.4) | 7 (41.2) | 4 (26.7) |
| Hispanic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 1 (3.1) | 0 (0.0) | 1 (6.7) |
| Education, n (%) | |||
| High school graduate | 4 (12.5) | 3 (17.6) | 1 (6.7) |
| Some college | 10 (31.3) | 6 (35.3) | 4 (26.7) |
| 4-year college degree | 11 (34.4) | 2 (11.8) | 9 (60.0) |
| Graduate school | 7 (21.9) | 6 (35.3) | 1 (6.7) |
| Married, n (%) | 28 (87.5) | 16 (94.1) | 12 (80.0) |
| Employment, n (%) | |||
| Full-time | 11 (34.4) | 5 (29.4) | 6 (40.0) |
| Part-time | 1 (3.1) | 1 (5.9) | 0 (0.0) |
| Self-employed | 1 (3.1) | 0 (0.0) | 1 (6.7) |
| Unemployed | 2 (6.3) | 2 (11.8) | 0 (0.0) |
| Retired | 15 (46.9) | 9 (52.9) | 6 (40.0) |
| Student | 1 (3.1) | 0 (0.0) | 1 (6.7) |
| Homemaker | 2 (6.3) | 0 (0.0) | 2 (13.3) |
| Disabled | 3 (9.4) | 2 (11.8) | 1 (6.7) |
| Annual income, n (%) [n = 31] | |||
| <USD 35,000 | 2 (6.5) | 2 (12.5) | 0 (0.0) |
| USD 35,000–49,999 | 1 (3.2) | 0 (0.0) | 1 (6.7) |
| USD 50,000–74,999 | 3 (9.7) | 2 (12.5) | 1 (6.7) |
| ≥USD 75,000 | 25 (80.6) | 12 (75.0) | 13 (86.7) |
| Transplant-related factors | |||
| Years after transplantation median (IQR) | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Age at the time of transplantation in years, mean (SD) | 59 (7) | 61 (6) | 57 (9) |
| Healthcare system access factors | |||
| Medical mistrust, median (IQR) A higher score indicates greater medical mistrust (1–5) | 1.8 (1.0–2.0) | 1.3 (1.0–2.0) | 1.8 (1.7–2.0) |
| Post-transplant treatment-related factors | |||
| Comorbidities | |||
| Obesity BMI ≥ 30, n (%) [n = 64] | 7 (21.9) | 6 (35.3) | 1 (6.7) |
| Diabetes, n (%) | 16 (50.0) | 12 (70.6) | 4 (26.7) |
| Ever diagnosed with COVID-19 n (%) | 8 (25.0) | 6 (35.3) | 2 (13.3) |
| Patient-related psychosocial factors | |||
| Physical Health | |||
| Physical activity, median (IQR) ≥24: high 14–23: moderate < 14: low | 30 (20–56) | 21 (16–48) | 35 (21–56) |
| WELL Diet score, median (IQR) Range: 0–120 Higher score indicates better diet quality | 73 (63–79) | 76 (68–82) | 71 (57–76) |
| Sleep quality, n (%) | 15 (46.9) | 9 (52.9) | 6 (40.0) |
| Smoking status, n (%) | 0 | 0 | 0 |
| Mental Health | |||
| Perceived stress, median (IQR) Range: 0–16 A higher score indicates greater perceived stress | 2.5 (1.0–5.0) | 2.0 (1.0–5.0) | 3.0 (1.0–5.0) |
| Everyday discrimination, median (IQR) | |||
| Situation Range: 0–5 Higher scores reflect greater median number of types of discrimination events experienced | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Frequency Range: 5–30 Higher scores reflect higher median number of discrimination events | 8 (6–11) | 8 (6–11) | 8 (6–10) |
| Chronicity Range: Higher scores reflect higher median number of discrimination events experienced annually | 3 (1–8) | 3 (1–7) | 3 (1–10) |
| Reason for Discrimination, n (%) [n = 18] Higher score corresponds to more participants | |||
| Age | 3 (16.7) | 2 (22.2) | 1 (11.1) |
| Gender | 1 (5.6) | 1 (11.1) | 0 (0.0) |
| Race/skin color | 4 (22.2) | 2 (22.2) | 2 (22.2) |
| Weight or height | 2 (11.1) | 0 (0.0) | 2 (22.2) |
| Education or income | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Physical disability | 1 (5.6) | 0 (0.0) | 1 (11.1) |
