Investigating Drug Treatment Costs and Patient Characteristics of Female Breast, Cervical, Colorectal, and Prostate Cancers in Antigua and Barbuda: A Retrospective Data Study (2017–2021)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Area and Population
2.2. Sample Size
2.3. Data Collection
2.4. Data and Cost Variables
2.5. Data Management
2.6. Data Analysis
2.7. Ethical Considerations
3. Results
3.1. Descriptive Information
3.2. Univariate Linear Regression
3.3. Multivariable Linear Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IARC | International Agency for Research on Cancer |
| SLBMC | Sir Lester Bird Medical Centre |
| TCCEC | The Cancer Centre of the Eastern Caribbean |
| MBS | Medical Benefits Scheme |
| ICD-10 | International Classification of Diseases Tenth Edition |
| AJCC 8Ed | American Joint Committee on Cancer Classification Staging Manual Eight Edition |
| FIGO | International Federation of Gynecology and Obstetrics |
| NCD | Noncommunicable Disease |
| PSA | Prostate-Specific Antigen |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Simon, L.; Gaskin, P.; Daniel, G.; Samuel, J.; Goodwin, S. Antigua/Barbuda Cancer Incidence Study. WIMJ Open 2014, 1, 84–87. [Google Scholar] [CrossRef]
- Bovell, A.A.N.; Ramaliba, T.; Goodwin, S.O.; Phillip, J.C.; Ncayiyana, J.; Ginindza, T.G. Incidence, trends and patterns of female breast, cervical, colorectal and prostate cancers in Antigua and Barbuda, 2017–2021: A retrospective study. BMC Cancer 2025, 25, 72. [Google Scholar] [CrossRef]
- Rhudd, A.R. The current state of prostate cancer in Antigua & Barbuda-2021. Ecancermedicalscience 2021, 15, ed112. [Google Scholar] [CrossRef]
- Bovell, A.A.N.; Ngcamphalala, C.; Abbott, D.; Ncayiyana, J.; Ginindza, T.G. Cost Analysis Related to Diagnosis, Treatment and Management of Cervical Cancer in Antigua and Barbuda: A Prevalence-Based Cost-of-Illness Study. Int. J. Environ. Res. Public Health 2024, 21, 1685. [Google Scholar] [CrossRef]
- Bovell, A.A.N.; Ngcamphalala, C.; Rhudd, A.; Ncayiyana, J.; Ginindza, T.G. The Economic Burden of Prostate Cancer in Antigua and Barbuda: A Prevalence-Based Cost-of-Illness Analysis from the Healthcare Provider Perspective. Int. J. Environ. Res. Public Health 2024, 21, 1527. [Google Scholar] [CrossRef]
- Bovell, A.A.N.; Ncayiyana, J.; Ginindza, T.G. Analysis of the Direct Medical Costs of Colorectal Cancer in Antigua and Barbuda: A Prevalence-Based Cost-of-Illness Study. Int. J. Environ. Res. Public Health 2025, 22, 552. [Google Scholar] [CrossRef]
- Bovell, A.A.N.; Ngcamphalala, C.; Brizan-St. Martin, R.; Ncayiyana, J.; Ginindza, T.G. Cost Analysis of Female Breast Cancer in Antigua and Barbuda: A Prevalence-Based Study. Discipline of Public Health Medicine, School of Nursing and Public Health. University of KwaZulu-Natal: Durban, South Africa, 2025; (manuscript in preparation; to be submitted). [Google Scholar]
- Moye-Holz, D.; Vogler, S. Comparison of Prices and Affordability of Cancer Medicines in 16 Countries in Europe and Latin America. Appl. Health Econ. Health Policy 2022, 20, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Dane, A.; van Leeuwen, R.; Hoedemakers, M.; van der Kuy, H.; Sleijfer, S. Combatting the rising costs of cancer drugs; interventions from a university hospital’s perspective. Front. Pharmacol. 2023, 14, 1264951. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M. Weighing Drug Costs and Patient Values in Cancer Care. Am. J. Manag. Care 2025, 31, SP268–SP271. [Google Scholar]
- Xiang, X.; Li, Y.; Liang, N.; Wang, B.; Wang, H. Assessing healthcare payment reforms’ effects on economic inequities and catastrophic expenditures among cancer patients in ethnic minority regions of China. BMC Med. 2025, 23, 208. [Google Scholar] [CrossRef]
- Lambert, L.K.; Horrill, T.C.; Beck, S.M.; Bourgeois, A.; Browne, A.J.; Cheng, S.; Howard, A.F.; Kaur, J.; McKenzie, M.; Stajduhar, K.I.; et al. Health and healthcare equity within the Canadian cancer care sector: A rapid scoping review. Int. J. Equity Health 2023, 22, 20. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, F.; Lin, R. Global burden of cancer and associated risk factors in 204 countries and territories, 1980–2021: A systematic analysis for the GBD 2021. J. Hematol. Oncol. 2024, 17, 119. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Sun, J.; Song, M.; Wang, L.; Yuan, S.; Zhu, Y.; Wan, Z.; Larsson, S.; Tsilidis, K.; et al. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. 2023, 2, e000049. [Google Scholar] [CrossRef]
- Brandão, M.; Morais, S.; Lopes-Conceição, L.; Fontes, F.; Araújo, N.; Dias, T.; Pereira, D.; Borges, M.; Pereira, S.; Lunet, N. Healthcare use and costs in early breast cancer: A patient-level data analysis according to stage and breast cancer subtype. ESMO Open 2020, 5, e000984. [Google Scholar] [CrossRef]
- Hennis, A.J.; Hambleton, I.R.; Wu, S.-Y.; Leske, M.C.; Nemesure, B. Breast cancer incidence and mortality in a Caribbean population: Comparisons with African-Americans. Int. J. Cancer 2009, 124, 429–433. [Google Scholar] [CrossRef]
- Ngcamphalala, C.; Östensson, E.; Ginindza, T.G. The economic burden of prostate cancer in Eswatini. BMC Health Serv. Res. 2022, 22, 483. [Google Scholar] [CrossRef]
- Akari, S.; Mateti, U.V.; Kunduru, B.R. Health-care cost of diabetes in South India: A cost of illness study. J. Res. Pharm. Pract. 2013, 2, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Statistic Division Ministry of Finance the Economy Public Administration Public Broadcasting and Information. Consumer Price Index. Stat Div Minist Financ Antig Barbuda 2022. Available online: https://statistics.gov.ag/wp-content/uploads/2022/01/Monthly-CPI-December-2021.pdf (accessed on 8 March 2024).
- Chow, R.D.; Bradley, E.H.; Gross, C.P. Comparison of Cancer-Related Spending and Mortality Rates in the US vs 21 High-Income Countries. JAMA Health Forum 2022, 3, e221229. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.R.; Spaulding, A.C.; Rinker, B.D.; Huayllani, M.T.; Boczar, D.; Torres-Guzman, R.A.; Maita, K.C.; Ho, O.A.; Forte, A.J. Demographic Characteristics Influence Treatment Costs of Invasive Melanoma in Florida. Ann. Plast. Surg. 2023, 90, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hennis, A.J.M.; Hambleton, I.R.; Wu, S.-Y.; Skeete, D.H.-A.; Nemesure, B.; Leske, M.C. Prostate Cancer Incidence and Mortality in Barbados, West Indies. Prostate Cancer 2011, 2011, 565230. [Google Scholar] [CrossRef]
- The International Agency for Research on Cancer. Cancer Incidence in Five Continents Volume XI Chapter 7: Age Standardization. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://publications.iarc.fr/_publications/media/download/3753/609d0d7711047dd76d7f3dbaa25d7f041fcd013e.pdf&ved=2ahUKEwifvMazquuNAxUG1QIHHXsEKtgQFnoECBcQAQ&usg=AOvVaw3TDNCcXIKXlL2M0ZZ-vTqk (accessed on 23 May 2025).
