Pharmacological and Non-Pharmacological Interventions to Improve Sleep in People with Cognitive Impairment: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Quality and Risk of Bias Assessment
2.5. Data Extraction
2.6. Data Synthesis and Analysis
3. Results
3.1. Study Selection
3.2. Quality and Risk of Bias Assessment
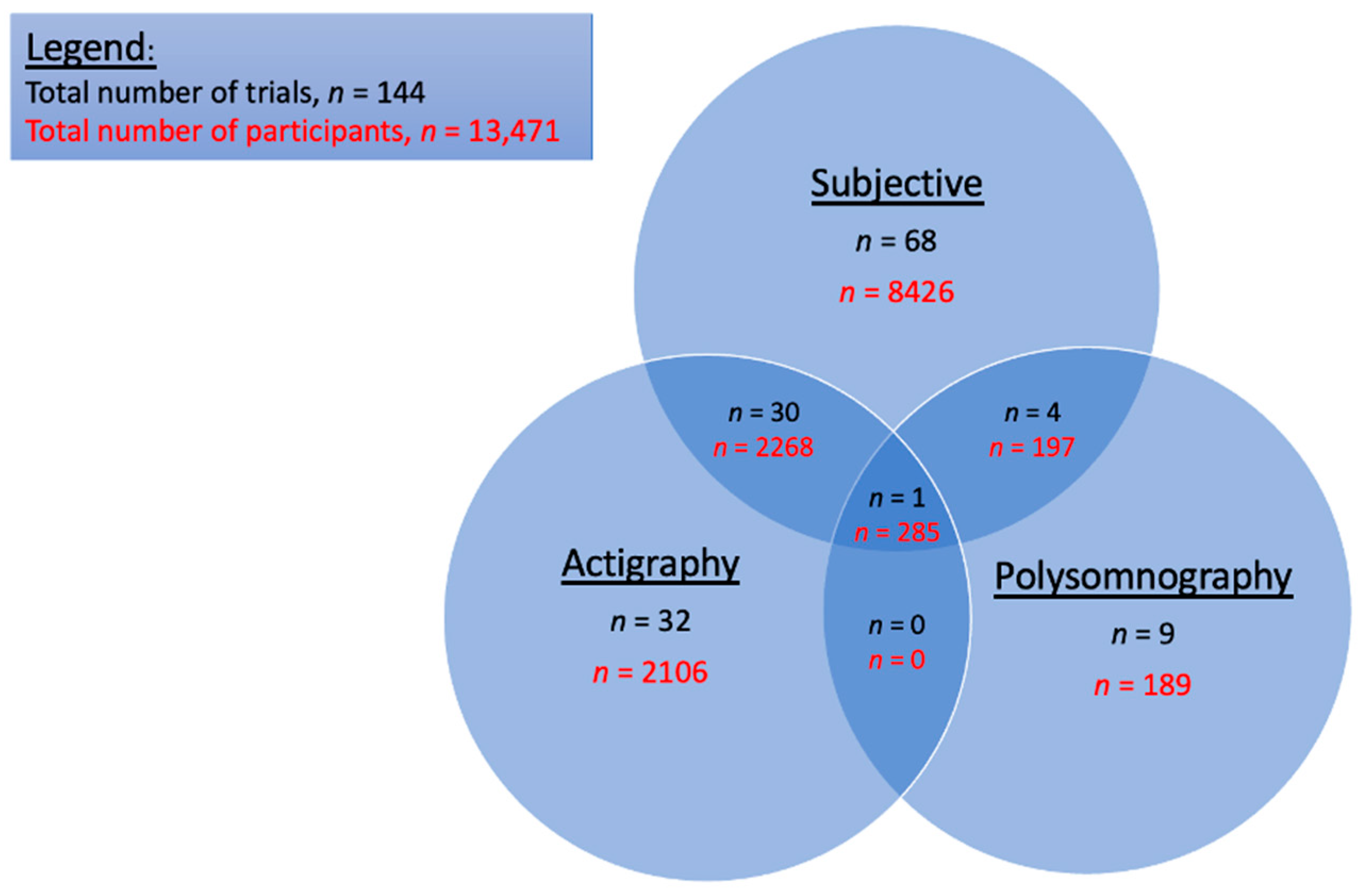
3.3. Sleep Measurement Tools
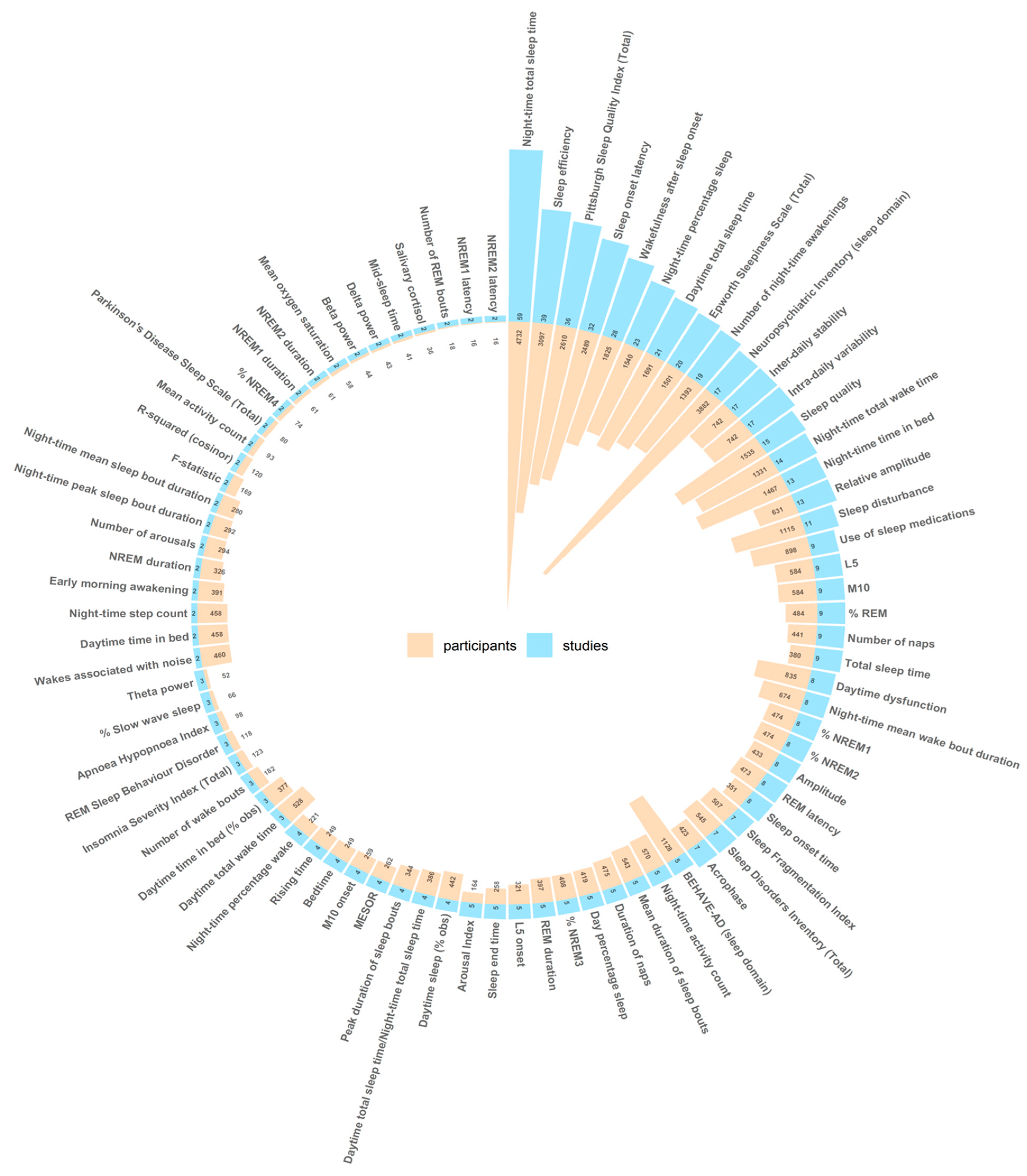
3.4. Sleep Outcome Measures
3.5. Other Non-Sleep Outcome Measures
3.6. Interventions (Non-Pharmacological and Pharmacological)
3.6.1. Non-Pharmacological Interventions
Bright Light Therapy
Multi-Modal Interventions
Sensory Stimulation
Physical Activity
Continuous Positive Airway Pressure (CPAP)
PARO Robotic Seal
Acupressure and Massage
Tai Chi
Cognitive Behavioural Therapy for Insomnia (CBT-I)
Music
Other Non-Pharmacological Interventions
3.6.2. Pharmacological Interventions
Melatonin
Cholinesterase Inhibitors
Memantine
Trazadone
Orexin-Receptor Antagonists
Non-Benzodiazepine Sedative-Hypnotics
Anti-Psychotics
Other Pharmacological Interventions
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Liang, L.; Zheng, F.; Shi, L.; Zhong, B.; Xie, W. Association Between Sleep Duration and Cognitive Decline. JAMA Netw. Open 2020, 3, e2013573. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.J.; Ma, M.Y.; Bao, Y.P.; Han, Y.; Want, Y.M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep Disturbances Increase the Risk of Dementia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef]
- D’Rozario, A.L.; Chapman, J.L.; Phillips, C.L.; Palmer, J.R.; Hoyos, C.M.; Mowszowski, L.; Duffy, S.L.; Marshall, N.S.; Benca, R.; Mander, B.; et al. Objective Measurement of Sleep in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2020, 52, 101308. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Guo, Y.; Liu, Y.; Dong, X. Sleep Structure Assessed by Objective Measurement in Patients with Mild Cognitive Impairment: A Meta-Analysis. Sleep Med. 2024, 113, 397–405. [Google Scholar] [CrossRef]
- Hita-Yañez, E.; Atienza, M.; Cantero, J.L. Polysomnographic and Subjective Sleep Markers of Mild Cognitive Impairment. Sleep 2013, 36, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.E.; McLeland, J.S.; Toedebusch, C.D.; Xiong, C.; Fagan, A.M.; Duntley, S.P.; Morris, J.C.; Holtzman, D.M. Sleep Quality and Preclinical Alzheimer Disease. JAMA Neurol. 2013, 70, 587–593. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Jagust, W.J.; Walker, M.P. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016, 39, 552–566. [Google Scholar] [CrossRef]
- Spira, A.P.; Chen-Edinboro, L.P.; Wu, M.N.; Yaffe, K. Impact of Sleep on the Risk of Cognitive Decline and Dementia. Curr. Opin. Psychiatry 2014, 27, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P. It’s Complicated: The Relationship between Sleep and Alzheimer’s Disease in Humans. Neurobiol. Dis. 2020, 144, 105031. [Google Scholar] [CrossRef]
- Huang, Y.; Potter, R.; Sigurdson, W.; Santacruz, A.; Shih, S.; Ju, Y.E.; Kasten, T.; Morris, J.C.; Mintun, M.; Duntley, S.; et al. Effects of Age and Amyloid Deposition on Aβ Dynamics in the Human Central Nervous System. Arch. Neurol. 2012, 69, 51–58. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Ooms, S.; Overeem, S.; Besse, K.; Olde Rikkert, M.; Verbeek, M.; Claassen, J.A.H.R. Effect of 1 Night of Total Sleep Deprivation on Cerebrospinal Fluid β-Amyloid 42 in Healthy Middle-Aged Men: A Randomized Clinical Trial. JAMA Neurol. 2014, 71, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The Sleep-Wake Cycle Regulates Brain Interstitial Fluid Tau in Mice and CSF Tau in Humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Bubu, O.M.; Brannick, M.; Mortimer, J.; Umasabor-Bubu, O.; Sebastião, Y.V.; Wen, Y.; Schwartz, S.; Borenstein, A.R.; Wu, Y.; Morgan, D.; et al. Sleep, Cognitive Impairment, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Sleep 2017, 40, zsw032. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbaek, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Minakawa, E.N.; Wada, K.; Nagai, Y. Sleep Disturbance as a Potential Modifiable Risk Factor for Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 803. [Google Scholar] [CrossRef]
- Liguori, C.; Placidi, F.; Izzi, F.; Spanetta, M.; Mercuri, N.B.; Di Pucchio, A. Sleep Dysregulation, Memory Impairment, and CSF Biomarkers during Different Levels of Neurocognitive Functioning in Alzheimer’s Disease Course. Alzheimers Res. Ther. 2020, 12, 5. [Google Scholar] [CrossRef]
- Prinz, P.N.; Vitaliano, P.P.; Vitiello, M.V.; Bokan, J.; Raskind, M.; Peskind, E.; Gerber, C. Sleep, EEG and Mental Function Changes in Senile Dementia of the Alzheimer’s Type. Neurobiol. Aging 1982, 3, 361–370. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar] [CrossRef]
- Vitiello, M.V.; Borson, S. Sleep Disturbances in Patients with Alzheimer’s Disease: Epidemiology, Pathophysiology and Treatment. CNS Drugs 2001, 15, 777–796. [Google Scholar] [CrossRef]
- Donaldson, C.; Tarrier, N.; Burns, A. Determinants of Carer Stress in Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 1998, 13, 248–256. [Google Scholar] [CrossRef]
- Pudelewicz, A.; Talarska, D.; Bączyk, G. Burden of Caregivers of Patients with Alzheimer’s Disease. Scand. J. Caring Sci. 2019, 33, 336–341. [Google Scholar] [CrossRef] [PubMed]
