Clinical and Behavioural Heterogeneity Among Women at Increased Risk for Gestational Diabetes: A Four-Country Analysis
Abstract
1. Introduction
2. Materials and Methods
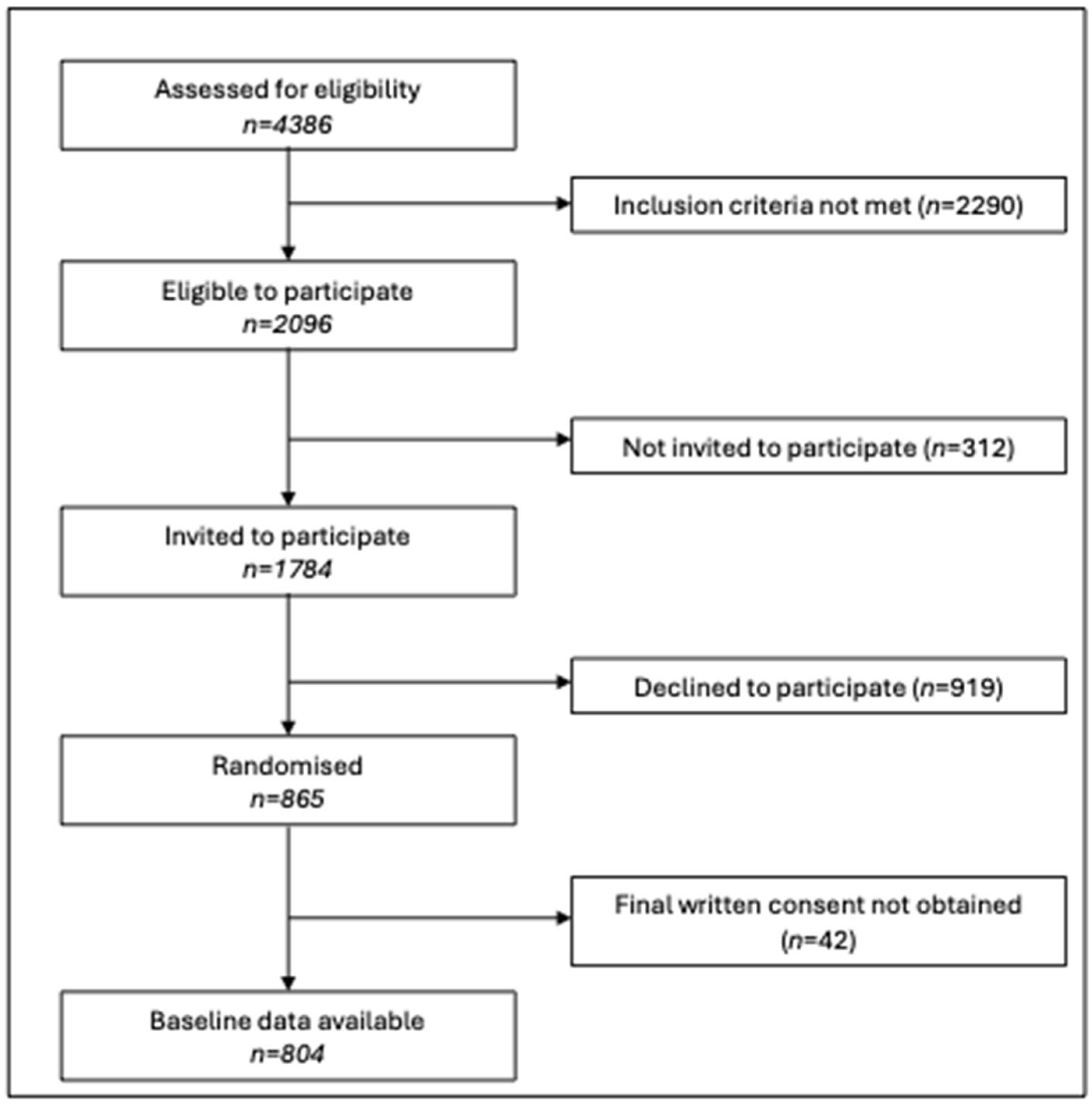
2.1. Study Design and Participants
2.2. Recruitment and Data Collection
2.3. Measures
2.4. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Blood Pressure
3.3. Health Behaviours
3.4. Mental Health and Sleep Quality
3.5. Health Literacy
3.6. BMI Associations
3.6.1. Bivariate Analyses
3.6.2. Multivariate Analyses
Dublin
Bristol
Granada
Melbourne
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| GDM | Gestational diabetes mellitus |
| CI | Confidence interval |
| B2B&Me | Bump2Baby and Me |
| ANOVA | Analysis of variance |
| PPAQ | Pregnancy Physical Activity Questionnaire |
| EPDS | Edinburgh Postnatal Depression Scale |
| BMR | Basal metabolic rate |
References
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Harrison, C.L.; Teh, W.T.; Paul, E.; Allan, C.A. Gestational diabetes: Development of an early risk prediction tool to facilitate opportunities for prevention. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.-F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef]
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes with Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef]
- Lowe, L.P.; Perak, A.M.; Kuang, A.; Lloyd-Jones, D.M.; ASacks, D.; Deerochanawong, C.; Maresh, M.; Ma, R.C.; Lowe, W.L.; EMetzger, B.; et al. Associations of glycemia and lipid levels in pregnancy with dyslipidemia 10–14 years later: The HAPO follow-up study. Diabetes Res. Clin. Pract. 2022, 185, 109790. [Google Scholar] [CrossRef]
- Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Hamilton, J.; Lawrence, J.M.; Lebenthal, Y.; Brickman, W.J.; Clayton, P.; Ma, R.C.; McCance, D.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 381–392. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Bardugo, A.; Bendor, C.D.; Rotem, R.S.; Tsur, A.M.; Derazne, E.; Gerstein, H.C.; Tzur, D.; Pinhas-Hamiel, O.; Cukierman-Yaffe, T.; Raz, I.; et al. Glucose intolerance in pregnancy and risk of early-onset type 2 diabetes: A population-based cohort study. Lancet Diabetes Endocrinol. 2023, 11, 333–344. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Ratner, R.E.; Christophi, C.A.; Metzger, B.E.; Dabelea, D.; Bennett, P.H.; Pi-Sunyer, X.; Fowler, S.; Kahn, S.E.; The Diabetes Prevention Program Research Group. Prevention of Diabetes in Women with a History of Gestational Diabetes: Effects of Metformin Lifestyle Interventions. J. Clin. Endocrinol. Metab. 2008, 93, 4774–4779. [Google Scholar] [CrossRef] [PubMed]
