Compensation for Patients with Work-Related Lung Cancers: Value of Specialised Occupational Disease Consultations to Reduce Under-Recognition
Abstract
1. Introduction
2. Literature Review
2.1. Lung Cancer Due to Occupational Exposures
2.2. Compensation Measures Provided in France and Across the Globe for Patients Presenting with LC
2.3. Screening Procedures for Occupational Lung Cancer
3. Material and Methods
3.1. Study Design and Ethical Considerations
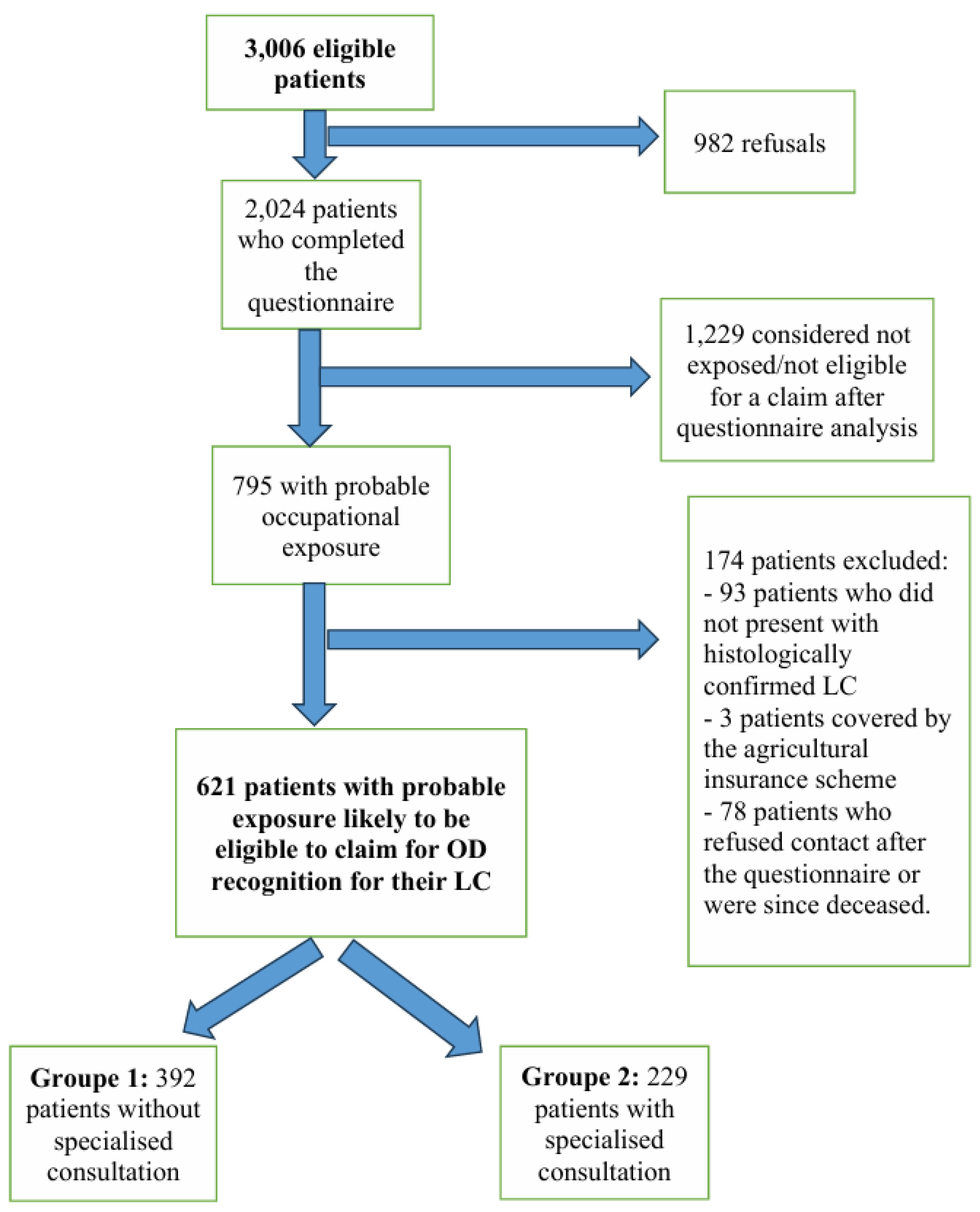
3.2. Study Population
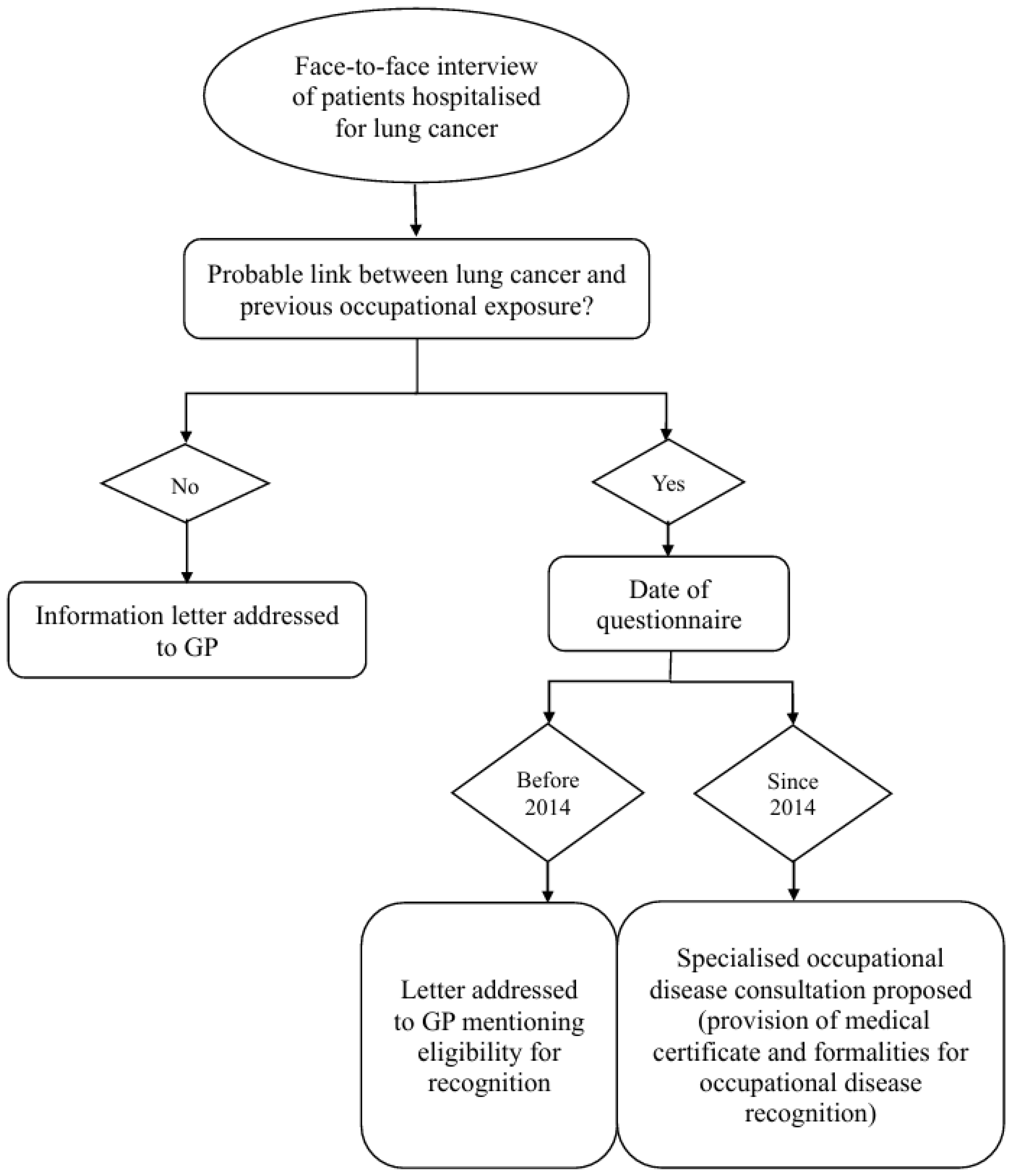
3.3. Systematic Screening Procedure
3.4. Data Collection
- patient socio-demographic data (date of birth, sex)
- histological cancer type
- smoking status
- exposure to the most relevant lung carcinogen for the OD recognition process (since, in France, only one carcinogen can be retained for this procedure)
- recognition as an OD: on the grounds of an OD table for a given carcinogen, or on the grounds of an ‘unlisted’ disease in the absence of an OD table for the carcinogen in question
3.5. Statistical Analysis Method
4. Results
- Group 1: 392 patients who did not benefit from a specialised OD consultation but for whom a letter was addressed to their general practitioner with advice on OD reporting;
- Group 2: 229 subjects who did benefit from a specialised OD consultation and for whom a medical certificate for an OD claim was provided at the end of the consultation.