- McCurry, S.M.; Vitiello, M.V.; Gibbons, L.E.; Logsdon, R.G.; Teri, L. Factors Associated with Caregiver Reports of Sleep Disturbances in Persons with Dementia. Am. J. Geriatr. Psychiatry 2006, 14, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, J.E.; Edwards, A.B.; Femia, E.E.; Zarit, S.H.; Stephens, M.A.; Townsend, A.; Greene, R. Predictors of Institutionalization of Cognitively Impaired Elders: Family Help and the Timing of Placement. J. Gerontol. Ser. B 2000, 55, P247–P255. [Google Scholar] [CrossRef]
- Pollak, C.P.; Perlick, D.; Linsner, J.P.; Wenston, J.; Hsieh, F. Sleep Problems in the Community Elderly as Predictors of Death and Nursing Home Placement. J. Community Health 1990, 15, 123–135. [Google Scholar] [CrossRef]
- World Health Organisation. World Failing to Address Dementia Challenge. 2 September 2021. Available online: https://www.who.int/news/item/02-09-2021-world-failing-to-address-dementia-challenge (accessed on 25 April 2025).
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Blackman, J.; Morrison, H.D.; Lloyd, K.; Gimson, A.; Banerjee, L.V.; Green, S.; Cousins, R.; Rudd, S.; Harding, S.; Coulthard, E. The Past, Present, and Future of Sleep Measurement in Mild Cognitive Impairment and Early Dementia-Towards a Core Outcome Set: A Scoping Review. Sleep 2022, 45, zac073. [Google Scholar] [CrossRef]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring Sleep Quality in Older Adults: A Comparison Using Subjective and Objective Methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef]
- Camargos, E.F.; Louzada, F.M.; Nóbrega, O.T. Wrist Actigraphy for Measuring Sleep in Intervention Studies with Alzheimer’s Disease Patients: Application, Usefulness, and Challenges. Sleep Med. Rev. 2013, 17, 475–488. [Google Scholar] [CrossRef]
- Kudrnáčová, M.; Kudrnáč, A. Better Sleep, Better Life? Testing the Role of Sleep on Quality of Life. PLoS ONE 2023, 18, e0282085. [Google Scholar] [CrossRef]
- Luyster, F.S.; Strollo, P.J.; Zee, P.C. Sleep: A Health Imperative. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Mannion, H.; Sezgin, D.; O’Donovan, M.R.; Liew, A.; Molloy, D.W. Non-Pharmacological Treatments for Sleep Disturbance in Mild Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis. Maturitas 2019, 127, 82–94. [Google Scholar] [CrossRef]
- Bedward, A.; Kaur, J.; Seedat, S.; Donohue, H.; Kow, C.S.; Rasheed, M.K.; Javed, M.; Hasan, S.S. Pharmacological Interventions to Improve Sleep in People with Alzheimer’s Disease: A Meta-Analysis of Randomized Controlled Trials. Expert Rev. Neurother. 2024, 24, 527–539. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Sharpley, A.L. Pharmacotherapies for Sleep Disturbances in Dementia. Cochrane Database Syst. Rev. 2020, 11, Cd009178. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hao, J.; Song, Y.; Cao, H.; Chen, Y.; Yang, H. Effectiveness of Non-Pharmacological Interventions for Sleep Disturbances in People Living with Dementia: A Systematic Review and Meta-Analysis. Geriatr. Nurs. 2023, 51, 76–83. [Google Scholar] [CrossRef]
- Wilfling, D.; Calo, S.; Dichter, M.N.; Meyer, G.; Möhler, R.; Köpke, S. Non-Pharmacological Interventions for Sleep Disturbances in People with Dementia. Cochrane Database Syst. Rev. 2023, 1, CD011881. [Google Scholar] [CrossRef]
- Blackman, J.; Swirski, M.; Clynes, J.; Harding, S.; Leng, Y.; Coulthard, E. Pharmacological and Non-Pharmacological Interventions to Enhance Sleep in Mild Cognitive Impairment and Mild Alzheimer’s Disease: A Systematic Review. J. Sleep Res. 2021, 30, e13229. [Google Scholar] [CrossRef]
- Crowley, P.; Flanagan, E.; O’Caoimh, R. Protocol for the Creation of a Core Outcome Set for Clinical Trials of Interventions to Improve Sleep in People with Cognitive Impairment: The Sleep in Cognitive Impairment Core Outcome Set (SCICOS) Study. HRB Open Res. 2024, 7, 67. [Google Scholar] [CrossRef]
- Crowley, P.; Flanagan, E.; O’Caoimh, R. A Protocol for the Systematic Review and Meta-Analysis of Clinical Trials of Interventions to Improve Sleep in People with Mild Cognitive Impairment or Dementia. HRB Open Res. 2024, 7, 63. [Google Scholar] [CrossRef]
- Glanville, J. ISSG Search Filter Resource. 2006. Available online: https://sites.google.com/a/york.ac.uk/issg-search-filters-resource/home (accessed on 25 April 2025).
- Helvik, A.S.; Engedal, K.; Benth, J.S.; Selbæk, G. Prevalence and Severity of Dementia in Nursing Home Residents. Dement. Geriatr. Cogn. Disord. 2015, 40, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Hsu, C.C.; Yang, Y.H. A Nationwide Survey of Dementia Prevalence in Long-Term Care Facilities in Taiwan. J. Clin. Med. 2022, 11, 1554. [Google Scholar] [CrossRef] [PubMed]
- Capezuti, E.; Zadeh, R.S.; Pain, K.; Basara, A.; Jiang, N.Z.; Krieger, A.C. A Systematic Review of Non-Pharmacological Interventions to Improve Nighttime Sleep Among Residents of Long-Term Care Settings. BMC Geriatr. 2018, 18, 143. [Google Scholar] [CrossRef]
- Alessi, C.A.; Schnelle, J.F.; MacRae, P.G.; Ouslander, J.G.; Al Samarrai, N.; Simmons, S.F.; Traub, S. Does Physical Activity Improve Sleep in Impaired Nursing Home Residents? J. Am. Geriatr. Soc. 1995, 43, 1098–1102. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Tufanaru, C. Systematic Reviews of Effectiveness. 2020. Available online: https://synthesismanual.jbi.global (accessed on 25 April 2025).
