Effects of Different Bedside Physiotherapy Frequencies in Hospitalized COVID-19 Patients, Focusing on Mild to Moderate Cases
Abstract
1. Introduction
2. Materials and Methods
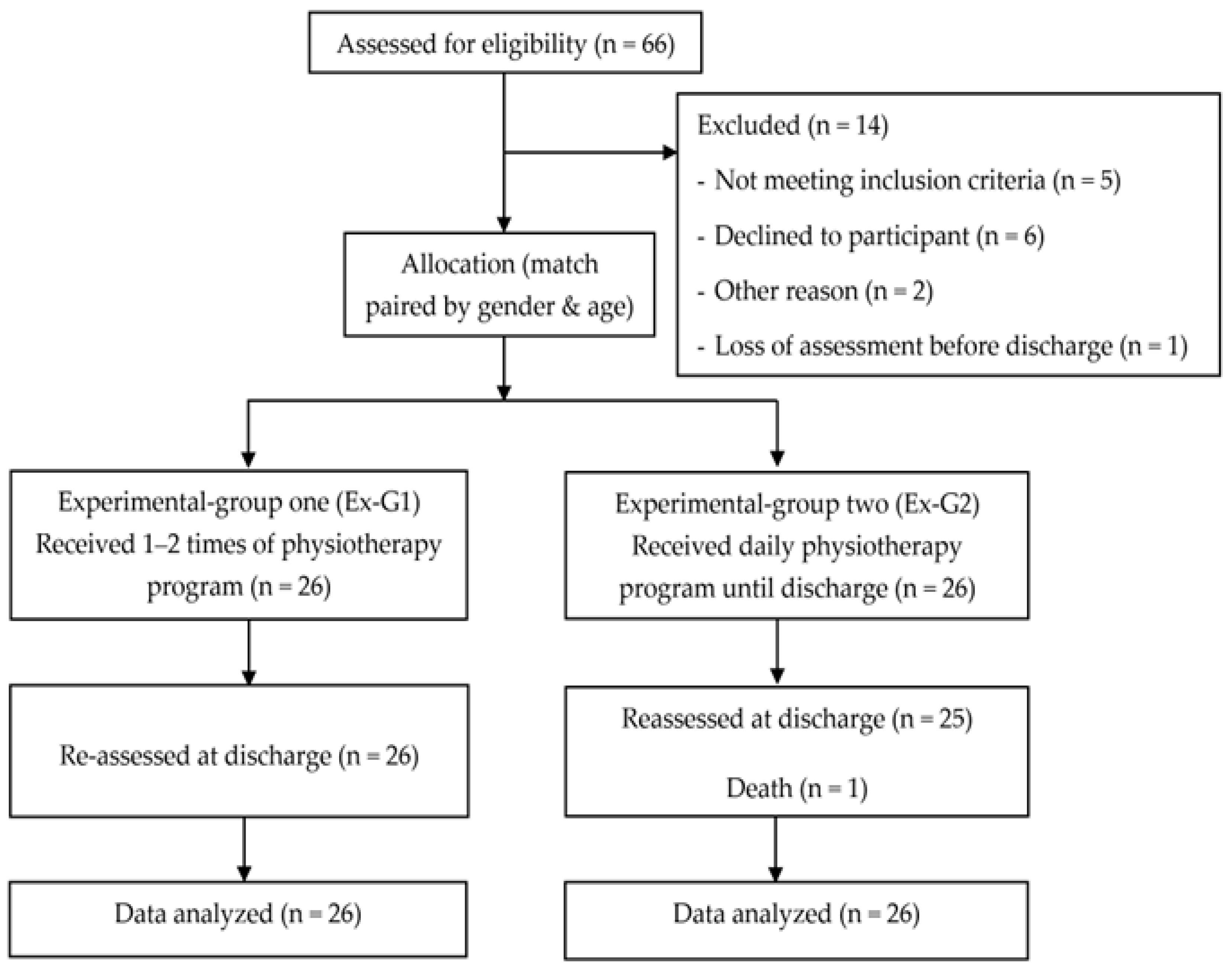
2.1. Study Design
2.2. Study Population
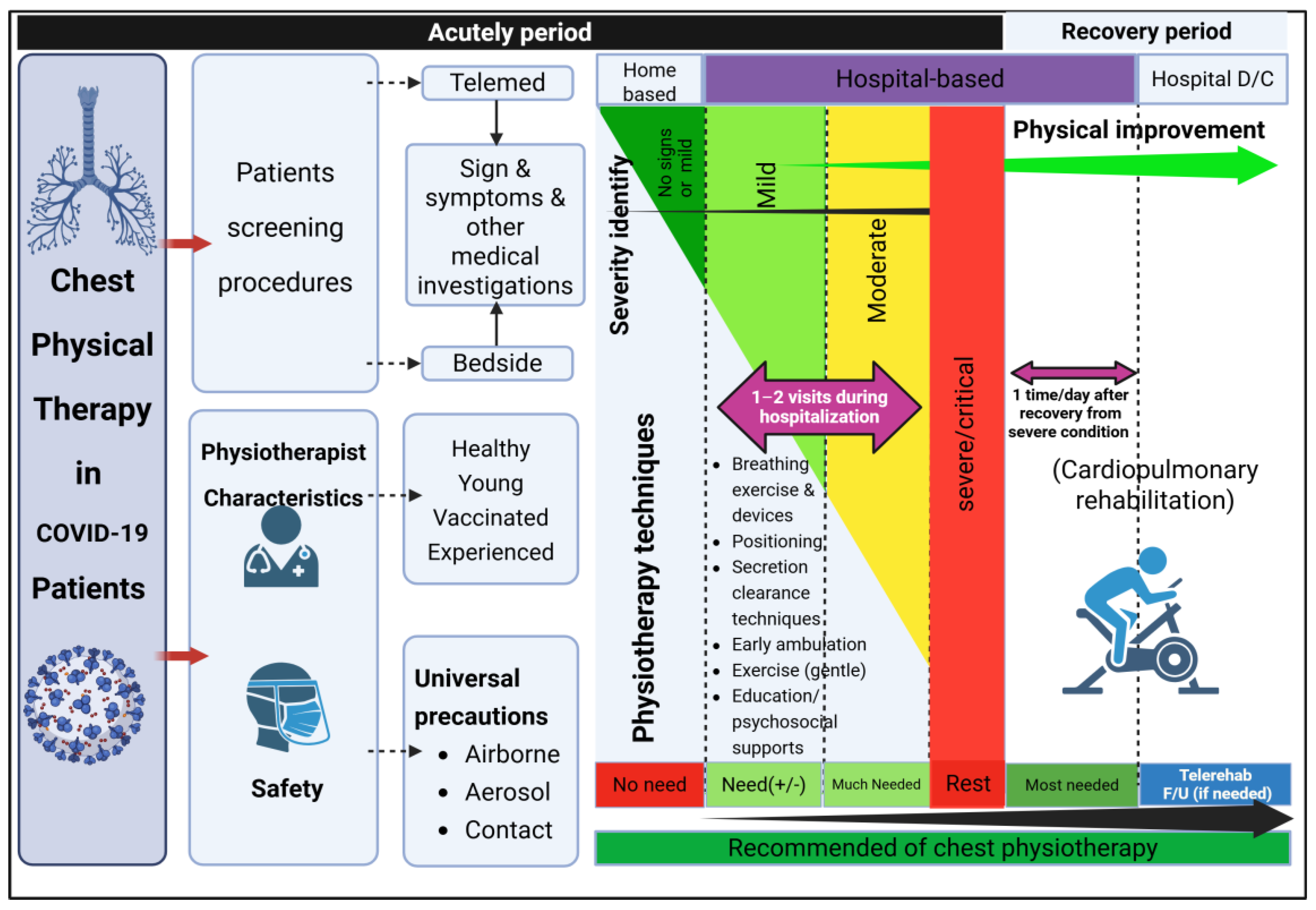
2.3. Characteristics and Classification of COVID-19 Disease Severity
2.4. Intervention, Physiotherapy Programs (PTPs)
2.5. Safety Considerations for COVID-19 Patients in Assigning Physiotherapy Programs
2.6. Safety Considerations for Physiotherapists to Prevent SARS-CoV-2 Transmission
2.7. Outcome Measures
2.8. Laboratory Tests
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. 2022. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 30 May 2020).