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | lung Cancer |
| OD | occupational Disease |
| CI | confidence interval |
| OR | odds ratio |
| IARC | international agency for research on cancer |
| SD | standard deviation |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 3 June 2025).
- Lapôtre-Ledoux, B.; Remontet, L.; Uhry, Z.; Dantony, E.; Grosclaude, P.; Molinié, F.; Woronoff, A.-S.; Lecoffre-Bernard, C.; Lafay, L.; Defossez, G.; et al. Main Cancers Incidence in Metropolitan France in 2023 and Trends since 1990. Bull. Epidemiol. Hebd. 2023, 12–13, 188–204. [Google Scholar]
- Yang, X.; Man, J.; Chen, H.; Zhang, T.; Yin, X.; He, Q.; Lu, M. Temporal Trends of the Lung Cancer Mortality Attributable to Smoking from 1990 to 2017: A Global, Regional and National Analysis. Lung Cancer 2021, 152, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Autier, P.; Boniol, M.; Boyle, P.; Hill, C.; Aurengo, A.; Masse, R.; de Thé, G.; Valleron, A.-J.; Monier, R.; et al. An Estimate of Cancers Attributable to Occupational Exposures in France. J. Occup. Environ. Med. 2010, 52, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Marant Micallef, C.; Shield, K.D.; Vignat, J.; Baldi, I.; Charbotel, B.; Fervers, B.; Gilg Soit Ilg, A.; Guénel, P.; Olsson, A.; Rushton, L.; et al. Cancers in France in 2015 Attributable to Occupational Exposures. Int. J. Hyg. Environ. Health 2019, 222, 22–29. [Google Scholar] [CrossRef]
- C155—Occupational Safety and Health Convention, 1981 (No. 155)|International Labour Organization. Available online: https://www.ilo.org/resource/c155-occupational-safety-and-health-convention-1981-no-155 (accessed on 3 June 2025).
- Over 37,000 Occupational Cancer Cases Recognised in the EU|Safety and Health at Work EU-OSHA. Available online: https://osha.europa.eu/en/oshnews/over-37000-occupational-cancer-cases-recognised-eu (accessed on 3 June 2025).
- Marant Micallef, C.; Charvat, H.; Houot, M.-T.; Vignat, J.; Straif, K.; Paul, A.; El Yamani, M.; Pilorget, C.; Soerjomataram, I. Estimated Number of Cancers Attributable to Occupational Exposures in France in 2017: An Update Using a New Method for Improved Estimates. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 125–131. [Google Scholar] [CrossRef]
- Rapport Annuel 2022 de L’Assurance Maladie—Risques Professionnels|L’Assurance Maladie. Available online: https://www.assurance-maladie.ameli.fr/etudes-et-donnees/2022-rapport-annuel-assurance-maladie-risques-professionnels (accessed on 10 June 2024).