- Bahri Khomami, M.; Teede, H.J.; Enticott, J.; O’Reilly, S.; Bailey, C.; Harrison, C.L. Implementation of Antenatal Lifestyle Interventions Into Routine Care: Secondary Analysis of a Systematic Review. JAMA Netw. Open 2022, 5, e2234870. [Google Scholar] [CrossRef]
- O’REilly, S.L.; McAuliffe, F.M.; Geraghty, A.A.; Burden, C.; Davies, A. Implementing weight management during and after pregnancy to reduce diabetes and CVD risk in maternal and child populations. Proc. Nutr. Soc. 2023, 84, 24–35. [Google Scholar] [CrossRef]
- Sperling, M.M.; Leonard, S.A.; Blumenfeld, Y.J.; Carmichael, S.L.; Chueh, J. Prepregnancy body mass index and gestational diabetes mellitus across Asian and Pacific Islander subgroups in California. AJOG Glob. Rep. 2022, 3, 100148. [Google Scholar] [CrossRef]
- Bahari, N.I.; Ahmad, N.; Mahmud, M.H.; Baharom, M.; Amir, S.M.; Peng, C.S.; Hassan, M.R.; Nawi, A.M. Issues and Challenges in the Primary Prevention of Type 2 Diabetes Mellitus: A Systematic Review. J. Prev. 2022, 44, 105–125. [Google Scholar] [CrossRef]
- Sørensen, K.; Pelikan, J.M.; Röthlin, F.; Ganahl, K.; Slonska, Z.; Doyle, G.; Fullam, J.; Kondilis, B.; Agrafiotis, D.; Uiters, E.; et al. Health literacy in Europe: Comparative results of the European health literacy survey (HLS-EU). Eur. J. Public Health 2015, 25, 1053–1058. [Google Scholar] [CrossRef]
- Baccolini, V.; Rosso, A.; Di Paolo, C.; Isonne, C.; Salerno, C.; Migliara, G.; Prencipe, G.P.; Massimi, A.; Marzuillo, C.; De Vito, C.; et al. What is the Prevalence of Low Health Literacy in European Union Member States? A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2021, 36, 753–761. [Google Scholar] [CrossRef]
- O’reilly, S.L.; Burden, C.; Campoy, C.; McAuliffe, F.M.; Teede, H.; Andresen, J.; Campbell, K.J.; Geraghty, A.A.; Harrison, C.L.; Laws, R.; et al. Bump2Baby and Me: Protocol for a randomised trial of mHealth coaching for healthy gestational weight gain and improved postnatal outcomes in high-risk women and their children. Trials 2021, 22, 963. [Google Scholar] [CrossRef]
- Fallaize, R.; Forster, H.; Macready, A.L.; Walsh, M.C.; Mathers, J.C.; Brennan, L.; Gibney, E.R.; Gibney, M.J.; ALovegrove, J. Online Dietary Intake Estimation: Reproducibility and Validity of the Food4Me Food Frequency Questionnaire Against a 4-Day Weighed Food Record. J. Med. Internet Res. 2014, 16, e190. [Google Scholar] [CrossRef]