- Department of Disease Control of Thailand. COVID-19 Infected Situation Updated Daily. 2022. Available online: https://ddc.moph.go.th/viralpneumonia/index.php (accessed on 30 May 2020).
- da Rosa Mesquita, R.; Francelino Silva Junior, L.C.; Santos Santana, F.M.; Farias de Oliveira, T.; Campos Alcântara, R.; Monteiro Arnozo, G.; Rodrigues da Silva Filho, E.; Galdino Dos Santos, A.G.; Oliveira da Cunha, E.J.; Salgueiro de Aquino, S.H.; et al. Clinical manifestations of COVID-19 in the general population: Systematic review. Wien. Klin. Wochenschr. 2021, 133, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Hussham Arshad, M.; et al. COVID-19 pandemic: From origins to outcomes. a comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez. Med. 2021, 29, 20–36. [Google Scholar] [PubMed]
- Grant, M.C.; Geoghegan, L.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS ONE 2020, 15, e0234765. [Google Scholar] [CrossRef]
- He, X.; Cheng, X.; Feng, X.; Wan, H.; Chen, S.; Xiong, M. Clinical symptom differences between mild and severe COVID-19 patients in China: A meta-analysis. Front. Public Health 2021, 8, 561264. [Google Scholar] [CrossRef]
- van der Lee, L.; Hill, A.M.; Jacques, A.; Patman, S. Efficacy of respiratory physiotherapy interventions for intubated and mechanically ventilated adults with pneumonia: A systematic review and meta-analysis. Physiother. Can. 2021, 73, 6–18. [Google Scholar] [CrossRef]
- Felten-Barentsz, K.M.; van Oorsouw, R.; Klooster, E.; Koenders, N.; Driehuis, F.; Hulzebos, E.H.J.; van der Schaaf, M.; Hoogeboom, T.J.; van der Wees, P.J. Recommendations for hospital-based physical therapists managing patients with COVID-19. Phys. Ther. 2020, 100, 1444–1457. [Google Scholar] [CrossRef]
- Thomas, P.; Baldwin, C.; Beach, L.; Bissett, B.; Boden, I.; Cruz, S.M.; Gosselink, R.; Granger, C.L.; Hodgson, C.; Holland, A.E.; et al. Physiotherapy management for COVID-19 in the acute hospital setting and beyond: An update to clinical practice recommendations. J. Physiother. 2022, 68, 8–25. [Google Scholar] [CrossRef]
- Vitacca, M.; Carone, M.; Clini, E.M.; Paneroni, M.; Lazzeri, M.; Lanza, A.; Privitera, E.; Pasqua, F.; Gigliotti, F.; Castellana, G.; et al. Joint statement on the role of respiratory rehabilitation in the COVID-19 crisis: The Italian position paper. Respiration 2020, 99, 493–499. [Google Scholar] [CrossRef]
- Eggmann, S.; Kindler, A.; Perren, A.; Ott, N.; Johannes, F.; Vollenweider, R.; Balma, T.; Bennett, C.; Silva, I.N.; Jakob, S.M. Early physical therapist interventions for patients with COVID-19 in the acute care hospital: A case report series. Phys. Ther. 2021, 101, pzaa194. [Google Scholar] [CrossRef]
- Gaspari, C.H.; Assumpção, I.; Freire, R.; Silva, A.; Santiso, C.; Jaccoud, A.C. The first 60 days: Physical therapy in a neurosurgical center converted into a COVID-19 center in Brazil. Phys. Ther. 2020, 100, 2120–2126. [Google Scholar] [CrossRef]
- Wittmer, V.L.; Paro, F.M.; Duarte, H.; Capellini, V.K.; Barbalho-Moulim, M.C. Early mobilization and physical exercise in patients with COVID-19: A narrative literature review. Complement. Ther. Clin. Pract. 2021, 43, 101364. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, P.; Yang, M.; Xie, W.; Huang, L.; He, C.; Gosselink, R.; Wei, Q.; Jones, A.Y.M. Physical therapist management of COVID-19 in the intensive care unit: The west China hospital experience. Phys. Ther. 2021, 101, pzaa198. [Google Scholar] [CrossRef] [PubMed]