- Gislard, A.; Gramond, C.; Clin, B.; Paris, C.; Delva, F.; Brochard, P.; Laurent, F.; Benoist, J.; Andujar, P.; Chouaïd, C.; et al. Compensation of occupational diseases during monitoring of the ARDCO cohort. Rev. Mal. Respir. 2024, 41, 472–487. [Google Scholar] [CrossRef]
- Morelle, I.; Berghmans, T.; CsToth, I.; Sculier, J.-P.; Meert, A.-P. Identification of occupational exposure in thoracic oncology: A Belgian experience. Rev. Mal. Respir. 2014, 31, 221–229. [Google Scholar] [CrossRef]
- Orriols, R.; Isidro, I.; Abu-Shams, K.; Costa, R.; Boldu, J.; Rego, G.; Zock, J.-P.; Other Members of the Enfermedades Respiratorias Ocupacionales y Medioambientales (EROM) Group. Reported Occupational Respiratory Diseases in Three Spanish Regions. Am. J. Ind. Med. 2010, 53, 922–930. [Google Scholar] [CrossRef]
- De Lamberterie, G.; Maître, A.; Goux, S.; Brambilla, C.; Perdrix, A. How do we reduce the under-reporting of occupational primary lung cancer. Rev. Mal. Respir. 2002, 19 Pt 1, 190–195. [Google Scholar]
- Cellier, C.; Charbotel, B.; Carretier, J.; Rebattu, P.; Fayette, J.; Pérol, M.; Claude, L.; Philip, T.; Fervers, B. Identification of occupational exposures among patients with lung cancer. Bull. Cancer 2013, 100, 661–670. [Google Scholar] [CrossRef]
- Varin, M.; Charbotel, B.; Pérol, O.; Perrier, L.; Massardier-Pilonchéry, A.; Bonnand, S.; Belladame, E.; Fort, E.; Avrillon, V.; Rebattu, P.; et al. Assessment of a self-administered questionnaire identifying occupational exposures among lung cancer patients. Bull. Cancer 2017, 104, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Pérol, O.; Charbotel, B.; Perrier, L.; Bonnand, S.; Belladame, E.; Avrillon, V.; Rebattu, P.; Gomez, F.; Lauridant, G.; Pérol, M.; et al. Systematic Screening for Occupational Exposures in Lung Cancer Patients: A Prospective French Cohort. Int. J. Environ. Res. Public Health 2018, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Pérol, O.; Lepage, N.; Noelle, H.; Lebailly, P.; de Labrusse, B.; Clin, B.; Boulanger, M.; Praud, D.; Fournié, F.; Galvaing, G.; et al. A Multicenter Study to Assess a Systematic Screening of Occupational Exposures in Lung Cancer Patients. Int. J. Environ. Res. Public Health 2023, 20, 5068. [Google Scholar] [CrossRef] [PubMed]
- Curti, S.; Sauni, R.; Spreeuwers, D.; De Schryver, A.; Valenty, M.; Rivière, S.; Mattioli, S. Interventions to Increase the Reporting of Occupational Diseases by Physicians: A Cochrane Systematic Review. Occup. Environ. Med. 2016, 73, 353–354. [Google Scholar] [CrossRef]
- Loomis, D.; Guha, N.; Hall, A.L.; Straif, K. Identifying Occupational Carcinogens: An Update from the IARC Monographs. Occup. Environ. Med. 2018, 75, 593–603. [Google Scholar] [CrossRef]
- Nenonen, N.; Hämäläinen, P.; Takala, J.; Saarela, K.L.; Lim, S.L.; Lim, G.K.; Manickam, K. Global Estimates of Occupational Accidents and Fatal Work-Related Diseases in 2014 Based on 2010 and 2011 Data; ILO: Geneva, Switzerland, 2006. [Google Scholar]
- Takala, J.; Hämäläinen, P.; Saarela, K.L.; Yun, L.Y.; Manickam, K.; Jin, T.W.; Heng, P.; Tjong, C.; Kheng, L.G.; Lim, S.; et al. Global Estimates of the Burden of Injury and Illness at Work in 2012. J. Occup. Environ. Hyg. 2014, 11, 326–337. [Google Scholar] [CrossRef]
- GBD 2016 Occupational Carcinogens Collaborators. Global and Regional Burden of Cancer in 2016 Arising from Occupational Exposure to Selected Carcinogens: A Systematic Analysis for the Global Burden of Disease Study 2016. Occup. Environ. Med. 2020, 77, 151–159. [Google Scholar] [CrossRef]
- GBD 2017 Risk Factor Collaborators. Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Zou, B.; Wu, P.; Chen, J.; Luo, J.; Lei, Y.; Luo, Q.; Zhu, B.; Zhou, M. The Global Burden of Cancers Attributable to Occupational Factors, 1990–2021. BMC Cancer 2025, 25, 503. [Google Scholar] [CrossRef]
- Clin, B.; Ferrant, O.; Marquignon, M.-F.; Letourneux, M. Comparative Analysis of Medicolegal Compensation for Occupational Cancers in France and Other Western European Countries: Development Proposals. Med. Law 2009, 28, 615–636. [Google Scholar]
- EUROGIP. Incidence and Detection of Occupational Cancers in Nine European Countries Germany, Austria, Belgium, Denmark, Finland, France, Italy, Sweden and Switzerland; 141E; Eurogip Report: Paris, France, 2018. [Google Scholar]
- Spreeuwers, D.; de Boer, A.G.E.M.; Verbeek, J.H.A.M.; van Dijk, F.J.H. Evaluation of Occupational Disease Surveillance in Six EU Countries. Occup. Med. 2010, 60, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.P.; Marcin, J.P. Workers’ Compensation Benefits and Shifting Costs for Occupational Injury and Illness. J. Occup. Environ. Med. 2012, 54, 445–450. [Google Scholar] [CrossRef] [PubMed]
- FIVA—Indemnisation Pour les Victimes de L’Amiante. FIVA. Available online: https://www.fiva.fr/ (accessed on 4 June 2025).