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Reading, Mass. Addison-Wesley Pub. Co.: New York, NY, USA, 1977; ISBN 0201076160. [Google Scholar]
- StataCorp. Ladder of powers. In Stata Reference Manual; Stata Press: College Station, TX, USA, 2025. [Google Scholar]
- Kirkwood, B.R.; Sterne, J.A. Transformations. In Essential Medical Statistics, 2nd ed.; Blackwell Publishing Ltd.: Malden, MA, USA, 2003; pp. 118–128. ISBN 9780865428713. [Google Scholar]
- Sharma, H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of research results. Saudi J. Anaesth. 2021, 15, 431. [Google Scholar] [CrossRef]
- Webber, C.; Jiang, L.; Grunfeld, E.; Groome, P.A. Identifying predictors of delayed diagnoses in symptomatic breast cancer: A scoping review. Eur. J. Cancer Care 2017, 26, e12483. [Google Scholar] [CrossRef]
- Kirkwood, B.R.; Sterne, J.A.C. Regression modelling. In Essential Medical Statistics, 2nd ed.; Blackwell Publishing Ltd.: Malden, MA, USA, 2003; pp. 315–342. ISBN 9780865428713. [Google Scholar]
- Shrestha, N. Detecting Multicollinearity in Regression Analysis. Am. J. Appl. Math. Stat. 2020, 8, 39–42. [Google Scholar] [CrossRef]
- Miles, J. Tolerance and Variance Inflation Factor. In Encyclopedia of Statistics in Behavioral Science; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Su, H.; Berenson, M.L. Comparing Tests of Homoscedasticity in Simple Linear Regression. JSM Math. Stat. 2017, 4, 1017. [Google Scholar]
- StataCorp. Skewness and kurtosis tests for normality. In Stata Reference Manual; Stata Press: College Station, TX, USA, 2025. [Google Scholar]
- Mansournia, M.A.; Nazemipour, M.; Naimi, A.I.; Collins, G.S.; Campbell, M.J. Reflection on modern methods: Demystifying robust standard errors for epidemiologists. Int. J. Epidemiol. 2021, 50, 346–351. [Google Scholar] [CrossRef]
- Cinelli, C.; Hazlett, C. Making Sense of Sensitivity: Extending Omitted Variable Bias. J. R. Stat. Soc. Ser. B Stat. Methodol. 2020, 82, 39–67. [Google Scholar] [CrossRef]
- Ekdahl Hjelm, T.; Matovu, A.; Mugisha, N.; Löfgren, J. Breast cancer care in Uganda: A multicenter study on the frequency of breast cancer surgery in relation to the incidence of breast cancer. PLoS ONE 2019, 14, e0219601. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Quesnel-Crooks, S.; Sherman, R.; Joseph, R.; Kohler, B.; Andall-Brereton, G.; Ivey, M.A.; Edwards, B.K.; Mery, L.; Gawryszewski, V.; et al. Leading Causes of Cancer Mortality—Caribbean Region, 2003–2013. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.P.; Vedsted, P.; Sokolowski, I.; Søndergaard, J.; Olesen, F. Time intervals from first symptom to treatment of cancer: A cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv. Res. 2011, 11, 284. [Google Scholar] [CrossRef]
- Caglar Bilgin, B.; Kahramanca, S.; Akin, T.; Emre Gokce, I.; Akin, M.; Kucukpinar, T. Factors influencing cost, length of hospital stay and mortality in colorectal cancer. J. BUON Off. J. Balk. Union Oncol. 2015, 20, 1023–1029. [Google Scholar]
- Tramontano, A.C.; Chen, Y.; Watson, T.R.; Eckel, A.; Hur, C.; Kong, C.Y. Racial/ethnic disparities in colorectal cancer treatment utilization and phase-specific costs, 2000-2014. PLoS ONE 2020, 15, e0231599. [Google Scholar] [CrossRef]
- Corral, J.; Castells, X.; Molins, E.; Chiarello, P.; Borras, J.M.; Cots, F. Long-term costs of colorectal cancer treatment in Spain. BMC Health Serv. Res. 2016, 16, 56. [Google Scholar] [CrossRef]
- Corral, M.J.; Clopès, A.; Navarro, M.; Germà, J.R.; Borràs, J.M. Impact on budget of new drugs for colorectal cancer treatment. Med. Clin. 2007, 129, 134–136. [Google Scholar] [CrossRef]
- Schrag, D. The price tag on progress—Chemotherapy for colorectal cancer. N. Engl. J. Med. 2004, 351, 317–319. [Google Scholar] [CrossRef]
- Araújo, J.K.L.; Silva, L.M.D.; Santos, C.A.; Oliveira, I.D.S.; Fialho, G.M.; Giglio, A.D. Assessment of costs related to cancer treatment. Rev. Assoc. Med. Bras. 2020, 66, 1423–1430. [Google Scholar] [CrossRef]
- Zi, H.; He, S.-H.; Leng, X.-Y.; Xu, X.-F.; Huang, Q.; Weng, H.; Zhu, C.; Li, L.-Y.; Gu, J.-M.; Li, X.-H.; et al. Global, regional, and national burden of kidney, bladder, and prostate cancers and their attributable risk factors, 1990–2019. Mil. Med. Res. 2021, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, G.; Gray, S.C.; Hall, I.J.; Smith, J.L. Multimorbidity clusters in adults 50 years or older with and without a history of cancer: National Health Interview Survey, 2018. BMC Geriatr. 2024, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Ritchie, C.S.; Kvale, E.; Fisch, M.J. Multimorbidity: An issue of growing importance for oncologists. J. Oncol. Pract. 2011, 7, 371–374. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, B.; Zhang, S.; Huang, Q.; Zhang, M.; Liu, G. Prognostic impact of tumor size on patients with metastatic colorectal cancer: A large SEER-based retrospective cohort study. Updates Surg. 2023, 75, 1135–1147. [Google Scholar] [CrossRef]
- Karaca-Mandic, P.; McCullough, J.S.; Siddiqui, M.A.; Van Houten, H.; Shah, N.D. Impact of new drugs and biologics on colorectal cancer treatment and costs. J. Oncol. Pract. 2011, 7, e30s–e37s. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Kerr, D.J.; Jani, A.; Gray, S.M. Strategies for Sustainable Cancer Care. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e11–e15. [Google Scholar] [CrossRef]
- Cherny, N.I.; Sullivan, R.; Dafni, U.; Kerst, J.M.; Sobrero, A.; Zielinski, C.; de Vries, E.G.E.; Piccart, M.J. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1547–1573. [Google Scholar] [CrossRef]
- Choi, H.Y.; Chang, J.-E. Targeted Therapy for Cancers: From Ongoing Clinical Trials to FDA-Approved Drugs. Int. J. Mol. Sci. 2023, 24, 13618. [Google Scholar] [CrossRef]
- Kyle, M.A.; Dusetzina, S.B.; Keating, N.L. Evaluation of Trends in Oncology Drug Spending in Medicare, 2016 to 2020. JAMA Netw. Open 2022, 5, e2221468. [Google Scholar] [CrossRef] [PubMed]
- OECS. OECS Pharmaceutical Procurement Service (PPS) Grows from Strength to Strength. Organ East Caribb States 2017. Available online: https://pressroom.oecs.int/oecs-pharmaceutical-procurement-service-grows-from-strength-to-strength (accessed on 23 May 2025).