- Kiel, R.J.; Smith, F.E.; Chason, J.; Khatib, R.; Reyes, M.P. Coxsackievirus B3 myocarditis in C3H/HeJ mice: Description of an inbred model and the effect of exercise on virulence. Eur. J. Epidemiol. 1989, 5, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, M.; Lanza, A.; Bellini, R.; Bellofiore, A.; Cecchetto, S.; Colombo, A.; D’Abrosca, F.; Del Monaco, C.; Gaudiello, G.; Paneroni, M.; et al. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: A position paper of the Italian Association of Respiratory Physiotherapists (ARIR). Monaldi Arch. Chest Dis. 2020, 90, 1285. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Alawna, M. The effect of aerobic exercise on immune biomarkers and symptoms severity and progression in patients with COVID-19: A randomized control trial. J. Bodyw. Mov. Ther. 2021, 28, 425–432. [Google Scholar] [CrossRef]
- Martin, S.A.; Pence, B.D.; Woods, J.A. Exercise and respiratory tract viral infections. Exerc. Sport. Sci. Rev. 2009, 37, 157–164. [Google Scholar] [CrossRef]
- Leandro, C.G.; Ferreira E Silva, W.T.; Lima-Silva, A.E. COVID-19 and exercise-induced immunomodulation. Neuroimmunomodulation 2020, 27, 75–78. [Google Scholar] [CrossRef]
- Piquet, V.; Luczak, C.; Seiler, F.; Monaury, J.; Martini, A.; Ward, A.B.; Gracies, J.-M.; Motavasseli, D.; Piquet, V.; Luczak, C.; et al. Do patients with COVID-19 benefit from rehabilitation? functional outcomes of the first 100 patients in a COVID-19 rehabilitation unit. Arch. Phys. Med. Rehabil. 2021, 102, 1067–1074. [Google Scholar] [CrossRef]
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary rehabilitation in patients recovering from COVID-19. Respiration 2021, 100, 416–422. [Google Scholar] [CrossRef]
- Castro, A.A.M.; Calil, S.R.; Freitas, S.A.; Oliveira, A.B.; Porto, E.F. Chest physiotherapy effectiveness to reduce hospitalization and mechanical ventilation length of stay, pulmonary infection rate and mortality in ICU patients. Respir. Med. 2013, 107, 68–74. [Google Scholar] [CrossRef]
- Johnson, J.K.; Lapin, B.; Green, K.; Stilphen, M. Frequency of physical therapist intervention is associated with mobility status and disposition at hospital discharge for patients with COVID-19. Phys. Ther. 2021, 101, pzaa181. [Google Scholar] [CrossRef] [PubMed]
- Living Guidance for Clinical Management of COVID-19: Living Guidance, 23 November 2021; World Health Organization: Geneva, Switzerland, 2021.
- Abdullahi, A. Safety and efficacy of chest physiotherapy in patients with COVID-19: A critical review. Front. Med. 2020, 7, 454. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Anarte-Lazo, E.; Bernal-Utrera, C.; Perez-Ale, M.; Rodriguez-Blanco, C. Short-Term effects of a respiratory telerehabilitation program in confined COVID-19 patients in the acute phase: A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 7511. [Google Scholar] [CrossRef]