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Bach, P.B.; Mirkin, J.N.; Oliver, T.K.; Azzoli, C.G.; Berry, D.A.; Brawley, O.W.; Byers, T.; Colditz, G.A.; Gould, M.K.; Jett, J.R.; et al. Benefits and Harms of CT Screening for Lung Cancer: A Systematic Review. JAMA 2012, 307, 2418–2429. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Mazzone, P.J.; Naidich, D.P.; Bach, P.B. Screening for Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143 (Suppl. S5), e78S–e92S. [Google Scholar] [CrossRef]
- Roberts, H.; Walker-Dilks, C.; Sivjee, K.; Ung, Y.; Yasufuku, K.; Hey, A.; Lewis, N.; Lung Cancer Screening Guideline Development Group. Screening High-Risk Populations for Lung Cancer: Guideline Recommendations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2013, 8, 1232–1237. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Crinò, L.; Dooms, C.; Douillard, J.Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; Van Schil, P.; Veronesi, G.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Early-Stage Non-Small-Cell Lung Cancer Consensus on Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1462–1474. [Google Scholar] [CrossRef]
- Wender, R.; Fontham, E.T.H.; Barrera, E.; Colditz, G.A.; Church, T.R.; Ettinger, D.S.; Etzioni, R.; Flowers, C.R.; Gazelle, G.S.; Kelsey, D.K.; et al. American Cancer Society Lung Cancer Screening Guidelines. CA. Cancer J. Clin. 2013, 63, 107–117. [Google Scholar] [CrossRef]
- Haute Autorité de Santé. Suivi Post-Professionnel Après Exposition à L’Amiante—Audition Publique. 2011. Available online: https://www.has-sante.fr/jcms/c_935546/fr/suivi-post-professionnel-apres-exposition-a-l-amiante (accessed on 18 December 2023).
- Delva, F.; Margery, J.; Laurent, F.; Petitprez, K.; Pairon, J.-C.; RecoCancerProf Working Group. Medical Follow-up of Workers Exposed to Lung Carcinogens: French Evidence-Based and Pragmatic Recommendations. BMC Public Health 2017, 17, 191. [Google Scholar] [CrossRef][Green Version]
- Delva, F.; Laurent, F.; Paris, C.; Belacel, M.; Brochard, P.; Bylicki, O.; Chouaïd, C.; Clin, B.; Dewitte, J.-D.; Le Denmat, V.; et al. LUCSO-1-French Pilot Study of LUng Cancer Screening with Low-Dose Computed Tomography in a Smokers Population Exposed to Occupational Lung Carcinogens: Study Protocol. BMJ Open 2019, 9, e025026. [Google Scholar] [CrossRef]
- Britel, M.; Pérol, O.; Blois Da Conceiçao, S.; Ficty, M.; Brunet, H.; Avrillon, V.; Charbotel, B.; Fervers, B. Motivations and obstacles to occupational disease claims in lung cancer patients: An exploratory psychosocial study. Sante Publique 2017, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Porru, S.; di Carlo, A.S.; Placidi, D.; Arici, C.; Tassi, G.; Alessio, L. Occupational cancer. The role of the occupational physician in systematic search and aetiological diagnosis of lung cancer. Analysis of a case list. Med. Lav. 2006, 97, 565–580. [Google Scholar] [PubMed]
- Porru, S.; Carta, A.; Toninelli, E.; Bozzola, G.; Arici, C. Reducing the Underreporting of Lung Cancer Attributable to Occupation: Outcomes from a Hospital-Based Systematic Search in Northern Italy. Int. Arch. Occup. Environ. Health 2016, 89, 981–989. [Google Scholar] [CrossRef]