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Chichester, UK, 2019. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Reisberg, B.; Borenstein, J.; Franssen, E.; Salob, S.; Steinberg, G.; Shulman, E.; Ferris, S.H.; Georgotas, A. BEHAVE-AD: A clinical rating scale for the assessment of pharmacologically remediable behavioral symptomatology in Alzheimer’s disease. In Alzheimer’s Disease: Problems, Prospects, and Perspectives; Plenum Press: New York, NY, USA, 1987; pp. 1–16. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rosen, W.G.; Mohs, R.C.; Davis, K.L. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 1984, 141, 1356–1364. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Marx, M.S.; Rosenthal, A.S. A description of agitation in a nursing home. J. Gerontol. 1989, 44, M77–M84. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Abrams, R.C.; young, R.C.; Shamoian, C.A. Cornell Scale for Depression in Dementia. Biol. Psychiatry 1988, 23, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.; Bennett, D.; Sano, M.; Ernesto, C.; Thomas, R.; Grundman, R.; Ferris, S. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 1997, 11 (Suppl. S2), S33–S39. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. the index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Quality of life in Alzheimer’s disease: Patient and caregiver reports. J. Ment. Health Aging 1999, 5, 21–32. [Google Scholar]
- McCurry, S.M.; Pike, K.C.; Vitiello, M.V.; Logsdon, R.G.; Larson, E.B.; Teri, L. Increasing Walking and Bright Light Exposure to Improve Sleep in Community-Dwelling Persons with Alzheimer’s Disease: Results of a Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2011, 59, 1393–1402. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Martin, J.L.; Kripke, D.F.; Marler, M.; Klauber, M.R. Effect of Light Treatment on Sleep and Circadian Rhythms in Demented Nursing Home Patients. J. Am. Geriatr. Soc. 2002, 50, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Baandrup, L.; Jennum, P. Effect of a Dynamic Lighting Intervention on Circadian Rest-Activity Disturbances in Cognitively Impaired, Older Adults Living in a Nursing Home: A Proof-of-Concept Study. Neurobiol. Sleep Circadian Rhythm. 2021, 11, 100062. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Allen, H.; Tomenson, B.; Duignan, D.; Byrne, J. Bright Light Therapy for Agitation in Dementia: A Randomized Controlled Trial. Int. Psychogeriatr. 2009, 21, 711–721. [Google Scholar] [CrossRef]
- Cremascoli, R.; Sparasci, D.; Giusti, G.; Cattaldo, S.; Prina, E.; Roveta, F.; Bruno, F.; Ghezzi, C.; Cerri, S.; Picascia, M.; et al. Effects of Circadian Phase Tailored Light Therapy on Sleep, Mood, and Cognition in Alzheimer’s Disease: Preliminary Findings in a Pivotal Study. Front. Physiol. 2022, 12, 755322. [Google Scholar] [CrossRef]
- Dowling, G.A.; Mastick, J.; Hubbard, E.M.; Luxenberg, J.S.; Burr, R.L. Effect of Timed Bright Light Treatment for Rest-Activity Disruption in Institutionalized Patients with Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 2005, 20, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Fontana Gasio, P.; Kräuchi, K.; Cajochen, C.; VanSomeren, E.; Amrhein, I.; Pache, M.; Savaskan, E.; Wirz-Justice, A. Dawn-Dusk Simulation Light Therapy of Disturbed Circadian Rest-Activity Cycles in Demented Elderly. Exp. Gerontol. 2003, 38, 207–216. [Google Scholar] [CrossRef]
- Hickman, S.E.; Barrick, A.L.; Williams, C.S.; Zimmerman, S.; Connell, B.R.; Preisser, J.S.; Mitchell, C.M.; Sloane, P.D. The Effect of Ambient Bright Light Therapy on Depressive Symptoms in Persons with Dementia. J. Am. Geriatr. Soc. 2007, 55, 1817–1824. [Google Scholar] [CrossRef]
- Kolberg, E.; Pallesen, S.; Hjetland, G.J.; Nordhus, I.H.; Flo-Groeneboom, E. The Effect of Bright Light Treatment on Rest-Activity Rhythms in People with Dementia: A 24-Week Cluster Randomized Controlled Trial. Clocks Sleep 2021, 3, 449–464. [Google Scholar] [CrossRef]
- Linander, C.B.; Kallemose, T.; Joergensen, L.M.; Andersen, O.; Nehlin, J.O.; Jawad, B.N. The Effect of Circadian-Adjusted LED-Based Lighting on Sleep, Daytime Sleepiness and Biomarkers of Inflammation in a Randomized Controlled Cross-Over Trial by Pragmatic Design in Elderly Care Home Dwellers. Arch. Gerontol. Geriatr. 2020, 91, 104223. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.F.; Spira, A.P.; Hernandez, B.; Mather, C.; Sheikh, J.; Ancoli-Israel, S.; Yesavage, J.A.; Zeitzer, J.M. Differential Effects of Morning Light Treatment Combined with Sleep Hygiene Therapy on Memory-Impaired Individuals and Their Caregivers. Sleep 2011, 34, A217. [Google Scholar]
- Hopkins, S.; Morgan, P.L.; Schlangen, L.J.; Williams, P.; Skene, D.J.; Middleton, B. Blue-Enriched Lighting for Older People Living in Care Homes: Effect on Activity, Actigraphic Sleep, Mood, and Alertness. Curr. Alzheimer Res. 2017, 14, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Münch, M.; Schmieder, M.; Bieler, K.; Goldbach, R.; Fuhrmann, T.; Zumstein, N.; Vonmoos, P.; Scartezzini, J.L.; Wirz-Justice, A.; Cajochen, C. Bright Light Delights: Effects of Daily Light Exposure on Emotions, Rest-Activity Cycles, Sleep and Melatonin Secretion in Severely Demented Patients. Curr. Alzheimer Res. 2017, 14, 1063–1075. [Google Scholar]
- Figueiro, M.G.; Plitnick, B.; Roohan, C.; Sahin, L.; Kalsher, M.; Rea, M.S. Effects of a Tailored Lighting Intervention on Sleep Quality, Rest-Activity, Mood, and Behavior in Older Adults with Alzheimer Disease and Related Dementias: A Randomized Clinical Trial. J. Clin. Sleep Med. 2019, 15, 1757–1767. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Veiel, L.L.; Baker, A.; Steele, C. A Randomized, Controlled Trial of Bright Light Therapy for Agitated Behaviors in Dementia Patients Residing in Long-Term Care. Int. J. Geriatr. Psychiatry 1999, 14, 520–525. [Google Scholar] [CrossRef]
- Mishima, K.; Okawa, M.; Hishikawa, Y.; Hozumi, S.; Hori, H.; Takahasi, K. Morning Bright Light Therapy for Sleep and Behavior Disorders in Elderly Patients with Dementia. Acta Psychiatr. Scand. 1994, 89, 1–7. [Google Scholar] [CrossRef]
- Hjetland, G.J.; Kolberg, E.; Pallesen, S.; Thun, E.; Nordhus, I.H.; Bjorvatn, B.; Flo-Groeneboom, E. Ambient Bright Light Treatment Improved Proxy-Rated Sleep but Not Sleep Measured by Actigraphy in Nursing Home Patients with Dementia: A Placebo-Controlled Randomized Trial. BMC Geriatr. 2021, 21, 312. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.H.; Suh, I.B.; Jang, J.W.; Jhoo, J.H.; Lee, J.H. Positive Effect of Timed Blue-Enriched White Light on Sleep and Cognition in Patients with Mild and Moderate Alzheimer’s Disease. Sci. Rep. 2021, 11, 10174. [Google Scholar] [CrossRef]
- Sloane, P.D.; Figueiro, M.; Garg, S.; Cohen, L.W.; Reed, D.; Williams, C.S.; Preisser, J.; Zimmerman, S. Effect of Home-Based Light Treatment on Persons with Dementia and Their Caregivers. Light. Res. Technol. 2015, 2, 161–176. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Gehrman, P.; Martin, J.L.; Shochat, T.; Marler, M.; Corey-Bloom, J.; Levi, L. Increased Light Exposure Consolidates Sleep and Strengthens Circadian Rhythms in Severe Alzheimer’s Disease Patients. Behav. Sleep Med. 2003, 1, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-R.; Liou, Y.M.; Jou, J.-H. Ambient Bright Lighting in the Morning Improves Sleep Disturbances of Older Adults with Dementia. Sleep Med. 2022, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Riemersma-van der Lek, R.F.; Swaab, D.F.; Twisk, J.; Hol, E.M.; Hoogendijk, W.J.; Van Someren, E.J. Effect of Bright Light and Melatonin on Cognitive and Noncognitive Function in Elderly Residents of Group Care Facilities: A Randomized Controlled Trial. JAMA 2008, 299, 2642–2655. [Google Scholar] [CrossRef]
- Mishima, K.; Hishikawa, Y.; Okawa, M. Randomized, Dim Light Controlled, Crossover Test of Morning Bright Light Therapy for Rest-Activity Rhythm Disorders in Patients with Vascular Dementia and Dementia of Alzheimer’s Type. Chronobiol. Int. 1998, 15, 647–654. [Google Scholar] [CrossRef]