- PAHO. PAHO Calls on Latin American and Caribbean Countries to Improve Access to Essential Cancer Medicines and Supplies. Pan Am Heal Organ 2025. Available online: https://www.paho.org/en/news/3-2-2025-paho-calls-latin-american-and-caribbean-countries-improve-access-essential-cancer (accessed on 23 May 2025).
- Li, C.I.; Malone, K.E.; Daling, J.R. Differences in Breast Cancer Stage, Treatment, and Survival by Race and Ethnicity. Arch. Intern. Med. 2003, 163, 49. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.P.; Olesen, F.; Sørensen, H.T.; Sokolowski, I.; Søndergaard, J. Socioeconomic patient characteristics predict delay in cancer diagnosis: A Danish cohort study. BMC Health Serv. Res. 2008, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.M.; von Wagner, C.; Abel, G.A.; McPhail, S.; Rubin, G.P.; Lyratzopoulos, G. Typical and atypical presenting symptoms of breast cancer and their associations with diagnostic intervals: Evidence from a national audit of cancer diagnosis. Cancer Epidemiol. 2017, 48, 140–146. [Google Scholar] [CrossRef]
- Miller-Kleinhenz, J.M.; Collin, L.J.; Seidel, R.; Reddy, A.; Nash, R.; Switchenko, J.M.; McCullough, L.E. Racial Disparities in Diagnostic Delay Among Women With Breast Cancer. J. Am. Coll. Radiol. 2021, 18, 1384–1393. [Google Scholar] [CrossRef]
- Behnamfar, F.; Azadehrah, M. Factors associated with delayed diagnosis of cervical cancer in Iran—A survey in Isfahan City. Asian Pac. J. Cancer Prev. 2015, 16, 635–639. [Google Scholar] [CrossRef]
- WorldCat. Field Trials of Health Interventions in Developing Countries a Toolbox, 2nd ed.; Smith, P.G., Morrow, R.H., Eds.; Macmillan: London, UK, 1996; pp. 42–71. [Google Scholar]
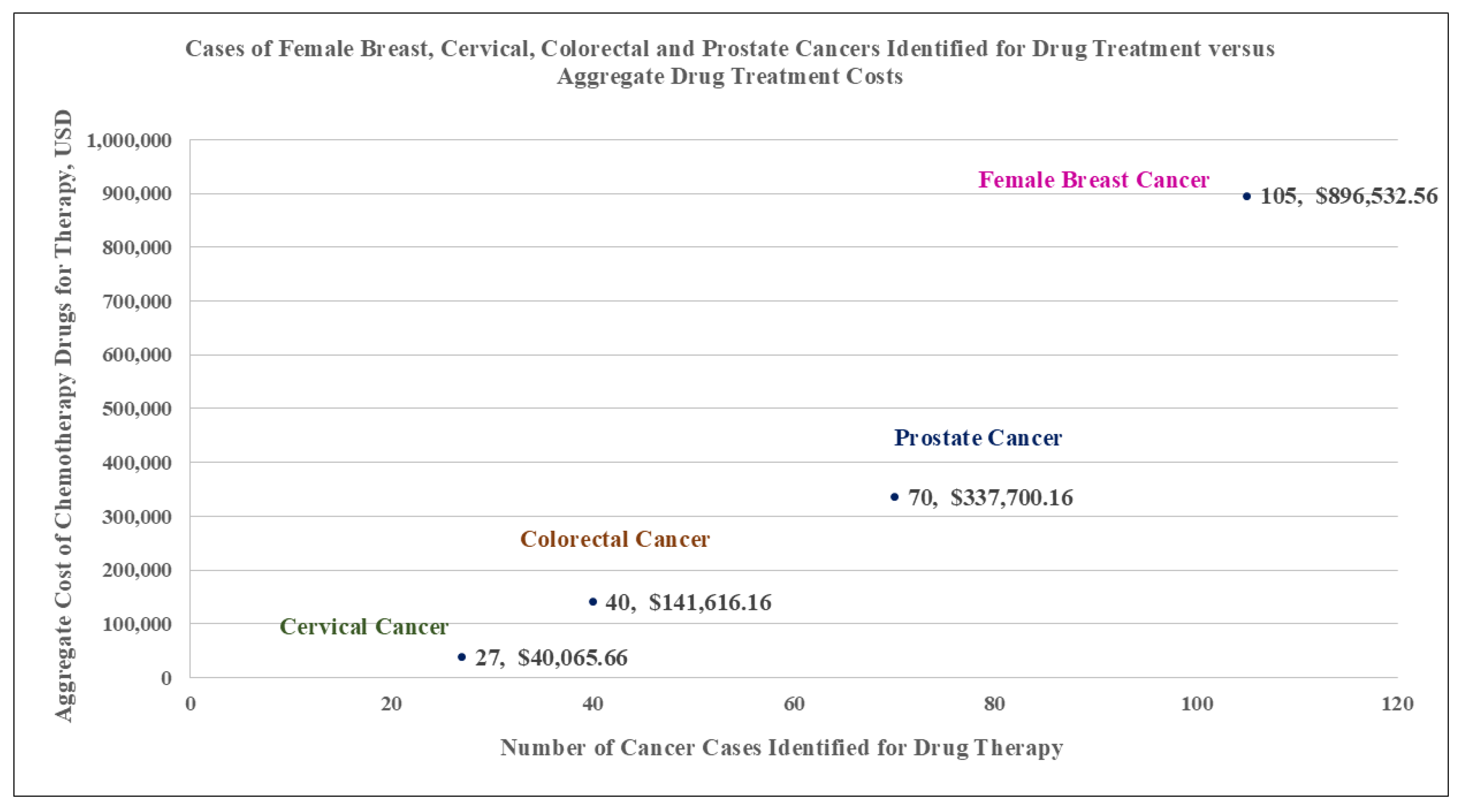
| Female Breast Cancer (n = 105) 43% | Cervical Cancer (n = 27) 11% | Colorectal Cancer (n = 40) 16% | Prostate Cancer (n = 70) 29% | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Female Breast N (%) | Characteristic | Cervical N (%) | Characteristic | Colorectal N (%) | Characteristic | Prostate N (%) |
| Age | Age | Age | Age | ||||
| Mean (SD) | 56.3 (12.4) | Mean (SD) | 52.5 (15.8) | Mean (SD) | 66.7 (11.6) | Mean (SD) | 67.1 (7.5) |
| 95% CI | 53.9–58.7 | 95% CI | 46.3–58.8 | 95% CI | 63.0–70.4 | 95% CI | 65.3–68.8 |
| Median (IQR) | 57.0 (17.0) | Median (IQR) | 51.0 (15.0) | Median (IQR) | 67.5 (13.5) | Median (IQR) | 68.0 (12.0) |
| Range | 31–94 | Range | 30–86 | Range | 42–87 | Range | 51–85 |
| Age Group | Age Group | Age Group | Age Group | ||||
| 30–34 | 6 (5.7) | 30–34 | 4 (14.8) | 30–34 | 0 | 30–34 | 0 |
| 35–39 | 3 (2.9) | 35–39 | 2 (7.4) | 35–39 | 0 | 35–39 | 0 |
| 40–44 | 7 (6.7) | 40–44 | 2 (7.4) | 40–44 | 3 (7.5) | 40–44 | 0 |
| 45–49 | 15 (14.3) | 45–49 | 5 (18.5) | 45–49 | 0 | 45–49 | 0 |
| 50–54 | 14 (13.3) | 50–54 | 3 (11.1) | 50–54 | 4 (10.0) | 50–54 | 1 (1.4) |
| 55–59 | 22 (21.0) | 55–59 | 5 (18.5) | 55–59 | 3 (7.5) | 55–59 | 12 (17.1) |
| 60–64 | 11 (10.5) | 60–64 | 0 | 60–64 | 5 (12.5) | 60–64 | 13 (18.6) |
| 65–69 | 14 (13.3) | 65–69 | 1 (3.7) | 65–69 | 8 (20.0) | 65–69 | 16 (22.9) |
| 70–74 | 8 (7.6) | 70–74 | 2 (7.4) | 70–74 | 9 (22.5) | 70–74 | 15 (21.4) |
| 75+ | 5 (4.8) | 75+ | 3 (11.1) | 75+ | 8 (20.0) | 75+ | 13 (18.6) |
| Vital Status | Vital Status | Vital Status | Vital Status | ||||
| Died | 11 (10.5) | Died | 11 (40.7) | Died | 3 (7.5) | Died | 10 (14.3) |
| Alive | 94 (89.5) | Alive | 16 (59.3) | Alive | 37 (92.5) | Alive | 60 (85.7) |
| Year | Year | Year | Year | ||||
| 2017–2019 | 58 (55.2) | 2017–2019 | 15 (55.6) | 2017–2019 | 11 (27.5) | 2017–2019 | 29 (41.4) |
| 2020–2021 | 47 (44.8) | 2020–2021 | 12 (44.4) | 2020–2021 | 29 (72.5) | 2020–2021 | 41 (58.6) |
| Parish (Area of Residence) | Parish (Area of Residence) | Parish (Area of Residence) | Parish (Area of Residence) | ||||
| Other Parishes | 47 (44.8) | Other Parishes | 10 (37.0) | Other Parishes | 20 (50.0) | Other Parishes | 29 (41.4) |
| St. John | 58 (55.2) | St. John | 17 (63.0) | St. John | 20 (50.0) | St. John | 41 (58.6) |
| Marital Status Known | Marital Status Known | Sex | Marital Status Known | ||||
| No | 64 (61.0) | No | 12 (44.4) | Female | 22 (55.0) | No | 25 (35.7) |
| Yes | 41 (39.1) | Yes | 15 (55.6) | Male | 18 (45.0) | Yes | 45 (64.3) |
| Family History Status Known | Family History Status Known | Disease stage | Family History Status Known | ||||
| No | 68 (64.8) | No | 13 (48.2) | Early Stage | 19 (47.5) | No | 15 (21.4) |
| Yes | 37 (35.2) | Yes | 14 (51.9) | Late Stage | 21 (52.5) | Yes | 55 (78.6) |