- Chiriac, C.-F.; Gavriliţă, L. When contronted with a new lung cancer case, are we often thinking of and searching for an occupational etiology? Pneumologia 2002, 51, 272–276. [Google Scholar]
- Del Bianco, A.; Demers, P.A. Trends in Compensation for Deaths from Occupational Cancer in Canada: A Descriptive Study. CMAJ Open 2013, 1, E91–E96. [Google Scholar] [CrossRef][Green Version]
- Markowitz, S.; Ringen, K.; Dement, J.M.; Straif, K.; Christine Oliver, L.; Algranti, E.; Nowak, D.; Ehrlich, R.; McDiarmid, M.A.; Miller, A.; et al. Occupational Lung Cancer Screening: A Collegium Ramazzini Statement. Am. J. Ind. Med. 2024, 67, 289–303. [Google Scholar] [CrossRef]
- Doria-Rose, V.P.; Silvestri, G.A.; Durham, D.D.; Connor, P.; Goldman, L.; Enewold, L.; Farjah, F.; Miller, E.A.; Simanowith, M.; Smith, R.A.; et al. The United States’ Early Experience With Lung Cancer Screening-Creation of a National Data Linkage: A Brief Report. JTO Clin. Res. Rep. 2025, 6, 100825. [Google Scholar] [CrossRef]
- Kistnasamy, B.; Yassi, A.; Yu, J.; Spiegel, S.J.; Fourie, A.; Barker, S.; Spiegel, J.M. Tackling Injustices of Occupational Lung Disease Acquired in South African Mines: Recent Developments and Ongoing Challenges. Glob. Health 2018, 14, 60. [Google Scholar] [CrossRef]
- Wild, P.; Gonzalez, M.; Bourgkard, E.; Courouble, N.; Clément-Duchêne, C.; Martinet, Y.; Févotte, J.; Paris, C. Occupational Risk Factors Have to Be Considered in the Definition of High-Risk Lung Cancer Populations. Br. J. Cancer 2012, 106, 1346–1352. [Google Scholar] [CrossRef]
- Verger, P.; Arnaud, S.; Ferrer, S.; Iarmarcovai, G.; Saliba, M.-L.; Viau, A.; Souville, M. Inequities in Reporting Asbestos-Related Lung Cancer: Influence of Smoking Stigma and Physician’s Specialty, Workload and Role Perception. Occup. Environ. Med. 2008, 65, 392–397. [Google Scholar] [CrossRef]
- Viau, A.; Arnaud, S.; Ferrer, S.; Iarmacovai, G.; Saliba, M.-L.; Souville, M.; Verger, P. Factors associated with physicians’ under-reporting of asbestos-related bronchopulmonary cancers. Telephone survey conducted among general practitioners and pulmonologists randomly selected in the French region of Provence-Alpes-Côte-d’Azur. Rev. Prat. 2008, 58 (Suppl. S19), 9–16. [Google Scholar] [PubMed]
- Gisquet, E.; Chamming’s, S.; Pairon, J.-C.; Gilg Soit Ilg, A.; Imbernon, E.; Goldberg, M. The determinants of under-reporting occupational diseases. The case of mesothelioma. Rev. D’Epidemiol. Sante Publique 2011, 59, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Thébaud-Mony, A. Construire la visibilité des cancers professionnels:Une enquête permanente en Seine-Saint-Denis. Rev. Française Aff. Soc. 2008, 2, 237–254. [Google Scholar] [CrossRef]
| Total Population (N = 621) | |
|---|---|
| Sex | |
| Men: n (%) | 608 (97.9%) |
| Women: n (%) | 13 (2.1%) |
| Mean age at completion of the questionnaire (years) | 64 (±8) |
| Carcinogenic agent: n (%) | |
| Asbestos | 521 (84%) |
| Silica | 17 (2.7%) |
| Welding fumes | 17 (2.7%) |
| Chromium | 5 (0.8%) |
| Iron | 5 (0.8%) |
| Coal tar | 9 (1.4%) |
| Polycyclic aromatic hydrocarbons from petroleum derivatives | 4 (0.6%) |
| Polycyclic aromatic hydrocarbons from coal combustion soot | 3 (0.5%) |