- Forbes, D.; Blake, C.M.; Thiessen, E.J.; Peacock, S.; Hawranik, P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst. Rev. 2014, 2014, Cd003946. [Google Scholar] [CrossRef]
- Tan, J.S.I.; Cheng, L.J.; Chan, E.Y.; Lau, Y.; Lau, S.T. Light therapy for sleep disturbances in older adults with dementia: A systematic review, meta-analysis and meta-regression. Sleep Med. 2022, 90, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Alessi, C.A.; Yoon, E.J.; Schnelle, J.F.; Al-Samarrai, N.R.; Cruise, P.A. A Randomized Trial of a Combined Physical Activity and Environmental Intervention in Nursing Home Residents: Do Sleep and Agitation Improve? J. Am. Geriatr. Soc. 1999, 47, 784–791. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Chan, P.C.; Li, L.C.; Wyrough, A.B.; Bennett, K.J.; Backhouse, D.; Liu-Ambrose, T. Effect of a Multimodal Lifestyle Intervention on Sleep and Cognitive Function in Older Adults with Probable Mild Cognitive Impairment and Poor Sleep: A Randomized Clinical Trial. J. Alzheimers Dis. 2020, 76, 179–193. [Google Scholar] [CrossRef]
- McCurry, S.M.; Gibbons, L.E.; Logsdon, R.G.; Vitiello, M.V.; Teri, L. Nighttime Insomnia Treatment and Education for Alzheimer’s Disease: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2005, 53, 793–802. [Google Scholar] [CrossRef]
- Alessi, C.A.; Martin, J.L.; Webber, A.P.; Kim, E.C.; Harker, J.O.; Josephson, K.R. Randomized, Controlled Trial of a Nonpharmacological Intervention to Improve Abnormal Sleep/Wake Patterns in Nursing Home Residents. J. Am. Geriatr. Soc. 2005, 53, 803–810. [Google Scholar] [CrossRef]
- Dowling, G.A.; Burr, R.L.; VanSomeren, E.J.; Hubbard, E.M.; Luxenberg, J.S.; Mastick, J.; Cooper, B.A. Melatonin and Bright-Light Treatment for Rest-Activity Disruption in Institutionalized Patients with Alzheimer’s Disease. J. Am. Geriatr. Soc. 2008, 56, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Leonpacher, A.K.; Peters, M.E.; Drye, L.T.; Makino, K.M.; Newell, J.A.; Devanand, D.P.; Frangakis, C.; Munro, C.A.; Mintzer, J.E.; Pollock, B.G.; et al. Effects of Citalopram on Neuropsychiatric Symptoms in Alzheimer’s Dementia: Evidence from the CitAD Study. Am. J. Psychiatry 2016, 173, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Barber, J.A.; Kinnunen, K.M.; Webster, L.; Kyle, S.D.; Cooper, C.; Espie, C.A.; Hallam, B.; Horsley, R.; Pickett, J.; et al. DREAMS-START (Dementia Related Manual for Sleep; Strategies for Relatives) for People with Dementia and Sleep Disturbances: A Single-Blind Feasibility and Acceptability Randomized Controlled Trial. Int. Psychogeriatr. 2019, 31, 251–265. [Google Scholar] [CrossRef]
- Ouslander, J.G.; Connell, B.R.; Bliwise, D.L.; Endeshaw, Y.; Griffiths, P.; Schnelle, J.F. A Nonpharmacological Intervention to Improve Sleep in Nursing Home Patients: Results of a Controlled Clinical Trial. J. Am. Geriatr. Soc. 2006, 54, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Schnelle, J.F.; Alessi, C.A.; Al-Samarrai, N.R.; Fricker, R.D., Jr.; Ouslander, J.G. The Nursing Home at Night: Effects of an Intervention on Noise, Light, and Sleep. J. Am. Geriatr. Soc. 1999, 47, 430–438. [Google Scholar] [CrossRef]
- Scherder, E.J.A.; Van Someren, E.J.W.; Swaab, D.F. Transcutaneous Electrical Nerve Stimulation (TENS) Improves the Rest–Activity Rhythm in Midstage Alzheimer’s Disease. Behav. Brain Res. 1999, 101, 105–107. [Google Scholar] [CrossRef]
- Van Someren, E.J.; Scherder, E.J.; Swaab, D.F. Transcutaneous electrical nerve stimulation (TENS) improves circadian rhythm disturbances in Alzheimer disease. Alzheimers Dis. Assoc. Disord. 1998, 12, 114–118. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Guo, C.; He, J.; Zhang, S.; Wang, Y.; Zhao, Y.; Li, L.; Wang, J.; Hou, L.; et al. The efficacy and safety of transcutaneous auricular vagus nerve stimulation in patients with mild cognitive impairment: A double blinded randomized clinical trial. Brain Stimul. 2022, 15, 1405–1414. [Google Scholar] [CrossRef]
- Ladenbauer, J.; Ladenbauer, J.; Külzow, N.; de Boor, R.; Avramova, E.; Grittner, U.; Flöel, A. Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment. J. Neurosci. 2017, 37, 7111–7124. [Google Scholar] [CrossRef]
- Hozumi, S.; Hori, H.; Okawa, M.; Hishikawa, Y.; Sato, K. Favorable Effect of Transcranial Electrostimulation on Behavior Disorders in Elderly Patients with Dementia: A Double-Blind Study. Int. J. Neurosci. 1996, 88, 1–10. [Google Scholar] [CrossRef]
- Scherder, E.; Knol, D.; Van Someren, E.; Deijen, J.B.; Binnekade, R.; Tilders, F.; Sergeant, J. Effects of Low-Frequency Cranial Electrostimulation on the Rest-Activity Rhythm and Salivary Cortisol in Alzheimer’s Disease. Neurorehabil. Neural Repair 2003, 17, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Scherder, E.; Knol, D.; Van Tol, M.J.; Van Someren, E.; Deijen, J.B.; Swaab, D.; Scheltens, P. Effects of High-Frequency Cranial Electrostimulation on the Rest-Activity Rhythm and Salivary Cortisol in Alzheimer’s Disease: A Pilot Study. Dement. Geriatr. Cogn. Disord. 2006, 22, 267–272. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Lv, S.; Li, Y.; Jia, S.; Niu, X.; Peng, D. Transcranial magnetic stimulation for sleep disorders in Alzheimer’s disease: A double-blind, randomized, and sham-controlled pilot study. Neurosci. Lett. 2022, 766, 136330. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jia, Y.; Sun, Y.; Ding, Y.; Huang, Z.; Liu, C.; Wang, Y. Efficacy and Safety of Simultaneous rTMS–tDCS Over Bilateral Angular Gyrus on Neuropsychiatric Symptoms in Patients with Moderate Alzheimer’s Disease: A Prospective, Randomized, Sham-Controlled Pilot Study. Brain Stimul. 2022, 15, 1530–1537. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; Qi, X.; Berman, M.H.; Dougal, G.; Dayawansa, S.; Wu, E.; Yi, S.S.; Stevens, A.B.; Huang, J.H. Transcranial Near Infrared Light Stimulation Improves Cognition in Patients with Dementia. Aging Dis. 2021, 12, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Papalambros, N.A.; Weintraub, S.; Chen, T.; Grimaldi, D.; Santostasi, G.; Paller, K.A.; Zee, P.C.; Malkani, R.G. Acoustic Enhancement of Sleep Slow Oscillations in Mild Cognitive Impairment. Ann. Clin. Transl. Neurol. 2019, 6, 1191–1201. [Google Scholar] [CrossRef]
- Cimenser, A.; Hempel, E.; Travers, T.; Strozewski, N.; Martin, K.; Malchano, Z.; Hajós, M. Sensory-Evoked 40-Hz Gamma Oscillation Improves Sleep and Daily Living Activities in Alzheimer’s Disease Patients. Front. Syst. Neurosci. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Taheri, M.; Irandoust, K.; Modabberi, S. An Acute Bout of Dynamic Sitting Exercises Improves Stroop Performance and Quality of Sleep in Older Adults with Cognitive Impairment. Int. Arch. Health Sci. 2019, 6, 126–130. [Google Scholar] [CrossRef]
- Song, D.; Yu, D.S.F. Effects of a Moderate-Intensity Aerobic Exercise Programme on the Cognitive Function and Quality of Life of Community-Dwelling Elderly People with Mild Cognitive Impairment: A Randomised Controlled Trial. Int. J. Nurs. Stud. 2019, 93, 97–105. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Tao, H.; Chai, N.; Zhao, X.; Zhen, X.; Zhou, X. Effects and mediating mechanisms of a structured limbs-exercise program on general cognitive function in older adults with mild cognitive impairment: A randomized controlled trial. Int. J. Nurs. Stud. 2020, 110, 103706. [Google Scholar] [CrossRef]
- Yu, D.J.; Yu, A.P.; Bernal, J.D.; Fong, D.Y.; Chan, D.K.; Cheng, C.P.; Siu, P.M. Effects of exercise intensity and frequency on improving cognitive performance in middle-aged and older adults with mild cognitive impairment: A pilot randomized controlled trial on the minimum physical activity recommendation from WHO. Front. Physiol. 2022, 13, 1021428. [Google Scholar] [CrossRef] [PubMed]