- Drake, T.M.; Riad, A.M.; Fairfield, C.J.; Egan, C.; Knight, S.R.; Pius, R.; Hardwick, H.E.; Norman, L.; Shaw, C.A.; McLean, K.A.; et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: A prospective, multicentre cohort study. Lancet 2021, 398, 223–237. [Google Scholar] [CrossRef]
- Minh, L.H.N.; Abozaid, A.A.-F.; Ha, N.X.; Le Quang, L.; Gad, A.G.; Tiwari, R.; Nhat-Le, T.; Quyen, D.K.; Al-Manaseer, B.; Kien, N.D.; et al. Clinical and laboratory factors associated with coronavirus disease 2019 (Covid-19): A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, e2288. [Google Scholar] [CrossRef]
- Menéndez, R.; Cremades, M.J.; Martínez-Moragón, E.; Soler, J.J.; Reyes, S.; Perpiñá, M. Duration of length of stay in pneumonia: Influence of clinical factors and hospital type. Eur. Respir. J. 2003, 22, 643. [Google Scholar] [CrossRef]
- Hartsgrove, C.; Guevarra-Fernandez, J.; Kendall, J.; Delauter, G.; Kirshblum, S. Measuring discharge outcomes, length of stay, and functional ADL score during COVID-19 in inpatient rehabilitation hospitals. Arch. Phys. Med. Rehabil. 2021, 102, 2291–2299. [Google Scholar] [CrossRef]
- Ahmed, I.; Mustafaoglu, R.; Yeldan, I.; Yasaci, Z.; Erhan, B. Effect of pulmonary rehabilitation approaches on dyspnea, exercise capacity, fatigue, lung functions and quality of life in patients with COVID-19: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 2051–2062. [Google Scholar] [CrossRef]
- Righetti, R.F.; Onoue, M.A.; Politi, F.V.A.; Teixeira, D.T.; Souza, P.N.; Kondo, C.S.; Moderno, E.V.; Moraes, I.G.; Maida, A.L.V.; Pastore Junior, L.; et al. Physiotherapy care of patients with Coronavirus Disease 2019 (COVID-19)—A Brazilian experience. Clinics 2020, 75, e2017. [Google Scholar] [CrossRef]
- Philip, K.E.J.; Owles, H.; McVey, S.; Pagnuco, T.; Bruce, K.; Brunjes, H.; Banya, W.; Mollica, J.; Lound, A.; Zumpe, S.; et al. An online breathing and wellbeing programme (ENO Breathe) for people with persistent symptoms following COVID-19: A parallel-group, single-blind, randomised controlled trial. Lancet Respir. Med. 2022, 10, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Z.; Zhou, Y.; Onoda, K.; Maruyama, H.; Hu, C.; Liu, Z. Summary of respiratory rehabilitation and physical therapy guidelines for patients with COVID-19 based on recommendations of World Confederation for Physical Therapy and National Association of Physical Therapy. J. Phys. Ther. Sci. 2020, 32, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, Y.; Sano, M.; Okawara, H.; Sawada, T.; Nakashima, D.; Ichihara, G.; Fukuda, K.; Sato, K.; Kobayashi, E. Laminar flow ventilation system to prevent airborne infection during exercise in the COVID-19 crisis: A single-center observational study. PLoS ONE 2021, 16, e0257549. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Dela Cruz, M.; Subramanyam, D.; Kumar, R.; Markan, S.; Parker, B.; Roy, H.K. Exercise-induced myokines downregulates the ACE2 level in bronchial epithelial cells: Implications for SARS-CoV-2 prevention. PLoS ONE 2022, 17, e0271303. [Google Scholar] [CrossRef]
- Semphuet, T.; Jianramas, N.; Nissapatorn, V.; Sivakorn, C.; De Lourdes Pereira, M.; Ratnarathon, A.; Salesingh, C.; Jaiyen, E.; Chaiyakul, S.; Piya-Amornphan, N.; et al. The effects of a home telerehabilitation-based program on the cardiopulmonary function and quality of life in post-COVID-19 patients. Heliyon 2024, 10, e40453. [Google Scholar] [CrossRef]