| Polycyclic aromatic hydrocarbons from cutting oils | 3 (0.5%) |
| Diesel engine fumes | 16 (2.6%) |
| Painting trade | 15 (2.4%) |
| Passive smoking | 3 (0.5%) |
| Ionising radiation | 2 (0.3%) |
| Cobalt dust | 1 (0.2%) |
| Claim filed for occupation disease recognition | |
| Yes | 202 (32.6%) |
| No | 419 (67.4%) |
| Specialised occupational disease consultation | |
| Yes | 229 (36.9%) |
| No | 392 (63.1%) |
| Smoking status | |
| Non-smoker | 22 (3.6%) |
| Former smoker (smokefree) | 410 (66%) |
| Active smoker | 178 (28.7%) |
| Unknown | 11 (1.8%) |
| Histology | |
| Adenocarcinoma | 326 (52.5%) |
| Epidermoid carcinoma | 168 (27.0%) |
| Large cell undifferentiated carcinoma | 45 (7.3%) |
| Adenosquamous carcinoma | 7 (1.1%) |
| Sarcomatoid carcinoma | 2 (0.3%) |
| Neuro-endocrine tumour | 57 (9.2%) |
| Typical or atypical carcinoid tumour | 10 (1.6%) |
| Other (pleiomorphic, small cell anaplasic) | 2 (0.3%) |
| Unknown | 4 (0.7%) |
| Group 1 (Patients Without Specialised Consultation) | Group 2 (Patients with Specialised Consultation) | p-Value (Group 1/Group 2) | |
|---|---|---|---|
| N = 392 | N = 229 | ||
| Sex | |||
| Men: n (%) | 385 (98.2%) | 223 (97.4%) | 0.564 |
| Women: n (%) | 7 (1.8%) | 6 (2.6%) | |
| Age at study (years) | 63 (±9) | 65 (±7) | 0.00038 |
| Carcinogenic agent from MPI * table: n (%) | NA | ||
| Chrome | 4 (1.0%) | 2 (0.9%) | |
| Coal tar | 9 (2.3%) | 3 (1.3%) | |
| Crystalline silica | 14 (3.6%) | 1 (0.4%) | |
| Asbestos | 309 (78.8%) | 212 (92.6%) | |
| Radon in iron ore mines | 5 (1.3%) | 0 (0) | |
| Ionising radiation | 0 (0) | 1 (0.4%) | |
| Cobalt | 0 (0) | 1 (0.4%) | |
| Other (welding fumes, diesel engine fumes, hydrocarbons, painting trade) | 51 (13.0%) | 9 (4.0%) | |
| Claim filed for occupational disease recognition | |||
| Yes | 45 (11.5) | 157 (68.5) | 2.87.10−48 |
| No | 347 (88.5) | 73 (31.9) | |
| Smoking status | |||
| Non-smoker | 10 (2.5%) | 13 (5.7%) | 8.9.10−14 |
| Former smoker (smokefree) | 222 (56.7%) | 187 (81.6%) | |
| Active smoker | 148 (37.7%) | 29 (12.7%) | |
| Unknown | 12 (3.1%) | 0 (0) | |
| Histology | NA ** | ||
| Adenocarcinoma | 194 (49.4%) | 132 (57.6%) | |
| Epidermoid carcinoma | 112 (28.6%) | 56 (24.5%) | |
| Large cell undifferentiated carcinoma | 35 (9.0%) | 10 (4.4%) | |
| Adenosquamous carcinoma | 4 (1.0%) | 3 (1.3%) | |
| Sarcomatoid carcinoma | 2 (0.5%) | 0 (0) | |
| Neuro-endocrine tumour | 35 (9.0%) | 22 (9.6%) | |
| Typical or atypical carcinoid tumour | 4 (1.0%) | 6 (2.6%) | |
| Other (pleiomorphic, small cell anaplasic) | 2 (0.5%) | 0 (0) | |
| Unknown | 4 (1.0%) | 0 (0) |
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| OR [95% CI] | p-Value | OR [95% CI] | p-Value | |
| Sex Women vs. Men | 1.43 [0.48–4.32] | 0.360 | - | |
| Age at study (years) | 1.03 [1.01–1.05] | 0.034 | 1.05 [1.02–1.07] | <0.001 |
| Claim filed for ODR 1 Yes vs. No | 16.32 [10.73–24.82] | <0.001 | 18.13 [11.47–28.64] | <0.001 |
| Smoking status | <0.001 | <0.001 | ||
| Non-smoker vs. Active smoker | 6.63 [2.66–16.57] | <0.001 | 4.90 [1.54–15.53] | 0.007 |
| Former smoker vs. Active smoker | 4.30 [2.76–6.70] | <0.001 | 3.08 [1.79–5.28] | <0.001 |