| Religion | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 7 (38.9) | 4 (44.4) | 3 (33.3) |
| Coping with Discrimination, n (%) Higher score corresponds to more participants | |||
| Tried to do something about it | 13 (40.6) | 8 (47.1) | 5 (33.3) |
| Accepted it as a fact of life | 25 (78.1) | 13 (76.5) | 12 (80.0) |
| Worked harder to prove them wrong | 12 (37.5) | 5 (29.4) | 7 (46.7) |
| Realized you brought it on yourself | 4 (12.5) | 1 (5.9) | 3 (20.0) |
| Talked to someone about how you were feeling | 12 (37.5) | 4 (23.5) | 8 (53.3) |
| Expressed anger or got mad | 14 (43.8) | 5 (29.4) | 9 (60.0) |
| Prayed about the situation | 15 (46.9) | 6 (35.3) | 9 (60.0) |
| Life discrimination events median (IQR) | 1 (0–3) | 2 (0–3) | 0 (0–3) |
| Discrimination in medical care, n (%) | 3 (9.4) | 0 (0.0) | 3 (20.0) |
| Internalized racism, median (IQR) [n = 31] | 2.0 (1.4–2.6) | 1.8 (1.4–2.2) | 2.3 (1.8–2.8) |
| Community stress, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| ACES, median (IQR) | 0 (0–2) | 0 (0–1) | 0 (0–2) |
| Financial stress, median (IQR) Range = 1–5 Higher score reflects more financial stress | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) |
| Global health ratings (PROMIS) | |||
| Pain in past week, median (IQR) Range = 0–10 Higher score reflects higher pain level | 2 (1–5) | 2 (2–5) | 1 (0–5) |
| Physical health, n (%) | |||
| Poor/fair | 5 (15.6) | 3 (17.6) | 2 (13.3) |
| Good | 12 (37.5) | 7 (41.2) | 5 (33.3) |
| Very good/excellent | 15 (46.9) | 7 (41.2) | 8 (53.3) |
| Mostly/completely able to carry out daily physical activities, n (%) | 26 (81.3) | 14 (82.4) | 12 (80.0) |
| Very good/excellent mental health, n (%) | 23 (71.9) | 11 (64.7) | 12 (80.0) |
| Very good/excellent satisfaction with social activities and relationships, n (%) [n = 64] | 23 (74.2) | 11 (64.7) | 12 (85.7) |
| Social support level, n (%) [n = 31] | |||
| Low | 2 (6.5) | 1 (5.9) | 1 (7.1) |
| Moderate | 17 (54.8) | 8 (47.1) | 9 (64.3) |
| High | 12 (38.7) | 8 (47.1) | 4 (28.6) |
| Depression (PHQ-2), n (%) Range = 0–6 Scores of 2 or more indicate high likelihood of major depressive disorder | |||
| 0–1 | 26 (81.3) | 15 (88.2) | 11 (73.3) |
| 2 | 5 (15.6) | 2 (11.8) | 3 (20.0) |
| 3–4 | 1 (3.1) | 0 (0.0) | 1 (6.7) |
| Characteristics | Overall N = 33 | Adherent N = 16, 53.1% | Nonadherent N = 17, 46.9% |
|---|---|---|---|
| Sociodemographic factors | |||
| Age, mean (SD) | 61 (10) | 61 (10) | 62 (10) |
| Female, n (%) | 15 (45.5) | 9 (56.3) | 6 (35.3) |
| Race/ethnicity, n (%) | |||
| White | 27 (81.8) | 14 (87.5) | 13 (76.5) |
| Black | 3 (9.1) | 1 (6.3) | 2 (11.8) |
| 1 (3.0) | 0 (0.0) | 1 (5.9) | |
| Other | 2 (6.1) | 1 (6.3) | 1 (5.9) |
| Education, n (%) | |||
| High school graduate | 4 (12.1) | 2 (12.5) | 2 (11.8) |
| Some college | 11 (33.3) | 5 (31.3) | 6 (35.3) |
| 4-year college degree | 8 (24.2) | 5 (31.3) | 3 (17.6) |
| Graduate school | 10 (30.3) | 4 (25.0) | 6 (35.3) |
| Married, n (%) | 28 (84.8) | 13 (81.3) | 15 (88.2) |
| Employment, n (%) | |||
| Full-time | 6 (18.2) | 3 (18.8) | 3 (17.6) |