- Forster, H.; Fallaize, R.; Gallagher, C.; O’dOnovan, C.B.; Woolhead, C.; Walsh, M.C.; Macready, A.L.; ALovegrove, J.; Mathers, J.C.; Gibney, M.J.; et al. Online Dietary Intake Estimation: The Food4Me Food Frequency Questionnaire. J. Med. Internet Res. 2014, 16, e150. [Google Scholar] [CrossRef] [PubMed]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and Validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2004, 36, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Osborne, R.H.; Batterham, R.W.; Elsworth, G.R.; Hawkins, M.; Buchbinder, R. The grounded psychometric development and initial validation of the Health Literacy Questionnaire (HLQ). BMC Public Health 2013, 13, 658. [Google Scholar] [CrossRef]
- Kayser, L.; Karnoe, A.; Furstrand, D.; Batterham, R.; Christensen, K.B.; Elsworth, G.; Osborne, R.H. A Multidimensional Tool Based on the eHealth Literacy Framework: Development and Initial Validity Testing of the eHealth Literacy Questionnaire (eHLQ). J. Med. Internet Res. 2018, 20, e36. [Google Scholar] [CrossRef]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat Metab Disord. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Vickers, A.J. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med. Res. Methodol. 2005, 5, 35. [Google Scholar] [CrossRef]
- Eurostat. Overweight and Obesity—BMI Statistics. Obesity in the EU: Gender Differences 2019. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity_-_BMI_statistics#Obesity_in_the_EU:_gender_differences (accessed on 17 December 2024).
- Australian Bureau of Statistics. Waist Circumference and BMI 2022. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/waist-circumference-and-bmi/latest-release#body-mass-index-bmi-prevalence (accessed on 17 December 2024).
- Cao, M.; Cui, B. Association of Educational Attainment With Adiposity, Type 2 Diabetes, and Coronary Artery Diseases: A Mendelian Randomization Study. Front. Public Health 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- O’bRien, E.C.; Segurado, R.; AGeraghty, A.; Alberdi, G.; Rogozinska, E.; Astrup, A.; Carballo, R.B.; Bogaerts, A.; Cecatti, J.G.; Coomarasamy, A.; et al. Impact of maternal education on response to lifestyle interventions to reduce gestational weight gain: Individual participant data meta-analysis. BMJ Open 2019, 9, e025620. [Google Scholar] [CrossRef] [PubMed]
- Euro-Peristat Network. European Perinatal Health Report: Core indicators of the Health and Care of Pregnant Women and Babies in Europe from 2015 to 2019. 2022. Available online: https://www.europeristat.com/publications/european-perinatal-health-report-2015-2019/ (accessed on 27 May 2025).
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies; Australian Institute of Health and Welfare: Canberra, Australia, 2021. [Google Scholar]
- Instituto Nacional de Estadística. Vital Statistics: Births 2021 Demographic Phenomena. 2021. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=6566#_tabs-tabla (accessed on 27 May 2025).