- Bademli, K.; Lok, N.; Canbaz, M.; Lok, S. Effects of Physical Activity Program on Cognitive Function and Sleep Quality in Elderly with Mild Cognitive Impairment: A Randomized Controlled Trial. Perspect. Psychiatr. Care 2019, 55, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Connell, B.R.; Sanford, J.A.; Lewis, D. Therapeutic Effects of an Outdoor Activity Program on Nursing Home Residents with Dementia. J. Hous. Elder. 2007, 21, 195–209. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; Radanovic, M.; de Mello, P.C.; Buchain, P.C.; Vizzotto, A.D.; Harder, J.; Stella, F.; Piersol, C.V.; Gitlin, L.N.; Forlenza, O.V. An Intervention to Reduce Neuropsychiatric Symptoms and Caregiver Burden in Dementia: Preliminary Results from a Randomized Trial of the Tailored Activity Program–Outpatient Version. Int. J. Geriatr. Psychiatry 2019, 34, 1301–1307. [Google Scholar] [CrossRef]
- Nascimento, C.M.C.; Ayan, C.; Cancela, J.M.; Gobbi, L.T.; Gobbi, S.; Stella, F. Effect of a Multimodal Exercise Program on Sleep Disturbances and Instrumental Activities of Daily Living Performance on Parkinson’s and Alzheimer’s Disease Patients. Geriatr. Gerontol. Int. 2014, 14, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ohman, H.; Savikko, N.R.; Strandberg, T.E.; Kautiainen, H.; Raivio, M.M.; Laakkonen, M.L.; Tilvis, R.; Pitkala, K.H. Effects of Frequent and Long-Term Exercise on Neuropsychiatric Symptoms in Patients with Alzheimer’s Disease—Secondary Analyses of a Randomized, Controlled Trial (FINALEX). Eur. Geriatr. Med. 2017, 8, 153–157. [Google Scholar] [CrossRef]
- Cooke, J.R.; Ancoli-Israel, S.; Liu, L.; Loredo, J.S.; Natarajan, L.; Palmer, B.W.; He, F.; Corey-Bloom, J. Continuous Positive Airway Pressure Deepens Sleep in Patients with Alzheimer’s Disease and Obstructive Sleep Apnea. Sleep Med. 2009, 10, 1101–1106. [Google Scholar] [CrossRef]
- Cooke, J.R.; Ayalon, L.; Palmer, B.W.; Loredo, J.S.; Corey-Bloom, J.; Natarajan, L.; Liu, L.; Ancoli-Israel, S. Sustained Use of CPAP Slows Deterioration of Cognition, Sleep, and Mood in Patients with Alzheimer’s Disease and Obstructive Sleep Apnea: A Preliminary Study. J. Clin. Sleep Med. 2009, 5, 305–309. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Zhang, M.; Qiu, J.; Cheng, H.; Mou, X.; Chen, Q.; Li, T.; Peng, J.; Li, B. Sleep Quality Improvement Enhances Neuropsychological Recovery and Reduces Blood Abeta 42/40 Ratio in Patients with Mild-Moderate Cognitive Impairment. Medicina 2021, 57, 1366. [Google Scholar] [CrossRef]
- Chong, M.S.; Ayalon, L.; Marler, M.; Loredo, J.S.; Corey-Bloom, J.; Palmer, B.W.; Liu, L.; Ancoli-Israel, S. Continuous Positive Airway Pressure Reduces Subjective Daytime Sleepiness in Patients with Mild to Moderate Alzheimer’s Disease with Sleep Disordered Breathing. J. Am. Geriatr. Soc. 2006, 54, 777–781. [Google Scholar] [CrossRef]
- Gehrman, P.R.; Connor, D.J.; Martin, J.L.; Shochat, T.; Corey-Bloom, J.; Ancoli-Israel, S. Melatonin Fails to Improve Sleep or Agitation in a Double-Blind Randomized Placebo-Controlled Trial of Institutionalized Patients with Alzheimer Disease. Am. J. Geriatr. Psychiatry 2009, 17, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Costa, Y.S.; Lim, A.S.; Thorpe, K.E.; Colelli, D.R.; Mitchell, S.; Masellis, M.; Lam, B.; Black, S.E.; Boulos, M.I. Investigating Changes in Cognition Associated with the Use of CPAP in Cognitive Impairment and Dementia: A Retrospective Study. Sleep Med. 2023, 101, 437–444. [Google Scholar] [CrossRef]
- Richards, K.C.; Gooneratne, N.; Dicicco, B.; Hanlon, A.; Moelter, S.; Onen, F.; Wang, Y.; Sawyer, A.; Weaver, T.; Lozano, A.; et al. CPAP Adherence May Slow 1-Year Cognitive Decline in Older Adults with Mild Cognitive Impairment and Apnea. J. Am. Geriatr. Soc. 2019, 67, 558–564. [Google Scholar] [CrossRef]
- Troussière, A.-C.; Charley, C.M.; Salleron, J.; Richard, F.; Delbeuck, X.; Derambure, P.; Pasquier, F.; Bombois, F. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1405–1408. [Google Scholar] [CrossRef]
- Shibata, T. Development and spread of therapeutic medical robot, PARO: Innovation of non-pharmacological therapy for dementia and mental health. J. Inf. Process. Manag. 2017, 60, 217–228. [Google Scholar]
- Jøranson, N.; Olsen, C.; Cologiuri, G.; Ihlebæk, C.; Pedersen, I. Effects on Sleep from Group Activity with a Robotic Seal for Nursing Home Residents with Dementia: A Cluster Randomized Controlled Trial. Int. Psychogeriatr. 2021, 33, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Moyle, W.; Jones, C.; Murfield, J.; Thalib, L.; Beattie, E.; Shum, D.; O’Dwyer, S.; Mervin, C.W.; Draper, B. Effect of a Robotic Seal on the Motor Activity and Sleep Patterns of Older People with Dementia, as Measured by Wearable Technology: A Cluster-Randomised Controlled Trial. Maturitas 2018, 110, 10–17. [Google Scholar] [CrossRef]
- Pu, L.; Moyle, W.; Jones, C.; Todorovic, M. The Effect of a Social Robot Intervention on Sleep and Motor Activity of People Living with Dementia and Chronic Pain: A Pilot Randomized Controlled Trial. Maturitas 2021, 144, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Thodberg, K.; Sørensen, L.U.; Christensen, J.W.; Poulsen, P.H.; Houbak, B.; Damgaard, V.; Keseler, I.; Edwards, D.; Videbech, P.B. Therapeutic Effects of Dog Visits in Nursing Homes for the Elderly. Psychogeriatrics 2016, 16, 289–297. [Google Scholar] [CrossRef]
- Harris, M.; Richards, K.C.; Grando, V.T. The Effects of Slow-Stroke Back Massage on Minutes of Nighttime Sleep in Persons with Dementia and Sleep Disturbances in the Nursing Home: A Pilot Study. J. Holist. Nurs. 2012, 30, 255–263. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.R.; Jung, Y.H.; Park, Y.H.; Seo, S.W. Effects of Electrical Automatic Massage on Cognition and Sleep Quality in Alzheimer’s Disease Spectrum Patients: A Randomized Controlled Trial. Yonsei Med. J. 2021, 62, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mansilla, J.; González-López-Arza, M.; Varela-Donoso, E.; Montanero-Fernández, J.; Jiménez-Palomares, M.; Garrido-Ardila, E.M. Ear Therapy and Massage Therapy in the Elderly with Dementia: A Pilot Study. J. Tradit. Chin. Med. 2013, 33, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Yu, D.S.; Choi, K.C.; Lee, D.T.; Sit, J.W.; Chan, H.Y. Tai Chi Qigong as a Means to Improve Night-Time Sleep Quality Among Older Adults with Cognitive Impairment: A Pilot Randomized Controlled Trial. Clin. Interv. Aging 2016, 11, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wang, Y.Q.; Ye, S.Q.; Cheng, Y.G.; Chen, Y.; Feng, X.Z. The Effects of Group-Based versus Individual-Based Tai Chi Training on Nonmotor Symptoms in Patients with Mild to Moderate Parkinson’s Disease: A Randomized Controlled Pilot Trial. Park. Dis. 2017, 2017, 7415870. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Y.; Pan, J.; Fu, C.; Wang, Y. Effect of simplified Tai Chi exercise on relieving symptoms of patients with mild to moderate Parkinson’s disease. J. Sports Med. Phys. Fit. 2020, 60, 282–288. [Google Scholar] [CrossRef]
- Cassidy-Eagle, E.; Siebern, A.; Unti, L.; Glassman, G.; O’Hara, R. Neuropsychological Functioning in Older Adults with Mild Cognitive Impairment and Insomnia Randomized to CBT-I or Control Group. Clin. Gerontol. 2018, 41, 136–144. [Google Scholar] [CrossRef]
- Naismith, S.L.; Pye, J.; Terpening, Z.; Lewis, S.; Bartlett, D. “Sleep Well, Think Well” Group Program for Mild Cognitive Impairment: A Randomized Controlled Pilot Study. Behav. Sleep Med. 2019, 17, 778–789. [Google Scholar] [CrossRef]
- Petrovsky, D.V.; Bradt, J.; McPhillips, M.V.; Sefcik, J.S.; Gitlin, L.N.; Hodgson, N.A. Tailored Music Listening in Persons with Dementia: A Feasibility Randomized Clinical Trial. Am. J. Alzheimer’s Dis. Other Dement. 2023, 38, 15333175231186728. [Google Scholar] [CrossRef]
- Weise, L.; Töpfer, N.F.; Deux, J.; Wilz, G. Feasibility and effects of individualized recorded music for people with dementia: A pilot RCT study. Nord. J. Music Ther. 2019, 29, 39–56. [Google Scholar] [CrossRef]
- Hsiao-Mei, C.; Tsai, L.J.; Chao, S.Y.; Clark, M.J. Study on the Effects of Individualized Learning Therapy on Cognitive Function and Behavioral and Psychological Symptoms of Dementia in the Institutionalized Older Adults. J. Nurs. Res. 2016, 24, 300–310. [Google Scholar]