| Disease stage | Disease stage | Histological Grade | Disease stage | ||||
| Early Stage | 52 (49.5) | Early Stage | 12 (44.4) | Grade 1 | 2 (5.0) | Early Stage | 31 (44.3) |
| Late Stage | 53 (50.5) | Late Stage | 15 (55.6) | Grade 2 | 30 (75.0) | Late Stage | 39 (55.7) |
| Histological Grade | Histological Grade | Grade 3 | 2 (5.0) | PSA Level (ng/mL) | |||
| Grade 1 | 19 (18.1) | Grade 2 | 21 (77.8) | Not Stated | 6 (15.0) | <10 | 5 (7.1) |
| Grade 2 | 55 (52.4) | Grade 3 | 6 (22.2) | Tumour Site | 10–20 | 11 (15.7) | |
| Grade 3 | 11 (10.5) | Morphological Description | Ascending/Transverse/Descending Colon | 16 (40.0) | >20 | 47 (67.1) | |
| Not Stated | 20 (19.1) | Adenocarcinoma | 3 (11.1) | Sigmoid colon/Rectum | 24 (60.0) | Not Stated | 10 |
| Morphological Description | Squamous Cell Carcinoma | 24 (88.9) | Greatest Dimensions (cm) | Gleason Score | |||
| In situ Carcinoma | 0 | Treatment Intention Status | ≤5 cm | 11 (27.5) | Not Stated | 14 (20.0) | |
| Invasive Carcinoma | 105 (100.0) | Unknown | 10 (37.0) | >5 cm | 10 (25.0) | 6–7 | 35 (50.0) |
| Subtypes | Known | 17 (63.0) | Not Stated | 19 (47.5) | 8–10 | 21 (30.0) | |
| Luminal A/Luminal B | 76 (72.4) | Had Radiation Therapy | Primary Tumour Status | Primary Tumour Status | |||
| Triple Negative Breast cancer/HERS/neu Enriched | 29 (27.6) | No | 4 (14.8) | Undetermined | 6 (15.0) | Undetermined | 46 (65.7) |
| Estrogen Receptor Status | Yes | 23 (85.2) | Determined | 34 (85.0) | Determined | 24 (34.3) | |
| ER-ve | 29 (27.6) | Had Diabetes at Presentation | Lymph Node Status | Lymph Node Status | |||
| ER+ve | 76 (72.4) | No | 25 (92.6) | Undetermined | 6 (15.0) | Undetermined | 49 (70.0) |
| Progesterone Receptor Status | Yes | 2 (7.4) | Determined | 34 (85.0) | Determined | 21 (30.0) | |
| PR-ve | 29 (27.6) | Had Hypertension at Presentation | Distant Metastases | Distant Metastases | |||
| PR+ve | 76 (72.4) | No | 23 (85.2) | Undetermined | 6 (15.0) | Undetermined | 52 (74.3) |
| HER2 Status | Yes | 4 (14.8) | Determined | 34 (85.0) | Determined | 18 (25.7) | |
| HER2-ve | 82 (78.1) | Had Cardiovascular Disease at Presentation | Radiation Therapy Status Known | Had Radiation Therapy | |||
| HER2+ve | 23 (21.9) | No | 24 (88.9) | No | 7 (17.5) | No | 25 (35.7) |
| Distant Metastases | Yes | 3 (11.1) | Yes | 33 (82.5) | Yes | 45 (64.3) | |
| Undetermined (Mx/Not Stated) | 37 (35.2) | No. of Tracked Payments Made at Hospital | Had Hypertension at Presentation | Had Diabetes at Presentation | |||
| Determined (M0/M1) | 68 (64.8) | ≤10 | 12 (44.4) | No | 26 (65.0) | No | 60 (85.7) |
| Regional Lymph node Status | >10 | 15 (55.6) | Yes | 14 (35.0) | Yes | 10 (14.3) | |
| Undetermined (Nx/Not Stated) | 38 (36.2) | Employment Status at Presentation | Had Diabetes at Presentation | Had Hypertension at Presentation | |||
| N0 (No lymph node metastases) | 23 (21.9) | Not Employed | 6 (22.2) | No | 34 (85.0) | No | 50 (71.4) |
| N1 (Metastases in 1 to 3 lymph nodes) | 30 (28.6) | Employed | 21 (77.8) | Yes | 6 (15.0) | Yes | 20 (28.6) |
| N2 (Metastases in 4 or more lymph nodes) | 14 (13.3) | Estimated Monthly Income at Presentation (USD) | Had Cardiovascular Disease | Had Cardiovascular Disease | |||
| Primary Tumour | ≤552 | 11 (40.7) | No | 32 (80.0) | No | 56 (80.0) | |
| Undetermined (Tx/Not Stated) | 25 (23.8) | >552 | 16 (59.3) | Yes | 8 (20.0) | Yes | 14 (20.0) |
| Determined (T1/T4) | 80 (76.2) | Cost of Chemotherapy Drugs (USD) | Evidence of NCD other than cancer | Evidence of NCD other than cancer | |||
| Had some form of Surgery | Mean (SD) | 1075.76 (1979.31) | No | 10 (25.0) | No | 45 (64.3) | |
| No | 15 (14.3) | 95% CI | 292.77–1858.75 | Yes | 30 (75.0) | Yes | 25 (35.7) |
| Yes | 90 (85.7) | Median (IQR) | 431.67 (1085.03) | No. of Tracked Payments Made at Hospital | No. of Tracked Payments Made at Hospital | ||
| Hormonal Therapy Status Known | Range | 0–10,052.39 | ≤10 | 16 (40.0) | ≤10 | 33 (47.1) | |
| No | 26 (24.8) | >10 | 24 (60.0) | >10 | 37 (52.9) | ||
| Yes | 79 (75.2) | Employment Status at Presentation | Employment Status at Presentation | ||||
| Radiation Therapy Status Known | Not Employed | 20 (50.0) | Not Employed | 24 (34.3) | |||
| No | 17 (16.2) | Employed | 20 (50.0) | Employed | 46 (65.7) | ||
| Yes | 88 (83.8) | Estimated Monthly Income at Presentation (USD) | Estimated Monthly Income at Presentation (USD) | ||||
| Evidence of NCD other than cancer | ≤552 | 24 (60.0) | ≤552 | 45 (64.3) | |||