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [CrossRef] [PubMed]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P.; Bhutta, Z.A. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef]
- Kim, J.Y.; Florez, M.; Botto, E.; Belgrave, X.; Grace, C.; Getz, K. The influence of socioeconomic status on individual attitudes and experience with clinical trials. Commun. Med. 2024, 4, 172. [Google Scholar] [CrossRef]
- Mohammed, S.H.; Habtewold, T.D.; Birhanu, M.M.; Sissay, T.A.; Tegegne, B.S.; Abuzerr, S.; Esmaillzadeh, A. Neighbourhood socioeconomic status and overweight/obesity: A systematic review and meta-analysis of epidemiological studies. BMJ Open 2019, 9, e028238. [Google Scholar] [CrossRef]
- Saurel-Cubizolles, M.-J.; Azria, E.; Blondel, B.; Regnault, N.; Deneux-Tharaux, C. Exploring the socioeconomic disparities of maternal body mass index: A national study in France. Eur. J. Public Health 2022, 32, 528–534. [Google Scholar] [CrossRef]
- Thériault, S.; Forest, J.-C.; Massé, J.; Giguère, Y. Validation of early risk-prediction models for gestational diabetes based on clinical characteristics. Diabetes Res. Clin. Pract. 2014, 103, 419–425. [Google Scholar] [CrossRef]
- Lamain-de Ruiter, M.; Kwee, A.; Naaktgeboren, C.A.; de Groot, I.; Evers, I.M.; Groenendaal, F.; Hering, Y.R.; Huisjes, A.J.; Kirpestein, C.; Monincx, W.M.; et al. External validation of prognostic models to predict risk of gestational diabetes mellitus in one Dutch cohort: Prospective multicentre cohort study. BMJ 2016, 354, i4338. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Salleh, R.; Nor, N.S.M.; Baharuddin, A.; Hasani, W.S.R.; Omar, A.; Jamil, A.T.; Appukutty, M.; Muda, W.A.M.W.; Aris, T. Comparison between self-reported physical activity (IPAQ-SF) and pedometer among overweight and obese women in the MyBFF@home study. BMC Women’s Health 2018, 18, 100. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef]
| Process | Dublin | Bristol | Granada | Melbourne |
|---|---|---|---|---|
| Notification of pregnancy | Direct contact to hospital, administrative staff managed | Community midwife contacted | Community health centre contacted | Direct contact to hospital, administrative staff managed |
| First antenatal visit booking and risk screening | Administrative staff and research team conducted on phone | Community midwife, conducted on phone | Community-based, conducted locally. Risk screening performed at first antenatal visit | Administrative staff and research team conducted by email and/or phone |
| First antenatal visit | Midwife-led, in-person with venepuncture (non-fasting) | Dating scan visit, in person, venepuncture possible (non-fasting) | Midwife or clinician-led, in person, venepuncture possible (non-fasting) | Midwife or clinician-led via telehealth, venepuncture request sent for remote phlebotomy (non-fasting or fasting) |
| Characteristics | Dublin | Bristol | Granada | Melbourne | Total | p |
|---|---|---|---|---|---|---|
| Age, mean (SD) (n = 804) | 35.5 (4.1) a | 34.5 (4.2) ac | 36.7 (4.0) b | 33.8 (4.3) c | 35.2 (4.3) | <0.001 |