- Keramtinejad, M.; Azadi, A.; Taghinejad, H.; Khorshidi, A. The Effectiveness of Cognitive Training on Improving Cognitive Function and Sleep Quality in Community-Dwelling Elderly in Iran. Sleep Sci. 2019, 12, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Z.; Lin, R.; Wang, X.X.; Yan, Y.J.; Li, H. Effects of Mindfulness in Patients with Mild Cognitive Impairment with Insomnia: A Double-Blind Randomized Controlled Trial. Geriatr. Nurs. 2022, 47, 239–246. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosomatics 1971, 12, 371–379. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Fatemeh, G.; Sajjad, M.; Niloufar, R.; Neda, S.; Leila, S.; Khadijeh, M. Effect of melatonin supplementation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2022, 269, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Aguilar, M.A.; Ramírez-Salado, I.; Cruz-Ulloa, C.; Benítez-King, G. Efectos de la Melatonina sobre la Macro-Arquitectura del Sueño en Pacientes con Demencia Tipo Alzheimer = Melatonin Effects on Macro Architecture Sleep in Alzheimer’s Disease Patients. Salud Ment. 2013, 36, 271–277. [Google Scholar] [CrossRef]
- Cruz-Aguilar, M.A.; Ramírez-Salado, I.; Guevara, M.A.; Hernández-González, M.; Benítez-King, G. Melatonin Effects on EEG Activity during Sleep Onset in Mild-to-Moderate Alzheimer’s Disease: A Pilot Study. J. Alzheimers Dis. Rep. 2018, 2, 55–65. [Google Scholar] [CrossRef]
- Asayama, K.; Yamadera, H.; Ito, T.; Suzuki, H.; Kudo, Y.; Endo, S. Double Blind Study of Melatonin Effects on the Sleep-Wake Rhythm, Cognitive and Non-Cognitive Functions in Alzheimer Type Dementia. J. Nippon Med. Sch. 2003, 70, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; von Gizycki, H.; Zizi, F. Melatonin Effects on Sleep, Mood, and Cognition in Elderly with Mild Cognitive Impairment. J. Pineal Res. 1998, 25, 177–183. [Google Scholar] [CrossRef]
- Morales-Delgado, R.; Cámara-Lemarroy, C.R.; Salinas-Martínez, R.; Gámez-Treviño, D.; Arredondo-Jaime, A.; Hernández-Maldonado, E.; Guajardo-Álvarez, G. A Randomized Placebo-Controlled Trial Evaluating the Effect of Melatonin on Sleep Quality in Patients with Mild–Moderate Dementia. Eur. Geriatr. Med. 2018, 9, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.; Tractenberg, R.E.; Kaye, J.; Schafer, K.; Gamst, A.; Grundman, M.; Thomas, R.; Thal, L.J.; Alzheimer’s Disease Cooperative Study. A Multicenter, Placebo-Controlled Trial of Melatonin for Sleep Disturbance in Alzheimer’s Disease. Sleep 2003, 26, 893–901. [Google Scholar] [CrossRef]
- Wade, A.G.; Farmer, M.; Harair, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: A 6-month, randomized, placebo-controlled, multicenter trial. Clin. Interv. Aging 2014, 9, 947–961. [Google Scholar]
- Serfaty, M.; Kennell-Webb, S.; Warner, J.; Blizard, R.; Raven, P. Double Blind Randomised Placebo Controlled Trial of Low Dose Melatonin for Sleep Disorders in Dementia. Int. J. Geriatr. Psychiatry 2002, 17, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Shuang, J.; Pan, X.; Li, P.; Yang, Y.; Li, Y.P.; Zhao, Z.Q.; Huang, L.Q.; Zhao, Z.X. Low-Dose Atypical Antipsychotic Risperidone Improves the 5-Year Outcome in Alzheimer’s Disease Patients with Sleep Disturbances. Pharmacology 2015, 96, 155–162. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Brunetti, V.; Losurdo, A.; Testani, E.; Lapenta, L.; Mariotti, P.; Marra, C.; Rossini, P.M.; Marca, G.D. Rivastigmine for Refractory REM Behavior Disorder in Mild Cognitive Impairment. Curr. Alzheimer Res. 2014, 11, 267–273. [Google Scholar] [CrossRef]
- Cumbo, E.; Ligori, L.D. Differential Effects of Current Specific Treatments on Behavioral and Psychological Symptoms in Patients with Alzheimer’s Disease: A 12-Month, Randomized, Open-Label Trial. J. Alzheimers Dis. 2014, 39, 477–485. [Google Scholar] [CrossRef]
- Moretti, R.; Torre, P.; Antonello, R.M.; Cazzato, G.; Pizzolato, G. Different Responses to Rivastigmine in Subcortical Vascular Dementia and Multi-Infarct Dementia. Am. J. Alzheimer’s Dis. Other Dement. 2008, 23, 167–176. [Google Scholar] [CrossRef]
- Korucu, O.; Demiryürek, B.E.; Morkavuk, G.; Korucu, A.A. The Effect of Cholinesterase Inhibitors on Sleep in Patients with Alzheimer’s Disease: An Observational Prospective Study. Psychiatry Clin. Psychopharmacol. 2018, 28, 14–18. [Google Scholar] [CrossRef]
- Mok, V.; Wong, A.; Ho, S.; Leung, T.; Lam, W.W.; Wong, K.S. Rivastigmine in Chinese Patients with Subcortical Vascular Dementia. Neuropsychiatr. Dis. Treat. 2007, 3, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Naharci, M.I.; Ozturk, A.; Yasar, H.; Clintosun, U.; Kocak, N.; Bozoglu, E.; Tazci, I.; Doruk, H. Galantamine Improves Sleep Quality in Patients with Dementia. Acta Neurol. Belg. 2015, 115, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Rozankovic, P.B.; Rožanković, M.; Badžak, J.; Stojić, M.; Sporiš, I.S. Impact of Donepezil and Memantine on Behavioral and Psychological Symptoms of Alzheimer’s Disease: Six-Month Open-Label Study. Cogn. Behav. Neurol. 2021, 34, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Song, H.R.; Woo, Y.S.; Wang, H.R.; Jun, T.Y.; Bahk, W.M. Effect of the Timing of Acetylcholinesterase Ingestion on Sleep. Int. J. Neuropsychopharmacol. 2014, 17, 70. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Amatniek, J.; Ascher, S.; Sadik, K.; Ramaswamy, K. Effects of Galantamine versus Donepezil on Sleep in Patients with Mild to Moderate Alzheimer Disease and Their Caregivers: A Double-Blind, Head-to-Head, Randomized Pilot Study. Alzheimer Dis. Assoc. Disord. 2005, 19, 240–245. [Google Scholar] [CrossRef]
- dos Santos Moraes, W.A.; Poyares, D.R.; Guilleminault, C.; Ramos, L.R.; Bertolucci, P.H.; Tufik, S. The Effect of Donepezil on Sleep and REM Sleep EEG in Patients with Alzheimer Disease: A Double-Blind Placebo-Controlled Study. Sleep 2006, 29, 199–205. [Google Scholar] [CrossRef]
- Moraes, W.; Poyares, D.; Sukys-Claudino, L.; Guilleminault, C.; Tufik, S. Donepezil Improves Obstructive Sleep Apnea in Alzheimer Disease: A Double-Blind, Placebo-Controlled Study. Chest 2008, 133, 677–683. [Google Scholar] [CrossRef]
- Markowitz, J.S.; Gutterman, E.M.; Lilienfeld, S.; Papadopoulos, G. Sleep-Related Outcomes in Persons with Mild to Moderate Alzheimer Disease in a Placebo-Controlled Trial of Galantamine. Sleep 2003, 26, 602–606. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdageur, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the Treatment of Dementia: A Review on its Current and Future Applications. J. Alzheimers Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Takeda, M.; Winblad, B. The Glutamatergic System and Neurodegeneration in Dementia: Preventive Strategies in Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 1999, 14, 3–47. [Google Scholar] [CrossRef]
- Larsson, V.; Aarsland, D.; Ballard, C.; Minthon, L.; Londos, E. The Effect of Memantine on Sleep Behaviour in Dementia with Lewy Bodies and Parkinson’s Disease Dementia. Int. J. Geriatr. Psychiatry 2010, 25, 1030–1038. [Google Scholar] [CrossRef]
- Fleischhacker, W.W.; Buchgeher, A.; Schubert, H. Memantine in the Treatment of Senile Dementia of the Alzheimer Type. Prog. Neuropsychopharmacol. Biol. Psychiatry 1986, 10, 87–93. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kitamura, S.; Homma, A.; Shiosakai, K.; Matsui, D. Efficacy and Safety of Memantine in Patients with Moderate-to-Severe Alzheimer’s Disease: Results of a Pooled Analysis of Two Randomized, Double-Blind, Placebo-Controlled Trials in Japan. Expert Opin. Pharmacother. 2014, 15, 913–925. [Google Scholar] [CrossRef]
- Jaffer, K.Y.; Chang, T.; Vanle, B.; Dang, J.; Steiner, A.J.; Loera, N.; Abdelmesseh, M.; Danovitch, I.; Ishak, W.W. Trazodone for Insomnia: A Systematic Review. Innov. Clin. Neurosci. 2017, 14, 24–34. [Google Scholar] [PubMed]
- Camargos, E.F.; Louzada, L.L.; Quintas, J.L.; Naves, J.O.; Louzada, F.M.; Nóbrega, O.T. Trazodone Improves Sleep Parameters in Alzheimer Disease Patients: A Randomized, Double-Blind and Placebo-Controlled Study. Am. J. Geriatr. Psychiatry 2014, 22, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Zhao, C.; Han, H.; Chen, X.; Tao, J.; Lu, Z. Effects of Trazodone on Sleep Quality and Cognitive Function in Arteriosclerotic Cerebral Small Vessel Disease Comorbid with Chronic Insomnia. Front. Psychiatry 2020, 11, 580. [Google Scholar] [CrossRef]