| No | 44 (41.9) | >552 | 16 (40.0) | >552 | 25 (35.7) | ||
| Yes | 61 (58.1) | Cost of Chemotherapy Drugs (USD)* | Cost of Androgen Deprivation Therapy Drugs (USD) * | ||||
| No. of Tracked Payments Made at Hospital | Mean (SD) | 3549.53 (4787.15) | Mean (SD) | 4824.29 (2797.22) | |||
| ≤10 | 49 (46.7) | 95% CI | 2018.52 (5080.53) | 95% CI | 4157.31–5491.26 | ||
| >10 | 56 (53.3) | Median (IQR) | 1167.68 (5959.94) | Median (IQR) | 4594.56 | ||
| Employment Status at Presentation | Range | 0–24,302.47 | Range | 0–11,486.40 | |||
| Not Employed | 23 (21.9) | ||||||
| Employed | 82 (78.1) | ||||||
| Estimated Monthly Income at Presentation (USD) | |||||||
| ≤552 | 22 (21.0) | ||||||
| >552 | 83 (79.1) | ||||||
| Cost of Chemotherapy Drugs (USD) * | |||||||
| Mean (SD) | 8566.24 (19,759.81) | ||||||
| 95% CI | 4742.23–12,390.25 | ||||||
| Median (IQR) | 3290.99 (4074.92) | ||||||
| Range | 0–185,655.70 | ||||||
| Age Categories | Cancer Type | |||||||
|---|---|---|---|---|---|---|---|---|
| Female Breast Cancer | Cervical Cancer | Colorectal Cancer | Prostate Cancer | |||||
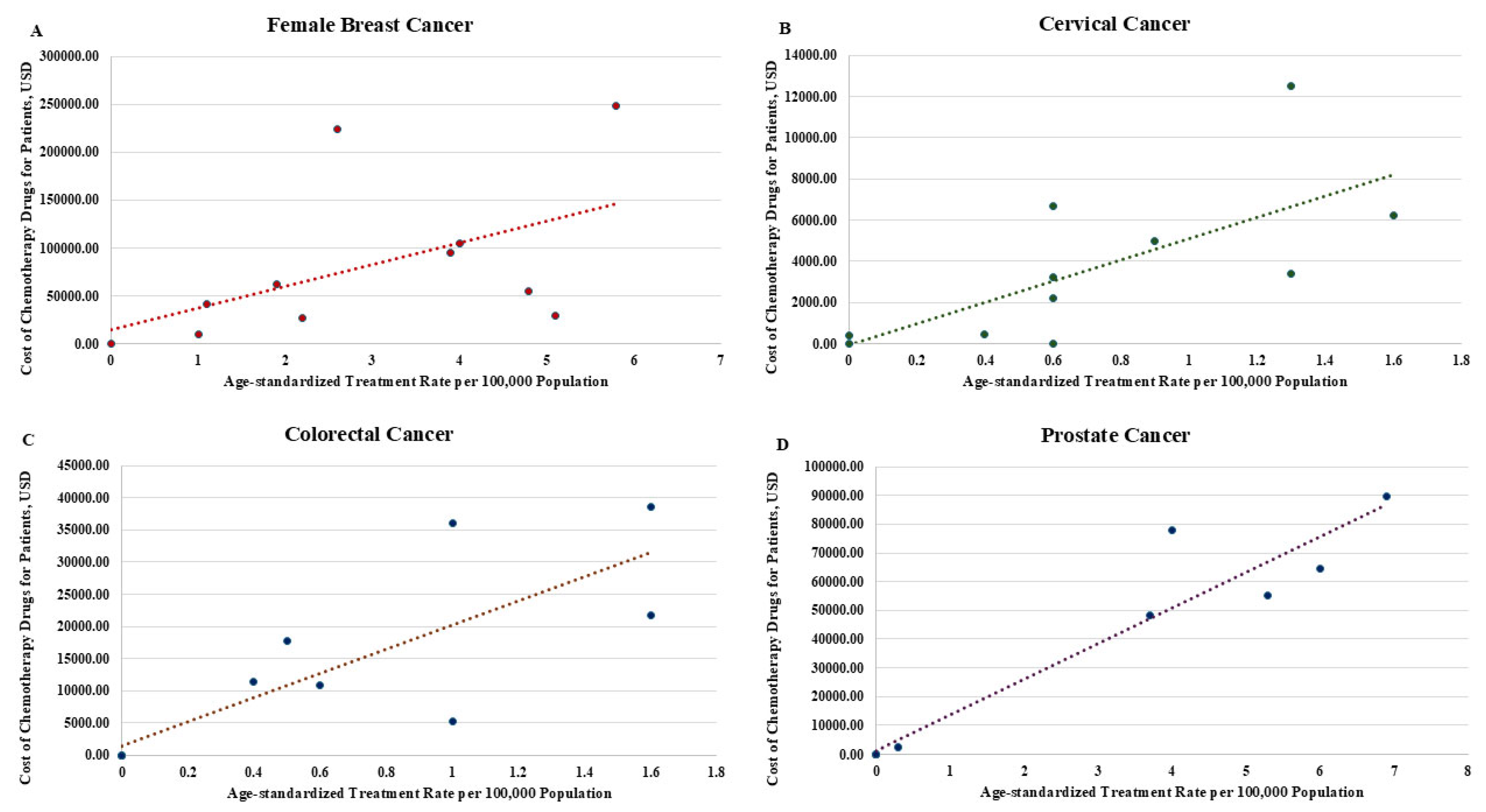
| Age-Standardized Treatment Rates | Aggregate Cost of Drug Treatment | Age-Standardized Treatment Rates | Aggregate Cost of Drug Treatment | Age-Standardized Treatment Rates | Aggregate Cost of Drug Treatment | Age-Standardized Treatment Rates | Aggregate Cost of Drug Treatment | |
| 25 to 29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 30 to 34 | 1.90 | 62,223.74 | 1.30 | 12,498.20 | 0.00 | 0.00 | 0.00 | 0.00 |
| 35 to 39 | 1.00 | 9830.50 | 0.60 | 2218.87 | 0.00 | 0.00 | 0.00 | 0.00 |
| 40 to 44 | 2.20 | 27,286.36 | 0.60 | 3243.90 | 0.50 | 17,704.34 | 0.00 | 0.00 |
| 45 to 49 | 4.80 | 54,830.29 | 1.60 | 6216.47 | 0.00 | 0.00 | 0.00 | 0.00 |
| 50 to 54 | 4.00 | 104,292.10 | 0.90 | 4997.08 | 0.60 | 10,789.14 | 0.30 | 2297.28 |
| 55 to 59 | 5.80 | 248,987.20 | 1.30 | 3400.93 | 0.40 | 11,407.09 | 3.70 | 48,242.88 |
| 60 to 64 | 3.90 | 95,008.63 | 0.00 | 380.95 | 1.00 | 36,018.24 | 5.30 | 55,134.72 |
| 65 to 69 | 5.10 | 29,043.02 | 0.40 | 431.67 | 1.60 | 38,600.84 | 6.90 | 89,593.92 |
| 70 to 74 | 2.60 | 224,073.10 | 0.60 | 6677.59 | 1.60 | 21,802.24 | 6.00 | 64,323.84 |
| 75+ | 1.10 | 40,957.62 | 0.60 | 0.00 | 1.00 | 5294.27 | 4.00 | 78,107.52 |
| Total | 32.40 | 896,532.56 | 7.90 | 40,065.66 | 6.70 | 141,616.16 | 26.20 | 337,700.16 |
| Female Breast Cancer (n = 105) | Cervical Cancer (n = 27) | ||||
|---|---|---|---|---|---|
| Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value | Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value |
| Age | Age | ||||
| 399.59 (−17,128.45–17,927.63) | 0.35 | 3383.94 (792.68–5975.20) | 0.06 | ||
| Age Group | Age Group | ||||
| 30–34 | ref | 30–34 | ref | ||
| 35–39 | 3276.83 (−19,103.00–25,656.65) | 35–39 | 198.85 (−2920.19–3317.89) | 0.74 | |
| 40–44 | 3898.05 (−10,752.98–18,549.08) | 0.26 | 40–44 | 1069.84 (−2049.20–4188.88) | |
| 45–49 | 3850.22 (−6158.34–13,858.78) | 45–49 | 1096.06 (−876.59–3068.72) | ||
| 50–54 | 7449.43 (−2910.41–17,809.28) | 50–54 | 1297.62 (−1249.06–3844.30) | ||
| 55–59 | 11,317.60 (3053.30–19,581.89) | 55–59 | 799.54 (−1173.11–2772.19) | ||
| 60–64 | 8637.14 (−3050.34–20,324.62) | 60–64 | 0 | ||
| 65–69 | 2074.50 (−8285.35–12,434.34) | 65–69 | 431.67 (−3979.32–4842.65) | ||
| 70–74 | 28,009.13 (14,304.35–41,713.92) | 70–74 | 287.74 (−2831.30–3406.78) | ||
| 75+ | 8181.52 (−9143.81–25,526.86) | 75+ | 0 | ||
| Vital Status | Vital Status | ||||
| Died | ref | Died | ref | ||
| Alive | 8607.07 (4545.54–12,668.60) | 0.95 | Alive | 1349.75 (325.61–2373.90) | 0.40 |
| Year | Year | ||||
| 2017–2019 | ref | 2017–2019 | ref | ||
| 2020–2021 | 5876.46 (176.19–11,576.73) | 0.21 | 2020–2021 | 1412.83 (227.30–2598.40) | 0.44 |
| Parish (Area of Residence) | Parish (Area of Residence) | ||||
| Other Parishes | ref | Other Parishes | ref | ||
| St. John | 6051.25 (933.02–11,169.48) | 0.15 | St. John | 770.21 (−216.62–1757.05) | 0.3 |
| Marital Status Known | Marital Status Known | ||||
| No | ref | No | ref | ||
| Yes | 11,385.07 (5275.76–17,494.39) | 0.24 | Yes | 1566.31 (536.60–2596.01) | 0.15 |
| Family History Status Known | Family History Status Known | ||||
| No | ref | No | ref | ||
| Yes | 11,461.39 (5025.85–17,896.92) | 0.27 | Yes | 1458.40 (370.81–2545.99) | 0.31 |
| Disease stage | Disease stage | ||||
| Early Stage | ref | Early Stage | ref | ||
| Late Stage | 10,954.39 (5586.12–16,322.67) | 0.21 | Late Stage | 703.97 (−344.54–1752.49) | 0.28 |