| Ex-G1 (n = 26) | Ex-G2 (n = 26) | p-Value | |
|---|---|---|---|
| Men | 12 (46%) | 12 (46%) | 1.000 |
| Age, mean (SD), years | 43.50 (13.34) | 45.92 (15.24) | 0.545 |
| Age > 60 year | 4 (16%) | 5 (19%) | 0.714 |
| Education level | |||
| Higher than bachelor degree | 4 (16%) | 1 (4%) | 0.168 |
| Bachelor degree | 9 (35%) | 7 (27%) | |
| Secondary school | 4 (16%) | 10 (38%) | |
| Primary school | 7 (27%) | 8 (31%) | |
| No formal education | 2 (8%) | 0 | |
| Oxygen device used | |||
| Nasal cannula | 1 (4%) | 3 (12%) | 0.350 |
| High-flow nasal cannula | 0 | 1 (4%) | |
| Mechanical ventilator | 0 | 0 | |
| Comorbidities | |||
| No | 4 (15%) | 7 (27%) | 0.308 |
| Yes a | 22 (85%) | 19 (73%) | |
| BW, median (IQR), kg | 69.50 (57.00, 89.25) | 68.00 (57.00, 85.00) | 0.905 |
| BMI, median (IQR), kg/m2 | 25.06 (22.83, 32.34) | 26.88 (23.18, 30.99) | 0.504 |
| Classification of BMI b | |||
| Obese | 13 (50%) | 16 (62%) | 0.606 |
| Overweight | 6 (23%) | 5 (19%) | |
| Normal | 7 (27%) | 5 (19%) | |
| COVID-19 disease severity | |||
| Mild | 15 (58%) | 11 (42%) | 0.538 |
| Moderate | 10 (38%) | 13 (50%) | |
| Severe | 1 (4%) | 2 (8%) | |
| Duration from admission to initiation PT, mean (SD), days | 1.62 (0.80) | 1.69 (1.09) | 0.773 |
| Antiviral medication use | |||
| Favipiravir | 24 (92%) | 22 (100%) | 1.000 |
| Mixed antiviral drug | 13 (50%) | 10 (40%) | 0.402 |
| Corticosteroid drug | 3 (12%) | 8 (32%) | 0.090 |
| Ex-G1 (n = 26) | Ex-G2 (n = 25 a) | p-Value | |
|---|---|---|---|
| ORF1ab gene, Ct | 20.81 (17.76, 23.64) | 20.56 (18.59, 24.10) | 0.859 |
| E gene, Ct | 21.70 (18.09, 24.09) | 21.07 (19.03, 23.93) | 0.861 |
| Complete blood count | |||
| WBC, mean (SD), ×103/uL | 6.90 (1.74) | 7.01 (2.44) | 0.856 |
| RBC, mean (SD), 106/uL | 5.07 (0.61) | 4.83 (0.64) | 0.167 |
| HGB, mean (SD), g/dL | 13.27 (1.56) | 13.4 (1.45) | 0.661 |
| HCT, mean (SD), % | 40.62 (4.44) | 40.84 (4.01) | 0.850 |
| RDW, % | 13.90 (13.00, 14.53) | 13.50 (12.60, 13.90) | 0.086 |
| Platelet, ×103/uL | 254.00 (215.50, 293.75) | 254.00 (207.50, 332.00) | 0.706 |
| Neutrophil, mean (SD), % | 60.77 (13.39) | 63.56 (12.94) | 0.453 |
| Lymphocyte, mean (SD), % | 28.46 (12.34) | 26.84 (12.83) | 0.647 |
| Monocyte, % | 7.50 (6.00, 10.00) | 6.00 (5.00, 9.50) | 0.209 |
| Eosinophil, % | 1.00 (0.00, 3.00) | 1.00 (0.00, 2.00) | 0.486 |
| Basophil, % | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 0.925 |
| Kidney function test | |||
| BUN, mg/dL | 12.50 (9.00, 15.00) | 11.00 (8.00, 17.00) | 0.769 |
| Creatinine, mg/dL | 0.74 (0.64, 0.88) | 0.71 (0.58, 1.04) | 0.850 |
| eGFR, mL/min/1.73 m2 | 106.01 (87.45, 115.83) | 107.06 (71.72, 122.14) | 0.880 |
| eGFR stage, n (%) | |||
| Stage I | 19 (73%) | 17 (68%) | 0.086 |
| Stage II | 7 (27%) | 4 (16%) | |
| Stage III | 0 | 4 (16%) | |
| Liver function test | |||
| Total protein, mean (SD), g/dL | 7.74 (0.75) | 7.63 (0.58) | 0.655 |
| Total bilirubin, mean (SD), mg/dL | 0.49 (0.18) | 0.52 (0.17) | 0.855 |
| Alkaline phosphate, U/L | 69.00 (55.00, 88.00) | 67.00 (52.00, 78.50) | 0.516 |
| AST/SGOT, U/L | 26.00 (22.50, 36.50) | 26.00 (23.00, 30.00) | 0.437 |