- Xia, L.; Liu, H.Y.; Wang, B.Y.; Lin, H.N.; Wang, M.C.; Ren, J.X. A Review of Physiological Functions of Orexin: From Instinctive Responses to Subjective Cognition. Medicine 2023, 102, e34206. [Google Scholar] [CrossRef]
- Herring, W.J.; Ceesay, P.; Snyder, E.; Bliwise, D.; Budd, K.; Hutzelmann, J.; Stevens, J.; Lines, C.; Michelson, D. Polysomnographic Assessment of Suvorexant in Patients with Probable Alzheimer’s Disease Dementia and Insomnia: A Randomized Trial. Alzheimers Dement. 2020, 16, 541–551. [Google Scholar] [CrossRef]
- Moline, M.; Thein, S.; Bsharat, M.; Rabbee, N.; Kemethofer-Waliczky, M.; Filippov, G.; Kubota, N.; Dhadda, S. Safety and Efficacy of Lemborexant in Patients with Irregular Sleep-Wake Rhythm Disorder and Alzheimer’s Disease Dementia: Results from a Phase 2 Randomized Clinical Trial. J. Prev. Alzheimer’s Dis. 2021, 8, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Cheng, L.; Li, S.; Xu, F. Effects of Eszopiclone on Sleep Quality and Cognitive Function in Elderly Patients with Alzheimer’s Disease and Sleep Disorder: A Randomized Controlled Trial. Brain Behav. 2022, 12, e2488. [Google Scholar] [CrossRef]
- Louzada, L.L.; Machado, F.V.; Quintas, J.L.; Ribeiro, G.A.; Silva, M.V.; Medonça-Silva, D.L.; Gonçalves, B.S.; Nóbrega, O.T.; Camargos, E.F. The Efficacy and Safety of Zolpidem and Zopiclone to Treat Insomnia in Alzheimer’s Disease: A Randomized, Triple-Blind, Placebo-Controlled Trial. Neuropsychopharmacology 2022, 47, 570–579. [Google Scholar] [CrossRef]
- Meguro, K.; Meguro, M.; Tanaka, Y.; Akanuma, K.; Yamaguchi, K.; Itoh, M. Risperidone is Effective for Wandering and Disturbed Sleep/Wake Patterns in Alzheimer’s Disease. J. Geriatr. Psychiatry Neurol. 2004, 17, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Schnitzler, C.; Schröder, C.; Cajochen, C.; Müller-Spahn, F.; Wirz-Justice, A. Treatment of Behavioural, Cognitive and Circadian Rest-Activity Cycle Disturbances in Alzheimer’s Disease: Haloperidol vs. Quetiapine. Int. J. Neuropsychopharmacol. 2006, 9, 507–516. [Google Scholar] [CrossRef]
- Anttila, S.A.; Leinonen, E.V. A Review of the Pharmacological and Clinical Profile of Mirtazapine. CNS Drug Rev. 2001, 7, 249–264. [Google Scholar] [CrossRef]
- Scoralick, F.M.; Louzada, L.L.; Quintas, J.L.; Naves, J.O.; Camargos, E.F.; Nóbrega, O.T. Mirtazapine Does Not Improve Sleep Disorders in Alzheimer’s Disease: Results from a Double-Blind, Placebo-Controlled Pilot Study. Psychogeriatrics 2017, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Morgan, K.; Lindesay, J. Effect of Institutional Respite Care on the Sleep of People with Dementia and Their Primary Caregivers. J. Am. Geriatr. Soc. 2007, 55, 252–258. [Google Scholar] [CrossRef]
- Son, G.; Neylan, T.C.; Grinberg, L.T. Neuronal and Glial Vulnerability of the Suprachiasmatic Nucleus in Tauopathies: Evidence from Human Studies and Animal Models. Mol. Neurodegener. 2024, 19, 4. [Google Scholar] [CrossRef]
- Pierce, M.; Linnebur, S.A.; Pearson, S.M.; Fixen, D.R. Optimal Melatonin Dose in Older Adults: A Clinical Review of the Literature. Sr. Care Pharm. 2019, 34, 419–431. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wurtman, R.J.; Regan, M.M.; Taylor, J.A.; Shi, J.P.; Leclair, O.U. Melatonin Treatment for Age-Related Insomnia. J. Clin. Endocrinol. Metab. 2001, 86, 4727–4730. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C. Orexin and Alzheimer’s Disease. Curr. Top. Behav. Neurosci. 2017, 33, 305–322. [Google Scholar]
- Ragsdale, S.M.; Radovich, J.M.; Coiduras, I.I.; McCall, V.W.; Grant, S.C.; Lee, C.; Wilber, A. Dual Orexin Receptor Antagonists as Promising Therapeutics for Alzheimer’s Disease. NPJ Biol. Timing Sleep 2025, 2, 11. [Google Scholar] [CrossRef]
- Rogowska, M.; Thornton, M.; Creese, B.; Velayudhan, L.; Aarsland, D.; Ballard, C.; Tsamakis, K.; Stewart, R.; Mueller, C. Implications of Adverse Outcomes Associated with Antipsychotics in Older Patients with Dementia: A 2011–2022 Update. Drugs Aging 2023, 40, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, V.; Silva, J.; Cauli, O. A Survey on Sleep Assessment Methods. PeerJ 2018, 6, e4849. [Google Scholar] [CrossRef] [PubMed]
- DiNapoli, E.A.; Gebara, M.A.; Kho, T.; Butters, M.A.; Gildengers, A.G.; Albert, S.M.; Dew, M.A.; Erickson, K.I.; Reynolds 3rd, C.F.; Karp, J.F. Subjective-Objective Sleep Discrepancy in Older Adults with MCI and Subsyndromal Depression. J. Geriatr. Psychiatry Neurol. 2017, 30, 316–323. [Google Scholar] [CrossRef]
- Most, E.I.; Aboudan, S.; Scheltens, P.; Van Someren, E.J. Discrepancy between Subjective and Objective Sleep Disturbances in Early- and Moderate-Stage Alzheimer Disease. Am. J. Geriatr. Psychiatry 2012, 20, 460–467. [Google Scholar] [CrossRef]
- Blytt, K.M.; Bjorvatn, B.; Husebo, B.; Flo, E. Clinically Significant Discrepancies between Sleep Problems Assessed by Standard Clinical Tools and Actigraphy. BMC Geriatr. 2017, 17, 253. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Waldhorn, R.; Werner, P.; Billig, N. Validation of Sleep Observations in a Nursing Home. Sleep 1990, 13, 512–525. [Google Scholar] [CrossRef]
- Jafari, B.; Mohsenin, V. Polysomnography. Clin. Chest Med. 2010, 31, 287–297. [Google Scholar] [CrossRef]
- Carpi, M.; Fernandes, M.; Mercuri, N.B.; Liguori, C. Sleep Biomarkers for Predicting Cognitive Decline and Alzheimer’s Disease: A Systematic Review of Longitudinal Studies. J. Alzheimers Dis. 2024, 97, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A., III. Sleep Assessment Methods. Monogr. Soc. Res. Child Dev. 2015, 80, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Klauber, M.R.; Jones, D.W.; Kripke, D.F.; Martin, J.; Mason, W.; Pat-Horenczyk, R.; Fell, R. Variations in Circadian Rhythms of Activity, Sleep, and Light Exposure Related to Dementia in Nursing-Home Patients. Sleep 1997, 20, 18–23. [Google Scholar] [PubMed]
- Bliwise, D.L. Sleep in Normal Aging and Dementia. Sleep 1993, 16, 40–81. [Google Scholar] [CrossRef]
- Agnew, H.W., Jr.; Webb, W.B.; Williams, R.L. The First Night Effect: An EEG Study of Sleep. Psychophysiology 1966, 2, 263–266. [Google Scholar] [CrossRef]
- Mohamed, M.; Mohamed, N.; Kim, J.G. Advancements in Wearable EEG Technology for Improved Home-Based Sleep Monitoring and Assessment: A Review. Biosensors 2023, 13, 1019. [Google Scholar] [CrossRef]
- Sadeh, A. The Role and Validity of Actigraphy in Sleep Medicine: An Update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef]
- Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Schneider, J.L.; Surovec, S.; Johnson, N.L.; Cauley, J.A.; Stone, K.L.; Study of Osteoporotic Fractures Research Group. Comparison of Sleep Parameters from Actigraphy and Polysomnography in Older Women: The SOF Study. Sleep 2008, 31, 283–291. [Google Scholar] [CrossRef]
- Martin, J.L.; Hakim, A.D. Wrist Actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef]
- Farina, N.; Sherlock, G.; Thomas, S.; Lowry, R.G.; Banerjee, S. Acceptability and Feasibility of Wearing Activity Monitors in Community-Dwelling Older Adults with Dementia. Int. J. Geriatr. Psychiatry 2019, 34, 617–624. [Google Scholar] [CrossRef]
- Guu, T.W.; Brem, A.K.; Albertyn, C.P.; Kandangwa, P.; Aarsland, D.; Ffytche, D. Wrist-Worn Actigraphy in Agitated Late-Stage Dementia Patients: A Feasibility Study on Digital Inclusion. Alzheimers Dement. 2024, 20, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.A.; Toedebusch, C.D.; Redrick, T.; Freund, D.; McLeland, J.S.; Morris, J.C.; Holtzman, D.M.; Lucey, B.P. Comparison of Single-Channel EEG, Actigraphy, and Sleep Diary in Cognitively Normal and Mildly Impaired Older Adults. Sleep Adv. 2020, 1, zpaa006. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M. Challenges in Involving People with Dementia as Study Participants in Research on Care and Services; Office of the Assistant Secretary for Planning and Evaluation: Washington, DC, USA, 2017.