| Subjects Who Did Not File an Occupational Disease Recognition Claim | Subjects Who Filed an Occupational Disease Recognition Claim | p-Value | |
|---|---|---|---|
| Number of subjects | 419 | 202 | |
| Sex | |||
| Men (n%) | 413 (98.6%) | 195 (96.5%) | 0.132 |
| Women (n%) | 6 (1.4%) | 7 (3.5%) | |
| Mean age at time of claim (years) | 64 (±9) | 63 (±8) | 0.34 |
| Carcinogenic agent: n (%) | NA * | ||
| Chromium | 3 (0.7%) | 3 (1.5%) | |
| Coal tar | 9 (2.2%) | 3 (1.5%) | |
| Crystaline silica | 12 (2.9%) | 3 (1.5%) | |
| Asbestos | 341 (81.3%) | 180 (89.1%) | |
| Radon in iron ore mines | 4 (1.0%) | 1 (0.5%) | |
| Ionising radiation | 1 (0.2%) | 0 (0) | |
| Cobalt | 0 (0) | 1 (0.5%) | |
| Other (welding fumes, diesel engine fumes, hydrocarbons, painting trade) | 49 (11.7%) | 11 (5.4%) | |
| Specialised occupational disease consultation | p < 0.01 | ||
| Yes before 2014 | 5 (1.2%) | 6 (3.0%) | |
| Yes since 2014 | 67 (16.0%) | 151 (74.7%) | |
| No before 2014 | 289 (69.0%) | 28 (13.9%) | |
| No since 2014 | 58 (13.8%) | 17 (8.4%) | |
| Smoking status | |||
| Non-smoker | 13 (3.1%) | 10 (5.0%) | 2.8.10−5 |
| Former smoker (smokefree) | 254 (60.6%) | 155 (76.7%) | |
| Active smoker | 142 (33.9%) | 35 (17.3%) | |
| Unknown | 10 (2.4%) | 2 (1.0%) | |
| Histology | NA * | ||
| Adenocarcinoma | 203 (48.4%) | 123 (60.9%) | |
| Epidermoid carcinoma | 124 (29.6%) | 44 (21.8%) | |
| Large cell undifferentiated carcinoma | 37 (8.8%) | 8 (3.9%) | |
| Adenosquamous carcinoma | 4 (1.0%) | 3 (1,5%) | |
| Sarcomatoid carcinoma | 2 (0.5%) | 0 (0) | |
| Neuro-endocrine tumour | 37 (8.8%) | 20 (9.9%) | |
| Typical or atypical carcinoid tumour | 7 (1.7%) | 3 (1.5%) | |
| Other (pleiomorphic, small cell anaplasic) | 2 (0.5%) | 0 (0) | |
| Unknown | 3 (0.7%) | 1 (0.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roux, C.; Fafin-Lefevre, M.; Morello, R.; Boullard, L.; Clin, B. Compensation for Patients with Work-Related Lung Cancers: Value of Specialised Occupational Disease Consultations to Reduce Under-Recognition. Int. J. Environ. Res. Public Health 2025, 22, 927. https://doi.org/10.3390/ijerph22060927
Roux C, Fafin-Lefevre M, Morello R, Boullard L, Clin B. Compensation for Patients with Work-Related Lung Cancers: Value of Specialised Occupational Disease Consultations to Reduce Under-Recognition. International Journal of Environmental Research and Public Health. 2025; 22(6):927. https://doi.org/10.3390/ijerph22060927
Chicago/Turabian StyleRoux, Clémence, Mélanie Fafin-Lefevre, Rémy Morello, Laurent Boullard, and Bénédicte Clin. 2025. "Compensation for Patients with Work-Related Lung Cancers: Value of Specialised Occupational Disease Consultations to Reduce Under-Recognition" International Journal of Environmental Research and Public Health 22, no. 6: 927. https://doi.org/10.3390/ijerph22060927
APA StyleRoux, C., Fafin-Lefevre, M., Morello, R., Boullard, L., & Clin, B. (2025). Compensation for Patients with Work-Related Lung Cancers: Value of Specialised Occupational Disease Consultations to Reduce Under-Recognition. International Journal of Environmental Research and Public Health, 22(6), 927. https://doi.org/10.3390/ijerph22060927