| Ethnicity (n = 804) | <0.001 | |||||
| Caucasian | 161 (75.6%) a | 162 (79.0%) a | 196 (92.9%) b | 69 (39.4%) c | 588 (73.1%) | |
| All other ethnic groups | 52 (24.4%) a | 43 (21.0%) a | 15 (7.1%) b | 106 (60.6%) c | 216 (26.9%) | |
| BMI (kg/m2), mean (SD) (n = 804) | 27.2 (5.9) abc | 28.2 (6.6) abc | 26.9 (5.5) b | 28.9 (8.2) c | 27.7 (6.6) | 0.014 d |
| BMI category (n = 804) | 0.472 | |||||
| Underweight | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | |
| Normal weight | 97 (45.5%) | 79 (38.5%) | 96 (45.5%) | 71 (40.6%) | 343 (42.7%) | |
| Overweight | 60 (28.2%) | 62 (30.2%) | 62 (29.4%) | 45 (25.7%) | 229 (28.5%) | |
| Obese | 56 (26.3%) | 63 (30.7%) | 53 (25.1%) | 59 (33.7%) | 231 (28.7%) | |
| Previous gestational diabetes (n = 804) | 5 (2.3%) a | 9 (4.4%) a | 11 (5.2%) a | 25 (14.3%) b | 50 (6.2%) | <0.001 |
| Family history of diabetes (n = 804) | 70 (32.9%) | 64 (31.2%) | 83 (39.3%) | 64 (36.6%) | 281 (35.0%) | 0.302 |
| GDM risk score, mode (minimum, maximum) (n = 804) | 3 (3,7) a | 3 (3, 7) a | 3 (3, 6) a | 3 (3, 8) b | 3 (3, 8) | <0.001 |
| Gestational age, mean (weeks) (n = 798) | 12.8 (1.6) a | 12.9 (1.3) a | 13.0 (2.7) a | 17.9 (2.3) b | 14.0 (2.9) | <0.001 d |
| Gravida (n = 797) | 0.225 | |||||
| 1 | 53 (25.4%) | 54 (26.5%) | 48 (22.7%) | 46 (26.6%) | 201 (25.2%) | |
| 2 | 59 (28.2%) | 82 (40.2%) | 79 (37.4%) | 49 (28.3%) | 269 (33.8%) | |
| ≥3 | 97 (46.4%) | 68 (33.3%) | 84 (39.8%) | 78 (45.1%) | 327 (41.0%) | |
| Parity (n = 797) | 0.216 | |||||
| 0 | 78 (37.3%) | 82 (40.2%) | 79 (37.4%) | 64 (37.0%) | 303 (38.0%) | |
| 1 | 76 (36.4%) | 97 (47.5%) | 99 (46.9%) | 76 (43.9%) | 348 (43.7%) | |
| 2 | 39 (18.7%) | 16 (7.8%) | 23 (10.9%) | 25 (14.5%) | 103 (12.9%) | |
| ≥3 | 16 (7.7%) | 9 (4.4%) | 10 (4.7%) | 8 (4.6%) | 43 (5.4%) | |
| Previous stillbirth (n = 596) | 3 (1.9%) | 3 (2.0%) | 3 (1.8%) | 5 (3.9%) | 14 (2.3%) | 0.661 e |
| Education (n = 743) | <0.001 | |||||
| Primary/secondary school | 8 (4.2%) a | 47 (25.5%) b | 23 (11.4%) c | 32 (19.4%) b | 110 (14.8%) | |
| Vocational qualifications | 32 (16.7%) a | 5 (2.7%) b | 52 (25.7%) c | 5 (3.0%) b | 94 (12.7%) | |
| University degree | 65 (33.9%) a | 85 (46.2%) b | 74 (36.6%) ab | 74 (44.8%) b | 298 (40.1%) | |
| Postgraduate degree | 87 (45.3%) a | 47 (25.5%) b | 53 (26.2%)b | 54 (32.7%) b | 241 (32.4%) | |
| Employment (n = 738) | <0.001 | |||||
| Homemaker | 18 (9.3%) ab | 8 (4.4%) b | 18 (9.0%) ab | 21 (12.8%) a | 65 (8.8%) | |
| Government assistance/disability support, student or casual employment | 7 (3.6%) a | 6 (3.3%) a | 24 (12.1%) b | 16 (9.8%) b | 53 (7.2%) | |
| Part-time employment | 17 (8.8%) a | 56 (30.8%) b | 28 (14.1%) a | 45 (27.4%) b | 146 (19.8%) | |
| Full-time employment | 151 (78.2%) a | 112 (61.5%) b | 129 (64.8%) b | 82 (50.0%) c | 474 (64.2%) | |
| Marital status (n = 745) | <0.001 | |||||
| Single/divorced/widowed | 11 (5.7%) a | 9 (4.8%) a | 42 (20.8%) b | 4 (2.4%) a | 66 (8.9%) | |