| Histological Grade | Histological Grade | ||||
| Grade 1 | ref | Grade 2 | ref | 0.46 | |
| Grade 2 | 6513.90 (1436.73–11,591.07) | 0.01 | Grade 3 | 535.62 (−1142.70–2213.93) | |
| Grade 3 | 27,034.13 (15,681.23–38,387.03) | Morphological Description | |||
| Not Stated | 5452.75 (−2966.79–13,872.29) | Adenocarcinoma | ref | ||
| Subtypes | Squamous Cell Carcinoma | 1148.45 (304.63–1992.27) | 0.60 | ||
| Luminal A/Luminal B | ref | Treatment Intention Status | |||
| Triple Negative Breast cancer/HERS/neu Enriched | 14,838.73 (7669.63–22,007.83) | 0.04 | Unknown | ref | |
| Estrogen Receptor Status | Known | 1455.78 (480.87–2430.69 | 0.20 | ||
| ER-ve | ref | Had Radiation Therapy | |||
| ER+ve | 6548.34 (2094.05–11,002.63) | 0.09 | No | ref | |
| Progesterone Receptor Status | Yes | 1166.08 (304.65–2027.51) | 0.58 | ||
| PR-ve | ref | Had Diabetes at Presentation | |||
| PR+ve | 7106.07 (2281.55–11,930.59) | 0.33 | No | ref | |
| HER2 Status | Yes | 5225.04 (2882.75–7567.34) | 0.001 | ||
| HER2-ve | ref | Had Hypertension at Presentation | |||
| HER2+ve | 24,188.26 (16,739.28–31,637.24) | <0.001 | No | ref | |
| Distant Metastases | Yes | 3575.78 (1822.01–5329.55) | 0.004 | ||
| Undetermined (Mx/Not Stated) | ref | Had Cardiovascular Disease at Presentation | |||
| Determined (M0/M1) | 6727.94 (1991.07–11,464.81) | 0.2 | No | ref | |
| Regional Lymph node Status | Yes | 4127.28 (2131.45–6123.12) | 0.003 | ||
| Undetermined (Nx/Not Stated) | ref | No. of Tracked Payments Made at Hospital | |||
| N0 (No lymph node metastases) | 4087.73 (−4094.99–12,270.45) | 0.43 | ≤10 | ref | |
| N1 (Metastases in 1 to 3 lymph nodes) | 13,040.15 (5875.40–20,204.90) | >10 | 1619.87 (600.49–2639.25) | 0.11 | |
| N2 (Metastases in 4 or more lymph nodes) | 8145.03 (−2343.09–18,633.16) | Employment Status at Presentation | |||
| Primary Tumour | Not Employed | ref | |||
| Undetermined (Tx/Not Stated) | ref | Employed | 1335.16 (456.76–2213.56) | 0.21 | |
| Determined (T1/T4) | 8893.40 (4492.67–13,294.12) | 0.76 | Estimated Monthly Income at Presentation (USDUSD) | ||
| Had some form of Surgery | ≤552 | ref | |||
| No | ref | >552 | 728.63 (−286.24–1743.50) | 0.28 | |
| Yes | 8989.76 (4844.66–13,134.86) | 0.59 | |||
| Hormonal Therapy Status Known | |||||
| No | ref | ||||
| Yes | 9417.73 (5000.00–13,835.55) | 0.44 | |||
| Radiation Therapy Status Known | |||||
| No | ref | ||||
| Yes | 9136.80 (4948.17–13,325.43) | 0.5 | |||
| Evidence of NCD other than cancer | |||||
| No | ref | ||||
| Yes | 9544.48 (4511.21–14,577.75) | 0.55 | |||
| No. of Tracked Payments Made at Hospital | |||||
| ≤10 | ref | ||||
| >10 | 6731.29 (1495.33–11,967.24) | 0.31 | |||
| Employment Status at Presentation | |||||
| Not Employed | ref | ||||
| Employed | 9297.71 (4959.79–13,635.62) | 0.48 | |||
| Estimated Monthly Income at Presentation (USDUSD) | |||||
| ≤552 | ref | ||||
| >552 | 7423.98 (3129.20–11,718.76) | 0.25 | |||
| Colorectal Cancer (n = 40) | Prostate Cancer (n = 70) | ||||
| Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value | Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value |
| Age | Age | ||||
| 19,546.84 (5294.02–42,791.06) | 0.006 | 1581.65 (15.60–6970.58) | 0.23 | ||
| Age Group | Age Group | ||||
| 30–34 | 0 | 30–34 | 0 | ||
| 35–39 | 0 | 35–39 | 0 | 0.50 | |
| 40–44 | ref | 0.06 | 40–44 | 0 | |
| 45–49 | 0 | 45–49 | 0 | ||
| 50–54 | 2104.06 (85.19–6809.55) | 50–54 | ref | ||
| 55–59 | 2559.35 (68.39–8632.27) | 55–59 | 3850.20 (2513.02–5471.56) | ||
| 60–64 | 4533.33 (1193.70–10,022.01) | 60–64 | 3984.13 (2669.79–5562.18) | ||
| 65–69 | 4014.49 (1402.50–7969.13) | 65–69 | 5172.49 (3793.33–6763.42) | ||
| 70–74 | 1164.17 (93.90–3428.10) | 70–74 | 3926.28 (2704.00–5375.82) | ||
| 75+ | 151.29 (185.23–1460.00 | 75+ | 5262.05 (3731.99–7054.32) | ||
| Vital Status | Vital Status | ||||
| Died | ref | Died | ref | ||
| Alive | 2211.82 (1153.28–3612.01) | 0.3 | Alive | 4273.24 (3607.20–4994.25) | 0.32 |
| Year | Year | ||||
| 2017–2019 | ref | 2017–2019 | ref | ||
| 2020–2021 | 2507.00 (1253.87–4189.97) | 0.21 | 2020–2021 | 3897.50 (3151.70–4722.44) | 0.06 |
| Parish (Area of Residence) | Parish (Area of Residence) | ||||
| Other Parishes | ref | Other Parishes | ref | ||
| St. John | 3123.69 (1486.10–5361.17) | 0.12 | St. John | 4087.04 (3310.85–4946.31) | 0.24 |
| Sex | Marital Status Known | ||||
| Female | ref | No | ref | ||
| Male | 2229.73 (796.93–4383.76) | 0.77 | Yes | 4243.22 (3479.82–5082.26) | 0.50 |
| Disease stage | Family History Status Known | ||||