| ALT/SGPT, U/L | 24.00 (17.00, 47.50) | 22.00 (14.00, 39.00) | 0.166 |
| LDH, U/L | 189.30 (159.50, 207.15) | 176.30 (163.55, 242.85) | 0.777 |
| Inflammation biomarkers | |||
| ESR, mean (SD), mm/h | 26.92 (13.06) | 31.12 (19.00) | 0.297 |
| CRP, mg/dL | 8.26 (3.56, 17.94) | 13.89 (5.80, 25.66) | 0.122 |
| Ex-G1 (n = 26) | Ex-G2 (n = 26) | p-Value | |
|---|---|---|---|
| Survival | 26 (100%) | 25 (96%) | 1.000 |
| Death | 0 | 1 (4%) | 1.000 |
| LoH, median (IQR), days | 10.00 (9.00, 11.80) | 10.00 (10.00, 12.00) | 0.117 |
| Patients referred to ICU | 0 | 1 (4%) | 0.313 |
| Complications | |||
| Influenza | 0 | 1 (4%) | 0.555 |
| Bacterial infections | 2 (8%) | 2 (8%) | |
| Cardiac arrest | 0 | 1 (4%) | |
| None | 24 (92%) | 22 (84%) | |
| Minor adverse event | |||
| Drop in SpO2 > 3% from baseline | 0 | 8 (31%) | 0.018 |
| Dizziness | 1 (4%) | 1 (4%) | |
| Nausea and vomiting | 2 (8%) | 0 | |
| Dyspnea a | 2 (8%) | 1 (4%) | |
| Dyspnea and drop in SpO2 > 3% from baseline a | 0 | 1 (4%) | |
| None | 21 (80%) | 15 (57%) | |
| Average of PT bedside, median (IQR) | 2.00 (1.00, 2.00) | 6.00 (5.00, 7.00) | <0.001 |
| Number of patients receiving each PT program | |||
| Breathing exercise, cough/huff training, active chest trunk mobilization, positioning, active exercise of UE and LE | 26 (100%) | 26 (100%) | 1.000 |
| Positive expiratory pressure devices | 0 | 26 (100%) | <0.001 |
| Out-of-bed exercise | 0 | 26 (100%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jianramas, N.; Semphuet, T.; Nissapatorn, V.; Sivakorn, C.; Pereira, M.d.L.; Ratnarathon, A.; Salesingh, C.; Jaiyen, E.; Chaiyakul, S.; Piya-Amornphan, N.; et al. Effects of Different Bedside Physiotherapy Frequencies in Hospitalized COVID-19 Patients, Focusing on Mild to Moderate Cases. Int. J. Environ. Res. Public Health 2025, 22, 931. https://doi.org/10.3390/ijerph22060931
Jianramas N, Semphuet T, Nissapatorn V, Sivakorn C, Pereira MdL, Ratnarathon A, Salesingh C, Jaiyen E, Chaiyakul S, Piya-Amornphan N, et al. Effects of Different Bedside Physiotherapy Frequencies in Hospitalized COVID-19 Patients, Focusing on Mild to Moderate Cases. International Journal of Environmental Research and Public Health. 2025; 22(6):931. https://doi.org/10.3390/ijerph22060931
Chicago/Turabian StyleJianramas, Netchanok, Thanaporn Semphuet, Veeranoot Nissapatorn, Chaisith Sivakorn, Maria de Lourdes Pereira, Anuttra (Chaovavanich) Ratnarathon, Chenpak Salesingh, Eittipad Jaiyen, Salinee Chaiyakul, Nitita Piya-Amornphan, and et al. 2025. "Effects of Different Bedside Physiotherapy Frequencies in Hospitalized COVID-19 Patients, Focusing on Mild to Moderate Cases" International Journal of Environmental Research and Public Health 22, no. 6: 931. https://doi.org/10.3390/ijerph22060931
APA StyleJianramas, N., Semphuet, T., Nissapatorn, V., Sivakorn, C., Pereira, M. d. L., Ratnarathon, A., Salesingh, C., Jaiyen, E., Chaiyakul, S., Piya-Amornphan, N., Thiangtham, T., Boontam, K., & Longlalerng, K. (2025). Effects of Different Bedside Physiotherapy Frequencies in Hospitalized COVID-19 Patients, Focusing on Mild to Moderate Cases. International Journal of Environmental Research and Public Health, 22(6), 931. https://doi.org/10.3390/ijerph22060931