| Married or de facto partner | 181 (94.3%) a | 177 (95.2%) a | 160 (79.2%) b | 161 (97.6%) a | 679 (91.1%) | |
| Living situation (n = 743) | 0.007 | |||||
| Own home | 117 (60.3%) a | 132 (71.4%) b | 151 (75.5%) b | 96 (58.5%) a | 496 (66.8%) | |
| Rented home | 65 (33.5%) a | 45 (24.3%) b | 42 (21.0%) b | 56 (34.1%) a | 208 (28.0%) | |
| Living with family or in emergency accommodation | 12 (6.2%) a | 8 (4.3%) a | 7 (3.5%) a | 12 (7.3%) a | 39 (5.2%) | |
| Children to care for throughout workday (n = 748) | 0.006 | |||||
| No | 65 (33.3%) a | 58 (31.2%) ab | 41 (20.3%) c | 39 (23.6%) bc | 203 (27.1%) | |
| Yes | 55 (28.2%) a | 67 (36.0%) ab | 87 (43.1%) b | 74 (44.8%) b | 283 (37.8%) | |
| No children to care for at present | 75 (38.5%) a | 61 (32.8%) a | 74 (36.6%) a | 52 (31.5%) a | 262 (35.0%) | |
| Current smoker (n = 748) | 5 (2.6%) a | 10 (5.4%) ab | 14 (6.9%) b | 3 (1.8%) a | 32 (4.3%) | 0.049 |
| Previous smoker (n = 724) | 55 (28.9%) a | 59 (32.4%) a | 84 (44.2%) b | 39 (24.1%) a | 237 (32.7%) | <0.001 |
| BP systolic, mean (SD) (n = 631) | 114.6 (11.0) a | 108.4 (11.5) b | 114.6 (11.4) a | 112.8 (14.5) a | 113.0 (12.1) | <0.001 |
| BP diastolic, mean (SD) (n = 631) | 70.6 (8.0) a | 65.8 (8.8) b | 70.8 (8.4) a | 70.7 (11.2) a | 69.7 (9.1) | <0.001 d |
| Dublin | Bristol | Granada | Melbourne | Total | p | |
|---|---|---|---|---|---|---|
| Dietary intake (n = 482) | ||||||
| Energy (kcal/day) | 2136.21 (508.90) a | 2048.51 (481.53) ab | 1881.88 (506.54) c | 1906.98 (498.34) bc | 1995.35 (508.51) | <0.001 |
| Fat (g/day) | ||||||
| Unadjusted mean (SD) | 93.63 (26.57) | 89.23 (24.52) | 83.83 (25.46) | 82.28 (26.83) | 87.44 (26.07) | |
| Adjusted mean (SE) | 87.25 (1.13) | 86.82 (1.10) | 88.97 (1.06) | 86.28 (1.28) | 0.355 d | |
| Protein (g/day) | ||||||
| Unadjusted mean (SD) | 87.42 (24.09) | 80.14 (24.04) | 86.07 (25.26) | 77.88 (20.87) | 83.26 (24.08) | |
| Adjusted mean (SE) | 82.23 (1.38) a | 78.18 (1.35) a | 90.25 (1.30) b | 81.13 (1.57) a | <0.001 d | |
| Carbohydrate (g/day) | ||||||
| Unadjusted mean (SD) | 243.59 (73.08) | 238.70 (66.77) | 202.05 (61.50) | 222.85 (71.67) | 226.32 (69.86) | |
| Adjusted mean (SE) | 227.46 (3.35) a | 232.61 (3.27) a | 215.05 (3.14) b | 232.98 (3.81) a | <0.001 d,e | |
| Activity (METs/week, n = 716) | ||||||
| Total | 35.40 (19.19) a | 35.24 (15.95) a | 41.77 (18.65) b | 34.86 (16.61) a | 36.99 (17.93) | <0.001 |
| Household activities | 15.04 (11.43) a | 14.77 (10.85) a | 20.31 (12.37) b | 14.95 (11.23) a | 16.40 (11.74) | <0.001 |
| Occupational activities | 8.22 (9.02) | 7.86 (6.42) | 8.64 (9.15) | 8.09 (7.23) | 8.21 (8.09) | 0.815 f |
| Sport/exercise activities | 5.52 (5.71) | 5.72 (4.94) | 6.05 (6.49) | 5.17 (4.13) | 5.64 (5.45) | 0.440 f |
| Transportation activities | 3.08 (2.90) | 3.08 (2.84) | 3.62 (2.67) | 2.91 (3.13) | 3.19 (2.88) | 0.092 |
| Sedentary activities | 7.70 (3.11) a | 7.84 (2.98) a | 6.45 (3.23) b | 8.18 (3.34) a | 7.50 (3.23) | <0.001 |
| EQ-5D visual analogue scale (n = 732) | 82.41 (15.04) | 80.85 (16.24) | 83.90 (14.69) | 83.37 (13.80) | 82.63 (15.02) | 0.218 |