| Early Stage | ref | No | ref | ||
| Late Stage | 3260.41 (1635.39–5440.54) | 0.04 | Yes | 4113.94 (3443.34–4842.77) | 0.08 |
| Histological Grade | Disease stage | ||||
| Grade 1 | ref | Early Stage | ref | ||
| Grade 2 | 1825.00 (765.63–3337.37) | Late Stage | 4902.80 (4036.06–5853.78) | 0.10 | |
| Grade 3 | 2632.72 (48.72–12,009.97) | 0.92 | PSA Level (ng/mL) | ||
| Not Stated | 2573.53 (291.73–7119.98) | Not Stated | ref | ||
| Tumour Site | ≤20 | 3003.04 (2025.90–4170.58) | 0.01 | ||
| Ascending/Transverse/Descending Colon | ref | >20 | 5070.86 (4290.25–5916.69) | ||
| Sigmoid colon/Rectum | 2557.32 (1179.92–4460.90) | 0.29 | Gleason Score | ||
| Greatest Dimensions (cm) | Not Stated | ref | |||
| ≤5 cm | ref | 6–7 | 4435.56 (3547.39–5422.85) | 0.93 | |
| >5 cm | 3620.43 (1369.00–6945.56) | 0.02 | 8–10 | 4522.56 (3382.59–5827.80) | |
| Not Stated | 732.24 (105.06–1924.58) | Primary Tumour Status | |||
| Primary Tumour Status | Undetermined | ref | |||
| Undetermined | ref | Determined | 5201.29 (4076.82–6462.55) | 0.09 | |
| Determined | 2010.63 (961.62–3442.17) | 0.90 | Lymph Node Status | ||
| Lymph Node Status | Undetermined | ref | |||
| Undetermined | ref | Determined | 5082.26 (3890.02–6433.64) | 0.19 | |
| Determined | 2010.63 (961.62–3442.17) | 0.90 | Distant Metastases | ||
| Distant Metastases | Undetermined | ref | |||
| Undetermined | ref | Determined | 5949.04 (4604.98–7466.69) | 0.01 | |
| Determined | 2010.63 (961.62–3442.17) | 0.90 | Had Radiation Therapy | ||
| Radiation Therapy Status Known | No | ref | |||
| No | ref | Yes | 4243.22 (3479.82–5082.26) | 0.50 | |
| Yes | 2452.23 (1288.81–3986.66) | 0.13 | Had Diabetes at Presentation | ||
| Had Hypertension at Presentation | No | ref | |||
| No | ref | Yes | 3951.38 (2480.04–5762.33) | 0.56 | |
| Yes | 1126.27 (157.00–2980.07) | 0.17 | Had Hypertension at Presentation | ||
| Had Diabetes at Presentation | No | ref | |||
| No | ref | Yes | 4174.36 (3042.63–5421.38) | 0.61 | |
| Yes | 3451.56 (685.39–8339.34) | 0.37 | Had Cardiovascular Disease | ||
| Had Cardiovascular Disease | No | ref | |||
| No | ref | Yes | 3794.56 (2562.38–5267.86) | 0.33 | |
| Yes | 574.08 (12.04–2640.93) | 0.09 | Evidence of NCD other than cancer | ||
| Evidence of NCD other than cancer | No | ref | |||
| No | ref | Yes | 4071.72 (3088.02–5191.20) | 0.44 | |
| Yes | 1846.42 (802.02–3321.22) | 0.55 | No. of Tracked Payments Made at Hospital | ||
| No. of Tracked Payments Made at Hospital | ≤10 | ref | |||
| ≤10 | ref | >10 | 4395.69 (3539.06–5343.61) | 0.97 | |
| >10 | 1143.79 (340.40–2419.66) | 0.02 | Employment Status at Presentation | ||
| Employment Status at Presentation | Not Employed | ref | |||
| Not Employed | ref | Employed | 4522.56 (3740.55–5378.76) | 0.63 | |
| Employed | 2668.76 (1147.85–4821.91) | 0.30 | Estimated Monthly Income at Presentation (USDUSD) | ||
| Estimated Monthly Income at Presentation (USDUSD) | ≤552 | ref | |||
| ≤552 | ref | >552 | 4542.76 (3496.36–5725.95) | 0.76 | |
| >552 | 2485.02 (887.44–4888.81) | 0.55 | |||
| Female Breast Cancer (n = 105) | Cervical Cancer (n = 27) | ||||
|---|---|---|---|---|---|
| Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value | Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value |
| Disease stage | Age | ||||
| Early Stage | ref | 1535.59 (−597.34–3668.51) | 0.15 | ||
| Late Stage | 11,121.21 (−8503.13–30,745.55) | 0.26 | Had Diabetes at Presentation | ||
| Histological Grade | No | ref | |||
| Grade 1 | ref | Yes | 4322.97 (1724.10–6921.84) | 0.002 | |
| Grade 2 | 10,077.35 (−9001.68–29,156.38) | 0.30 | Had Hypertension at Presentation | ||
| Grade 3 | 26,282.45 (2701.79–49,863.11) | 0.03 | No | ref | |
| Not Stated | 6295.25 (−14,233.99–26,824.48) | 0.54 | Yes | 3080.04 (350.91–5817.17) | 0.03 |
| Subtypes | Had Cardiovascular Disease at Presentation | ||||
| Luminal A/Luminal B | ref | No | ref | ||
| Triple Negative Breast cancer/HERS/neu Enriched | 6144.21 (−4892.85–17,181.26) | 0.27 | Yes | 3185.53 (144.36–6226.70) | 0.04 |
| Estrogen Receptor Status | |||||
| ER-ve | ref | ||||
| ER+ve | 2208.67 (−6370.92–10,788.25) | 0.61 | |||
| HER2 Status | |||||
| HER2-ve | ref | ||||
| HER2+ve | 29,283.06 (6299.87–52,266.25) | 0.01 | |||
| Colorectal Cancer (n = 40) | Prostate Cancer (n = 70) | ||||
| Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value | Characteristic | Mean Drug Treatment Cost (95% CI) * | F-Test p value |
| Age | Age | ||||
| 13,931.08 (2488.01–34,662.99) | 0.001 | 1709.00 (21.34–7621.29) | 0.07 | ||