- Cordone, S.; Annarumma, L.; Rossini, P.M.; De Gennaro, L. Sleep and β-Amyloid Deposition in Alzheimer Disease: Insights on Mechanisms and Possible Innovative Treatments. Front. Pharmacol. 2019, 10, 695. [Google Scholar] [CrossRef]
- Blytt, K.M.; Bjorvatn, B.; Husebo, B.; Flo, E. Effects of Pain Treatment on Sleep in Nursing Home Patients with Dementia and Depression: A Multicenter Placebo-Controlled Randomized Clinical Trial. Int. J. Geriatr. Psychiatry 2018, 33, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Wang, L.; Chen, K.; Mi, Q.; Zu, X.; Xu, Q.; Na, W.; He, W.; Li, X.; Xu, C.; et al. The Effects of a Multi-Disciplinary Team on Sleep Quality Assessment in Mild-to-Moderate Alzheimer’s Disease Patients with Sleep Disorders. Scott. Med. J. 2021, 66, 134–141. [Google Scholar] [CrossRef]
- Cummings, J.L.; Lyketsos, C.G.; Peskind, E.R.; Porsteinsson, A.P.; Mintzer, J.E.; Scharre, D.W.; De La Gandara, J.E.; Agronin, M.; Davis, C.S.; Nguyen, U.; et al. Dextromethorphan/Quinidine (AVP-923) for Treatment of Agitation in Patients with Alzheimer’s Disease: Analysis of Week 10 Results for Patients Treated Only with AVP-923 versus Patients Receiving Only Placebo (NCT01584440). Alzheimers Dement. 2015, 11, P291–P292. [Google Scholar] [CrossRef]
- Dimpfel, W.; Schombert, L.; Keplinger-Dimpfel, I.K.; Panossian, A. Effects of an Adaptogenic Extract on Electrical Activity of the Brain in Elderly Subjects with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Two-Armed Cross-Over Study. Pharmaceuticals 2020, 13, 45. [Google Scholar] [CrossRef]
- Fei, Y.; Wang, R.; Lu, J.; Peng, S.; yang, S.; Wang, Y.; Zheng, K.; Li, R.; Lin, L.; Li, M. Probiotic Intervention Benefits Multiple Neural Behaviors in Older Adults with Mild Cognitive Impairment. Geriatr. Nurs. 2023, 51, 167–175. [Google Scholar] [CrossRef]
- Femia, E.E.; Zarit, S.H.; Parris Stephens, M.A.; Greene, R. Impact of Adult Day Services on Behavioral and Psychological Symptoms of Dementia. Gerontologist 2007, 47, 775–788. [Google Scholar] [CrossRef]
- Fernandez, H.H.; Weintraub, D.; Macklin, E.; Litvan, I.; Schwarzschild, M.A.; Eberling, J.; Videnovic, A.; Kenney, C.J.; Parkinson Study Group SYNAPSE Investigators. Safety, Tolerability, and Preliminary Efficacy of SYN120, a Dual 5-HT6/5-HT2A Antagonist, for the Treatment of Parkinson Disease Dementia: A Randomized, Controlled, Proof-of-Concept Trial. Park. Relat. Disord. 2023, 114, 105511. [Google Scholar] [CrossRef]
- Foster, N.L.; Aldrich, M.S.; Bluemlein, L.; White, R.F.; Berent, S. Failure of Cholinergic Agonist RS-86 to Improve Cognition and Movement in PSP Despite Effects on Sleep. Neurology 1989, 39 Pt 1, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Froggatt, K.; Best, A.; Bunn, F.; Burnside, G.; Coast, J.; Dunleavy, L.; Goodman, C.; Hardwick, B.; Jackson, C.; Kinley, J.; et al. A Group Intervention to Improve Quality of Life for People with Advanced Dementia Living in Care Homes: The Namaste Feasibility Cluster RCT. Health Technol. Assess. 2020, 24, 1–140. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, H.; Hantikainen, V.; Ott, S.; Stark, M. Effectiveness of a Mobility Monitoring System Included in the Nursing Care Process to Enhance Sleep Quality of Nursing Home Residents with Cognitive Impairment. Health Technol. 2017, 7, 161–171. [Google Scholar] [CrossRef]
- Grove, R.A.; Harrington, C.M.; Mahler, A.; Beresford, I.; Maruff, P.; Lowy, M.T.; Nicholls, A.P.; Boardley, R.L.; Berges, A.C.; Nathan, P.J.; et al. A Randomized, Double-Blind, Placebo-Controlled, 16-Week Study of the H3 Receptor Antagonist, GSK239512, as a Monotherapy in Subjects with Mild-to-Moderate Alzheimer’s Disease. Curr. Alzheimer Res. 2014, 11, 47–58. [Google Scholar] [CrossRef]
- Hastings, S.N.; Mahanna, E.P.; Berkowitz, T.S.; Smith, V.A.; Choate, A.L.; Hughes, J.M.; Pavon, J.; Robinson, K.; Hendrix, C.; VanHoutven, C.; et al. Video-Enhanced Care Management for Medically Complex Older Adults with Cognitive Impairment. J. Am. Geriatr. Soc. 2021, 69, 77–84. [Google Scholar] [CrossRef]
- Ito, T.; Yamadera, H.; Ito, R.; Suzuki, H.; Asayama, K.; Endo, S. Effects of Vitamin B12 on Bright Light on Cognition and Sleep-Wake Rhythm in Alzheimer-Type Dementia. Psychiatry Clin. Neurosci. 2001, 55, 281–282. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.; Sohng, K.Y. The Effects of Footbath on Sleep among the Older Adults in Nursing Homes: A Quasi-Experimental Study. Complement. Ther. Med. 2016, 26, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kouzuki, M.; Kitao, S.; Kaju, T.; Urakami, K. Evaluation of the Effect of Aroma Oil as a Bath Salt on Cognitive Function. Psychogeriatrics 2020, 20, 163–171. [Google Scholar] [CrossRef]
- Li, J.; Grandner, M.A.; Chang, Y.P.; Jungquist, C.; Porock, D. Person-Centered Dementia Care and Sleep in Assisted Living Residents with Dementia: A Pilot Study. Behav. Sleep Med. 2017, 15, 97–113. [Google Scholar] [CrossRef]
- McCurry, S.M.; Gibbons, L.E.; Logsdon, R.G.; Vitiello, M.; Teri, L. Training Caregivers to Change the Sleep Hygiene Practices of Patients with Dementia: The NITE-AD Project. J. Am. Geriatr. Soc. 2003, 51, 1455–1460. [Google Scholar] [CrossRef]
- McCurry, S.M.; laFazia, D.M.; Pike, K.C.; Logsdon, R.G.; Teri, L. Development and Evaluation of a Sleep Education Program for Older Adults with Dementia Living in Adult Family Homes. Am. J. Geriatr. Psychiatry 2012, 20, 494–504. [Google Scholar] [CrossRef] [PubMed]
- McPhillips, M.V.; Li, J.; Petrovsky, D.V.; Brewster, G.S.; Ward 3rd, E.J.; Hodgson, N.; Gooneratne, N.S. Assisted Relaxation Therapy for Insomnia in Older Adults with Mild Cognitive Impairment: A Pilot Study. Int. J. Aging Hum. Dev. 2022, 97, 65–80. [Google Scholar] [CrossRef]
- Nacu, A.; Hoerr, R. Neuropsychiatric Symptoms in Dementia and the Effects of Ginkgo Biloba Extract EGB 761® Treatment: Additional Results from a 24-Week Randomized, Placebo-Controlled Trial. Open Access J. Clin. Trials 2016, 8, 1–6. [Google Scholar]
- Petit, D.; Montplaisir, J.; Lorrain, D.; Gauthier, S. THA Does Not Affect Sleep or EEG Spectral Power in Alzheimer’s Disease. Biol. Psychiatry 1993, 33, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Reverte-Villarroya, S.; Zaragoza, J.; Matamoros, C.; Escalante, S. Individual Therapeutic-Cognitive Intervention of the Psychological and Behavioural Symptoms of Patients with Dementia (PRESTA Study). Rev. Cient. Soc. Esp. Enferm. Neurol. 2020, 52, 7–18. [Google Scholar] [CrossRef]
- Richards, K.C.; Beck, C.; O’Sullivan, P.S.; Shue, V.M. Effect of Individualized Social Activity on Sleep in Nursing Home Residents with Dementia. J. Am. Geriatr. Soc. 2005, 53, 1510–1517. [Google Scholar] [CrossRef]
- Rosenberg, P.B.; Lanctôt, K.L.; Herrmann, N.; Mintzer, J.E.; Porsteinsson, A.P.; Sun, X.; Raman, R. Changes in Neuropsychiatric Inventory Associated with Semagacestat Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 54, 373–381. [Google Scholar] [CrossRef]
- Scripnikov, A.; Khomenko, A.; Napryeyenko, O. Effects of Ginkgo Biloba Extract EGb 761 on Neuropsychiatric Symptoms of Dementia: Findings from a Randomised Controlled Trial. Wien. Med. Wochenschr. 2007, 157, 295–300. [Google Scholar] [CrossRef]
- Spagnolo, C.; Dallasta, D.; Iannuccelli, M. A Controlled Double-Blind Trial Comparing Etoperidone with Thioridazine in the Management of Severe Senile Dementia. Drugs Exp. Clin. Res. 1983, 9, 873–880. [Google Scholar]
- Stefani, A.; Santamaria, J.; Iranzo, A.; Hackner, H.; Schenck, C.H.; Högl, B. Nelotanserin as Symptomatic Treatment for Rapid Eye Movement Sleep Behavior Disorder: A Double-Blind Randomized Study Using Video Analysis in Patients with Dementia with Lewy Bodies or Parkinson’s Disease Dementia. Sleep Med. 2021, 81, 180–187. [Google Scholar] [CrossRef]
- Stotsky, B. Multicenter Study Comparing Thioridazine with Diazepam and Placebo in Elderly, Nonpsychotic Patients with Emotional and Behavioral Disorders. Clin. Ther. 1984, 6, 546–559. [Google Scholar] [PubMed]
- Valtonen, M.; Niskanen, L.; Kangas, A.P.; Koskinen, T. Effects of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord. J. Psychiatry 2005, 59, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Battioui, C.; McCarthy, A.; Dang, X.; Zhang, H.; Man, A.; Zou, J.; Kyle, J.; Munsie, L.; Pugh, M.; et al. Evaluating the Use of Digital Biomarkers to Test Treatment Effects on Cognition and Movement in Patients with Lewy Body Dementia. J. Park. Dis. 2022, 12, 1991–2004. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, W.; Liu, D.; Zhang, S.S.; Tang, Y.; Song, J.; Long, J.; Yang, J.; Jiang, H.; Li, Y.; et al. Effects of Sport Stacking on Neuropsychological, Neurobiological, and Brain Function Performances in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Randomized Controlled Trial. Front. Aging Neurosci. 2022, 14, 910261. [Google Scholar] [CrossRef]
- Yehuda, S.; Rabinovtz, S.; Carasso, R.L.; Mostofsky, D.I. Essential fatty acids preparation (SR-3) improves Alzheimer’s patients quality of life. Int. J. Neurosci. 1996, 87, 141–149. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowley, P.; O’Donovan, M.R.; Leahy, P.; Flanagan, E.; O’Caoimh, R. Pharmacological and Non-Pharmacological Interventions to Improve Sleep in People with Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2025, 22, 956. https://doi.org/10.3390/ijerph22060956
Crowley P, O’Donovan MR, Leahy P, Flanagan E, O’Caoimh R. Pharmacological and Non-Pharmacological Interventions to Improve Sleep in People with Cognitive Impairment: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2025; 22(6):956. https://doi.org/10.3390/ijerph22060956
Chicago/Turabian StyleCrowley, Patrick, Mark R. O’Donovan, Peter Leahy, Evelyn Flanagan, and Rónán O’Caoimh. 2025. "Pharmacological and Non-Pharmacological Interventions to Improve Sleep in People with Cognitive Impairment: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 22, no. 6: 956. https://doi.org/10.3390/ijerph22060956
APA StyleCrowley, P., O’Donovan, M. R., Leahy, P., Flanagan, E., & O’Caoimh, R. (2025). Pharmacological and Non-Pharmacological Interventions to Improve Sleep in People with Cognitive Impairment: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 22(6), 956. https://doi.org/10.3390/ijerph22060956