| EPDS (n = 717) | 6.91 (4.30) | 7.29 (4.80) | 7.08 (4.31) | 7.47 (4.38) | 7.18 (4.45) | 0.641 |
| Pittsburgh sleep score (n = 706) | 6.26 (3.07) a | 6.75 (3.25) a | 7.56 (3.48) b | 6.47 (3.15) a | 6.79 (3.28) | <0.001 |
| Infant feeding intention (n = 712) | ||||||
| Breastfeed | 121 (66.1%) a | 116 (65.5%) a | 157 (80.1%) b | 100 (64.1%) a | 494 (69.4%) | <0.001 |
| Formula | 12 (6.6%) a | 4 (2.3%) b | 5 (2.6%) ab | 4 (2.6%) ab | 25 (3.5%) | |
| Mixed feeding | 40 (21.9%) a | 39 (22.0%) a | 16 (8.2%) b | 42 (26.9%) a | 137 (19.2%) | |
| No plan | 10 (5.5%) a | 18 (10.2%) a | 18 (9.2%) a | 10 (6.4%) a | 56 (7.9%) | |
| eHealth literacy scores (n = 713) | ||||||
| Using technology to process health information | 2.92 (0.44) a | 2.94 (0.54) a | 2.93 (0.52) a | 3.11 (0.51) b | 2.97 (0.50) | 0.003 |
| Motivated to engage with digital services | 2.81 (0.49) ab | 2.76 (0.56) a | 2.77 (0.48) a | 2.93 (0.48) b | 2.81 (0.51) | 0.038 |
| Feel safe and in control | 2.83 (0.46) a | 2.84 (0.52) ac | 3.06 (0.57) b | 2.96 (0.51) c | 2.93 (0.53) | <0.001 |
| Health literacy scores (n = 712) | ||||||
| Having sufficient information to manage health | 3.04 (0.48) | 2.97 (0.52) | 2.97 (0.52) | 3.00 (0.49) | 2.99 (0.50) | 0.468 |
| Actively managing health | 2.78 (0.49) | 2.72 (0.50) | 2.66 (0.53) | 2.75 (0.47) | 2.72 (0.50) | 0.063 |
| Ability to actively engage with healthcare providers | 3.92 (0.66) ac | 3.73 (0.71) b | 3.84 (0.68) ab | 4.05 (0.58) c | 3.88 (0.67) | <0.001 |
| Navigating the healthcare system | 3.62 (0.62) a | 3.51 (0.69) a | 3.60 (0.64) a | 3.77 (0.70) b | 3.62 (0.66) | 0.011 |
| Understand health information | 4.16 (0.50) a | 4.27 (0.56) b | 4.05 (0.55) a | 4.35 (0.51) b | 4.20 (0.54) | <0.001 |
| Dublin (n = 177) | Bristol (n = 122) | Granada (n = 191) | Melbourne (n = 92) | |||||
|---|---|---|---|---|---|---|---|---|
| B (95% CI) | VIF | B (95% CI) | VIF | B (95% CI) | VIF | B (95% CI) | VIF | |
| Education level (reference category: primary or secondary school level) | ||||||||
| Vocational qualification | −0.162 (−4.791, 4.466) | 4.505 | 7.322 (−3.012, 17.655) | 1.068 | −1.228 (−3.849, 1.393) | 2.821 | −1.720 (−9.668, 6.229) | 1.628 |
| Undergraduate education | −1.854 (−6.263, 2.555) | 6.391 | −3.432 (−5.897, −0.966) ** | 1.869 | −2.569 (−5.148, 0.011) | 3.509 | −4.413 (−9.189, 0.363) | 3.533 |
| Postgraduate education | −3.631 (−7.986, 0.724) | 6.807 | −3.004 (−5.957, −0.051) * | 1.988 | −1.488 (−4.169, 1.192) | 3.236 | −3.675 (−8.846, 1.497) | 3.813 |
| Age | −0.291 (−0.553, −0.030) * | 1.331 | ||||||
| Childcare responsibility | ||||||||
| Yes | 0.754 (−1.319, 2.828) | 1.219 | ||||||
| Living circumstances (reference category: own home) | ||||||||
| Rented home | 0.423 (−1.944, 2.790) | 1.155 | 1.083 (−0.634, 2.799) | 1.076 | ||||
| Living with family or in emergency accommodation | −2.599 (−8.801, 3.603) | 1.135 | 9.844 (5.564, 14.125) *** | 1.252 | ||||
| Marital status | ||||||||
| Single | 0.436 (−5.561, 6.433) | 1.061 | ||||||