| Disease stage | Year | ||||
| Early Stage | ref | 2017–2019 | ref | ||
| Late Stage | 16,594.59 (3671.15–39,037.86) | 0.001 | 2020–2021 | 1366.78 (94.48–6999.00) | 0.12 |
| Greatest Dimensions (cm) | Family History Status Known | ||||
| ≤5 cm | ref | No | ref | ||
| >5 cm | 10,455.06 (1293.84–28,402.36) | 0.004 | Yes | 1047.17 (198.81–6211.02) | 0.17 |
| Not Stated | 8047.88 (414.12–25,303.27) | 0.013 | Disease stage | ||
| Radiation Therapy Status Known | Early Stage | ref | |||
| No | ref | Late Stage | 1817.32 (4.45–7631.77) | 0.06 | |
| Yes | 20,471.89 (5858.37–43,940.54) | <0.001 | PSA Level (ng/mL) | ||
| Had Cardiovascular Disease | Not Stated | ref | |||
| No | ref | ≤20 | 1619.26 (6.50–6892.32) | 0.07 | |
| Yes | 8460.32 (346.70–27,340.62) | 0.016 | >20 | 2735.29 (60.37–9377.99) | 0.02 |
| No. of Tracked Payments Made at Hospital | Distant Metastases | ||||
| ≤10 | ref | Undetermined | ref | ||
| >10 | 11,006.11 (1023.36–31,623.51) | 0.01 | Determined | 2758.35 (28.62–9938.10) | 0.03 |
| Female Breast Cancer (n = 105) | Cervical Cancer (n = 27) | ||||
|---|---|---|---|---|---|
| Characteristic | Model Standard Error | Robust Standard Error | Characteristic | Model Standard Error | Robust Standard Error |
| Disease stage | Age | ||||
| Early Stage | - | 18.56 | 9.27 | ||
| Late Stage | 3593.37 | 1807.48 | Had Diabetes at Presentation | ||
| Histological Grade | No | - | |||
| Grade 1 | - | Yes | 1174.79 | 2660.56 | |
| Grade 2 | 4693.45 | 1831.29 | Had Hypertension at Presentation | ||
| Grade 3 | 7027.63 | 13,382.11 | No | - | |
| Not Stated | 5698.03 | 2454.33 | Yes | 887.57 | 853.13 |
| Subtypes | Had Cardiovascular Disease at Presentation | ||||
| Luminal A/Luminal B | - | No | - | ||
| Triple Negative Breast cancer/HERS/neu Enriched | 9544.80 | 9067.89 | Yes | 1028.57 | 1314.68 |
| Estrogen Receptor Status | |||||
| ER-ve | - | ||||
| ER+ve | 9338.75 | 10,809.07 | |||
| HER2 Status | |||||
| HER2-ve | - | ||||
| HER2+ve | 4357.17 | 6356.60 | |||
| Colorectal Cancer (n = 40) | Prostate Cancer (n = 70) | ||||
| Characteristic | Model Standard Error | Robust Standard Error | Characteristic | Model Standard Error | Robust Standard Error |
| Age | Age | ||||
| 0.46 | 0.37 | 0.31 | 0.30 | ||
| Disease stage | Year | ||||
| Early Stage | - | 2017–2019 | - | ||
| Late Stage | 11.12 | 11.76 | 2020–2021 | 4.94 | 5.70 |
| Greatest Dimensions (cm) | Family History Status Known | ||||
| ≤5 cm | - | No | - | ||
| >5 cm | 15.54 | 16.70 | Yes | 5.84 | 6.24 |
| Not Stated | 12.58 | 11.19 | Disease stage | ||
| Radiation Therapy Status Known | Early Stage | - | |||
| No | - | Late Stage | 5.32 | 6.20 | |
| Yes | 13.10 | 7.80 | PSA Level (ng/mL) | ||
| Had Cardiovascular Disease | Not Stated | - | |||
| No | - | ≤20 | 9.12 | 7.81 | |
| Yes | 13.18 | 12.21 | >20 | 7.62 | 6.46 |
| No. of Tracked Payments Made at Hospital | Distant Metastases | ||||
| ≤10 | - | Undetermined | - | ||
| >10 | 11.57 | 14.26 | Determined | 5.40 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bovell, A.A.N.; Ncayiyana, J.; Ginindza, T.G. Investigating Drug Treatment Costs and Patient Characteristics of Female Breast, Cervical, Colorectal, and Prostate Cancers in Antigua and Barbuda: A Retrospective Data Study (2017–2021). Int. J. Environ. Res. Public Health 2025, 22, 930. https://doi.org/10.3390/ijerph22060930
Bovell AAN, Ncayiyana J, Ginindza TG. Investigating Drug Treatment Costs and Patient Characteristics of Female Breast, Cervical, Colorectal, and Prostate Cancers in Antigua and Barbuda: A Retrospective Data Study (2017–2021). International Journal of Environmental Research and Public Health. 2025; 22(6):930. https://doi.org/10.3390/ijerph22060930
Chicago/Turabian StyleBovell, Andre A. N., Jabulani Ncayiyana, and Themba G. Ginindza. 2025. "Investigating Drug Treatment Costs and Patient Characteristics of Female Breast, Cervical, Colorectal, and Prostate Cancers in Antigua and Barbuda: A Retrospective Data Study (2017–2021)" International Journal of Environmental Research and Public Health 22, no. 6: 930. https://doi.org/10.3390/ijerph22060930
APA StyleBovell, A. A. N., Ncayiyana, J., & Ginindza, T. G. (2025). Investigating Drug Treatment Costs and Patient Characteristics of Female Breast, Cervical, Colorectal, and Prostate Cancers in Antigua and Barbuda: A Retrospective Data Study (2017–2021). International Journal of Environmental Research and Public Health, 22(6), 930. https://doi.org/10.3390/ijerph22060930