| Part-time | 1 (3.0) | 0 (0.0) | 1 (5.9) |
| Self-employed | 2 (6.1) | 2 (12.5) | 0 (0.0) |
| Unemployed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Retired | 20 (60.6) | 9 (56.3) | 11 (64.7) |
| Student | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Homemaker | 3 (9.1) | 2 (12.5) | 1 (5.9) |
| Disabled | 8 (24.2) | 5 (31.3) | 3 (17.6) |
| Annual income, n (%) [n = 31] | |||
| <USD 35,000 | 3 (9.7) | 1 (6.7) | 2 (12.5) |
| USD 35,000–49,999 | 3 (9.7) | 2 (13.3) | 1 (6.3) |
| USD 50,000–74,999 | 3 (9.7) | 1 (6.7) | 2 (12.5) |
| ≥USD 75,000 | 22 (71.0) | 11 (73.3) | 11 (68.8) |
| Transplant-related factors | |||
| Years after transplantation median (IQR) | 3 (2–5) | 4 (3–5) | 3 (2–5) |
| Age at the time of transplantation in years, mean (SD) | 58 (10) | 57 (10) | 59 (11) |
| Healthcare system access factors | |||
| Medical mistrust, median (IQR) A higher score indicates greater medical mistrust (1–5) | 1.3 (1.0–2.0) | 1.3 (1.0–1.8) | 1.3 (1.0–2.0) |
| Post-transplant treatment-related factors | |||
| Comorbidities | |||
| Obesity BMI ≥ 30, n (%) [n = 64] | 6 (18.8) | 4 (25.0) | 2 (12.5) |
| Diabetes, n (%) | 14 (42.4) | 8 (50.0) | 6 (35.3) |
| Ever diagnosed with COVID-19 n (%) | 5 (15.2) | 3 (18.8) | 2 (11.8) |
| Patient-related psychosocial factors | |||
| Physical Health | |||
| Physical activity, median (IQR) >24: high 14–23: moderate <14: low | 36 (25–42) | 33 (20–40) | 42 (27–48) |
| WELL Diet score, median (IQR) Range: 0–120 Higher score indicates better diet quality | 73 (66–84) | 78 (71–87) | 68 (59–81) |
| Sleep quality, n (%) | 26 (78.8) | 13 (81.3) | 13 (76.5) |
| Smoking status, n (%) | 0 | 0 | 0 |
| Mental Health | |||
| Perceived stress, median (IQR) Range: 0–16 A higher score indicates greater perceived stress | 2.0 (1.0–6.0) | 2.5 (1.0–6.0) | 2.0 (1.0–6.0) |
| Everyday discrimination, median (IQR) | |||
| Situation Range: 0–5 Higher scores reflect greater median number of types of discrimination events experienced | 1 (0–3) | 1 (0–2) | 1 (0–3) |
| Frequency Range: 5–30 Higher scores reflect higher median number of discrimination events | 7 (5–9) | 6 (5–9) | 7 (5–9) |
| Chronicity Range: Higher scores reflect higher median number of discrimination events experienced annually | 1 (0–4) | 1 (0–4) | 2 (0–4) |
| Reason for Discrimination, n (%) [n = 13] Higher score corresponds to more participants | |||
| Age | 1 (7.7) | 0 (0.0) | 1 (12.5) |
| Gender | 1 (7.7) | 1 (20.0) | 0 (0.0) |
| Race/skin color | 2 (15.4) | 0 (0.0) | 2 (25.0) |
| Weight or height | 1 (7.7) | 1 (20.0) | 0 (0.0) |
| Education or income | 1 (7.7) | 0 (0.0) | 1 (12.5) |
| Physical disability | 3 (23.1) | 2 (40.0) | 1 (12.5) |
| Religion | 1 (7.7) | 0 (0.0) | 1 (12.5) |
| Other | 3 (23.1) | 1 (20.0) | 2 (25.0) |
| Coping with Discrimination, n (%) Higher score corresponds to more participants | |||
| Tried to do something about it | 12 (36.4) | 5 (31.3) | 7 (41.2) |
| Accepted it as a fact of life | 19 (57.6) | 9 (56.3) | 10 (58.8) |
| Worked harder to prove them wrong | 12 (36.4) | 3 (18.8) | 9 (52.9) |
| Realized you brought it on yourself | 6 (18.2) | 2 (12.5) | 4 (23.5) |