| Employment status (reference category: full-time employment) | ||||||||
| Homemaker | −1.007 (−3.522, 1.508) | 1.152 | ||||||
| Student, casual employment, government or disability support | 2.719 (0.580, 4.859) * | 1.089 | ||||||
| Part-time employment | 1.435 (−0.537, 3.407) | 1.093 | ||||||
| Gravida | −0.361 (−1.300, 0.578) | 1.697 | ||||||
| Parity | 0.493 (−1.453, 2.438) | 1.675 | ||||||
| Ethnicity (reference category: Caucasian) | ||||||||
| All other ethnic groups | −4.494 (−7.251, −1.736) ** | 1.133 | ||||||
| Energy intake (Kcal) | 0.004 (0.002, 0.006) *** | 1.075 | 0.004 (0.001, 0.007) * | 1.333 | ||||
| Physical activity (METs/week) | ||||||||
| Activity level from sport | 0.137 (−0.017, 0.291) | 1.001 | ||||||
| Total physical activity level | 0.032 (−0.006, 0.069) | 1.100 | ||||||
| Health literacy scale (average score) | ||||||||
| Ability to actively engage with healthcare providers | −0.599 (−1.887, 0.689) | 1.073 | ||||||
| Actively managing my health | −2.426 (−4.333, −0.519) * | 1.148 | −2.339 (−3.616, −1.061) *** | 1.047 | −1.962 (−5.123, 1.198) | 1.367 | ||
| EQ-5D visual analogue scale | −0.049 (−0.109, 0.011) | 1.069 | −0.048 (−0.150, 0.055) | 1.253 | ||||
| Current smoking (yes) | 1.597 (−1.021, 4.215) | 1.046 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Reilly, S.L.; Greene, E.; McAuliffe, F.M.; Teede, H.; Campoy, C.; Burden, C.; Geraghty, A.; Bermúdez, M.G.; Davies, A.; Harrison, C.L.; et al. Clinical and Behavioural Heterogeneity Among Women at Increased Risk for Gestational Diabetes: A Four-Country Analysis. Int. J. Environ. Res. Public Health 2025, 22, 1022. https://doi.org/10.3390/ijerph22071022
O’Reilly SL, Greene E, McAuliffe FM, Teede H, Campoy C, Burden C, Geraghty A, Bermúdez MG, Davies A, Harrison CL, et al. Clinical and Behavioural Heterogeneity Among Women at Increased Risk for Gestational Diabetes: A Four-Country Analysis. International Journal of Environmental Research and Public Health. 2025; 22(7):1022. https://doi.org/10.3390/ijerph22071022
Chicago/Turabian StyleO’Reilly, Sharleen L., Ellen Greene, Fionnuala M. McAuliffe, Helena Teede, Cristina Campoy, Christy Burden, Aisling Geraghty, Mercedes G. Bermúdez, Anna Davies, Cheryce L. Harrison, and et al. 2025. "Clinical and Behavioural Heterogeneity Among Women at Increased Risk for Gestational Diabetes: A Four-Country Analysis" International Journal of Environmental Research and Public Health 22, no. 7: 1022. https://doi.org/10.3390/ijerph22071022
APA StyleO’Reilly, S. L., Greene, E., McAuliffe, F. M., Teede, H., Campoy, C., Burden, C., Geraghty, A., Bermúdez, M. G., Davies, A., Harrison, C. L., Terkildsen Maindal, H., Versace, V. L., Laursen, D. H., Skinner, T., & on behalf of the IMPACT DIABETES B2B Collaboration Group. (2025). Clinical and Behavioural Heterogeneity Among Women at Increased Risk for Gestational Diabetes: A Four-Country Analysis. International Journal of Environmental Research and Public Health, 22(7), 1022. https://doi.org/10.3390/ijerph22071022