| Talked to someone about how you were feeling | 16 (48.5) | 7 (43.8) | 9 (52.9) |
| Expressed anger or got mad | 9 (27.3) | 2 (12.5) | 7 (41.2) |
| Prayed about the situation | 10 (30.3) | 5 (31.3) | 5 (29.4) |
| Life discrimination events median (IQR) | 0 (0–2) | 1 (0–2) | 0 (0–1) |
| Discrimination in medical care, n (%) | 2 (6.1) | 1 (6.3) | 1 (5.9) |
| Internalized racism, median (IQR) [n = 32] | 2.3 (1.6–2.6) | 2.5 (1.7–2.8) | 1.9 (1.5–2.4) |
| Community stress, median (IQR) | 0 (0–1) | 0 (0–0) | 1 (0–1) |
| ACES, median (IQR) | 0 (0–2) | 0 (0–2) | 0 (0–2) |
| Financial stress, median (IQR) Range = 1–5 Higher score reflects more financial stress | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) |
| Global health ratings (PROMIS) | |||
| Pain in past week, median (IQR) Range = 0–10 Higher score reflects higher pain level | 3 (0–4) | 2 (1–5) | 3 (0–4) |
| Physical health, n (%) | |||
| Poor/fair | 8 (24.2) | 4 (25.0) | 4 (23.5) |
| Good | 16 (48.5) | 6 (37.5) | 10 (58.8) |
| Very good/excellent | 9 (27.3) | 6 (37.5) | 3 (17.6) |
| Mostly/completely able to carry out daily physical activities, n (%) | 31 (93.9) | 15 (93.8) | 16 (94.1) |
| Very good/excellent mental health, n (%) | 25 (75.8) | 11 (68.8) | 14 (82.4) |
| Very good/excellent satisfaction with social activities and relationships, n (%) [n = 64] | 22 (66.7) | 9 (56.3) | 13 (76.5) |
| Social support level, n (%) [n = 64] | |||
| Low | 1 (3.0) | 0 (0.0) | 1 (5.9) |
| Moderate | 25 (75.8) | 12 (75.0) | 13 (76.5) |
| High | 7 (21.2) | 4 (25.0) | 3 (17.6) |
| Depression (PHQ-2), n (%) Range = 0–6 Scores of 2 or more indicate high likelihood of major depressive disorder | |||
| 0–1 | 27 (81.8) | 13 (81.3) | 14 (82.4) |
| 2 | 3 (9.1) | 1 (6.3) | 2 (11.8) |
| 3–4 | 3 (9.1) | 2 (12.5) | 1 (5.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springfield-Trice, S.; Reddy, G.; Joyce, C.; Garcia, B.M.; Shah, P.; Agbor-Enoh, S.; Valantine, H. Barriers to Immunosuppressant Medication Adherence in Thoracic Transplant Recipients: Initial Findings. Int. J. Environ. Res. Public Health 2025, 22, 1090. https://doi.org/10.3390/ijerph22071090
Springfield-Trice S, Reddy G, Joyce C, Garcia BM, Shah P, Agbor-Enoh S, Valantine H. Barriers to Immunosuppressant Medication Adherence in Thoracic Transplant Recipients: Initial Findings. International Journal of Environmental Research and Public Health. 2025; 22(7):1090. https://doi.org/10.3390/ijerph22071090
Chicago/Turabian StyleSpringfield-Trice, Sparkle, Grishma Reddy, Cara Joyce, Benito M. Garcia, Palak Shah, Sean Agbor-Enoh, and Hannah Valantine. 2025. "Barriers to Immunosuppressant Medication Adherence in Thoracic Transplant Recipients: Initial Findings" International Journal of Environmental Research and Public Health 22, no. 7: 1090. https://doi.org/10.3390/ijerph22071090
APA StyleSpringfield-Trice, S., Reddy, G., Joyce, C., Garcia, B. M., Shah, P., Agbor-Enoh, S., & Valantine, H. (2025). Barriers to Immunosuppressant Medication Adherence in Thoracic Transplant Recipients: Initial Findings. International Journal of Environmental Research and Public Health, 22(7), 1090. https://doi.org/10.3390/ijerph22071090






